Abstract
Intravenous tissue plasminogen activator (tPA) remains the cornerstone of recanalization therapy for acute ischemic stroke (AIS), albeit with varying degrees of response. The triglyceride-glucose (TyG) index is a novel marker of insulin resistance, but association with outcomes among AIS patients who have received tPA has not been well elucidated. We studied 698 patients with AIS who received tPA from 2006 to 2018 in a comprehensive stroke centre. TyG index was calculated using the formula: ln[fasting triglycerides (mg/dL) × fasting glucose (mg/dL)/2]. TyG index was significantly lower in patients that survived at 90-days than those who died (8.61 [Interquartile Range: 8.27–8.99] vs 8.76 [interquartile range: 8.39–9.40], p = 0.007). In multivariate analysis, TyG index was significantly associated with 90-day mortality (OR: 2.12, 95% CI: 1.39–3.23, p = 0.001), poor functional outcome (OR: 1.41 95% CI: 1.05–1.90, p = 0.022), and negatively associated with early neurological improvement (ENI) (OR: 0.68, 95% CI: 0.52–0.89, p = 0.004). There was no association between TyG index and symptomatic intracranial hemorrhage. ‘High TyG’ (defined by TyG index ≥ 9.15) was associated with mortality, poor functional outcomes and no ENI. In conclusion, the TyG index, a measure of insulin resistance, was significantly associated with poorer clinical outcomes in AIS patients who received tPA.
Similar content being viewed by others
Introduction
While intravenous tissue plasminogen activator (IV tPA) remains the mainstay of acute ischemic stroke (AIS) treatment and its use continues to rise1, overall functional outcomes of AIS patients have not improved even with more successful recanalization2, where up to three-quarters of patients experience unfavorable outcomes3. Furthermore, patients also risk having symptomatic intracranial hemorrhage (SICH) following IV tPA4. These highlight a need for greater risk stratification to guide clinical decision making in thrombolysis. Meanwhile, it is estimated that a quarter of the world population suffers from metabolic syndrome5, but despite such a high burden of disease, there are a lack of specific guidelines to risk-stratify post-thrombolysis AIS patients with metabolic syndrome6. Furthermore, conflicting evidence of the relationship between metabolic syndrome (and its components) and outcomes post-thrombolysis further add to the lack of clarity7,8,9. Thus, a reliable and practical measure of metabolic disease should be utilized to re-assess this relationship with post-thrombolysis stroke outcomes.
While Insulin Resistance (IR), part of the underlying pathophysiology of disorders related to metabolic syndrome10, has shown correlation with poor post-stroke outcomes11, current measures of IR, such as the hyperglycemic clamp, the hyperinsulinemic-euglycemic clamp test (HEC) and homeostatic model assessment of insulin resistance (HOMA-IR), are not practical in a clinical setting. Alternative measures of IR that reflect various components of metabolic syndrome have been proposed, but among these, the Triglyceride-Glucose (TyG) Index was shown to be superior to other similar markers for identifying IR risk and risk of developing diabetes12,13. The TyG Index has recently been established as a reliable, cost-effective and easily accessible surrogate marker for IR14,15, and performed better than HOMA-IR in estimating IR when compared to the hyperglycemic clamp16. Furthermore, the TyG index performed superiorly to fasting glucose and triglycerides alone in predicting development of Type 2 diabetes17, and notably increased the risk of cardio-cerebrovascular disease18, suggesting the TyG index may potentially be a good marker of metabolic disease to predict post-stroke outcomes.
While the TyG index has demonstrated predictive effects in major adverse cardiovascular event outcomes in patients with acute coronary disease undergoing percutaneous coronary intervention19, and was superior to fasting glucose and hemoglobin A1c (HbA1C) in this setting20, it has not been well investigated in stroke populations. While previous authors have found an association between incidence of AIS and TyG index21,22, limited studies have reported the effect of the TyG index on post-stroke clinical outcomes or recurrence23,24,25, and the relationship of the TyG index in a thrombolyzed patient cohort has been studied scarcely. The only two studies that have investigated the effect of IR on AIS patients’ response to thrombolysis both utilized HOMA-IR and were limited by sample size or analyzed only non-diabetic patients26,27. Hence, further studies in a current, larger cohort of patients are required to establish the significance of a relationship between the TyG index and post-thrombolysis stroke outcomes. Therefore, we sought to understand the association of the TyG index, as a reflection of IR, in AIS patients who underwent thrombolysis.
Methods
Study design
This is a retrospective cohort study of consecutive patients who received IV tPA from September 2006 to June 2018 and was approved by the local institutional review board. Patients were assessed by a neurologist to be eligible to receive intravenous thrombolysis according to institutional protocol and American Heart Association/American Stroke Association guidelines at a standard dose of 0.9 mg/kg body weight6. All stroke patients who were thrombolyzed underwent standard non-contrast head Computed Tomography and Computed Tomography brain and neck angiography. Patients with valid laboratory readings of their fasting triglycerides and venous glucose levels, taken within 24 h of their AIS admission were included. Patients who were deemed unsuitable for IV tPA were excluded from the study. Other baseline demographics, clinical parameters and ischemic stroke characteristics were collected and tabulated within 24 h of AIS admission. Diabetes Mellitus (DM) was defined as pre-existing diagnosis of diabetes mellitus or an admitting fasting blood glucose level greater than or equal to 7.0 mmol/L (126 mg/dl) or an HbA1c greater than or equal to 6.5%. The presence of Large Vessel Occlusion (LVO) was defined as occlusions of the first and second segment of the middle cerebral artery (MCA), the Internal Carotid Artery (ICA) and as well as its terminus, tandem occlusions involving ICA-MCA, or occlusion of the basilar artery. The severity of stroke at presentation was assessed using the National Institute of Health Stroke Scale (NIHSS)28, recorded as admitting NIHSS. This assessment was made by credentialed nurses as part of the acute stroke response team. The Trial of Org 10172 in Acute Stroke Treatment (TOAST) criteria was used by the treating stroke neurologist to classify stroke subtypes29. A cut-off for TyG index of 9.15 was calculated using the internationally recognised triglyceride cut-off for hypertriglyceridemia of 150 mg/dL (1.7 mmol/L)30 and the cut-off of fasting glucose of 126 mg/dL (7.0 mmol/L), which was selected based on the fasting glucose cut-off for the diagnosis of diabetes31. All procedures were performed in accordance with the relevant guidelines.
The TyG Index was calculated using the formula: ln[fasting triglycerides (mg/dL) × fasting glucose (mg/dL)/2]32. The primary outcome studied was all-cause 90-day mortality. The secondary outcomes included poor functional outcome, for which the dichotomous definition of poor functional outcome was defined as having a 90-day modified Rankin Scale (mRS) of 3–6. The 90-day mRS was evaluated during the follow-up visit to the stroke clinic, and if patients were not able to attend the follow-up visit physically, mRS was evaluated via telephone call instead. Other secondary outcomes included early neurological improvement (ENI), defined as a decrease in NIHSS score by 4 points or more within 24 h or complete resolution of neurological deficits at 24 h, and SICH, based on the European Cooperative Acute Stroke Study (ECASS) II definition: any type of intracerebral hemorrhage on any post-treatment imaging after the start of thrombolysis and increase of ≥ 4 NIHSS points from baseline, or from the lowest value within 7 days, or leading to death33.
Data analysis
Statistical tests were performed using R version 4.0.2 (R Core Team, Vienna, Austria), and a p-value of < 0.05 was considered statistically significant. We performed statistical analysis using Mann–Whitney U test for non-normally distributed continuous variables, and Chi-square analysis for categorical variables. All continuous variables were non-normally distributed as determined by visual histogram and Shapiro–Wilk statistical tests, and median and interquartile range (IQR) were reported for each variable. A logistic regression model was then performed to identify independent predictors of mortality, 90-day functional outcome, ENI and SICH. Covariates that were selected a priori for variable adjustment were age, hypertension, NIHSS recorded at presentation (admitting NIHSS), and LVO status. The adjusted multivariate logistic regression model was then employed to evaluate the associations of TyG index with the respective outcomes and presented as adjusted odds ratio (OR) with 95% confidence intervals (CI).
Characteristics of patients with ‘Low TyG’ and ‘High TyG’ indices were then compared using ≥ 9.15 as a cut-off value, including the distributions of 90-day functional outcome (measured by mRS) between the two groups of patients. Receiver-operating characteristic (ROC) curve analysis was performed in R to determine the performance of the TyG index and the cut-off value in predicting the primary outcome of mortality. Logistic regression was again performed to determine the relationships between ‘High TyG’ and ‘Low TyG’ and dichotomous outcomes including mortality, ENI and SICH. For functional outcome, ordinal shift analysis was carried out to compare associations between patient cohorts with ‘Low TyG’ and ‘High TyG’ indices and ordinal functional outcome (mRS 0–6), where multivariate ordinal logistic regression was presented as OR with 95% CI. After analyzing the main cohort, subgroup analysis was conducted to investigate the relationship of TyG index in patients with and without diabetes respectively. Similarly, baseline characteristics and multivariate logistic regression were carried out for both cohorts of patients with and without diabetes.
Multivariate Imputation by Chained Equations (MICE) in R was employed for missing baseline data to conduct multivariate regression analyses. Variables with originally more than 25% missing data were excluded from multivariate analysis but were used as predictors in imputation. Missing baseline data was imputed with MICE. The multiple imputation method used was Predictive Mean Matching (PMM) for scale variables and logistic regression for categorical variables. Predictors used in the imputation included age, race, gender, smoking status, hypertension, hyperlipidemia, DM, atrial fibrillation (AF), fasting glucose, random plasma glucose, HbA1c, serum total cholesterol, low-density lipoprotein cholesterol (LDL), high-density lipoprotein cholesterol (HDL), triglycerides, the cholesterol:HDL (cholHDL) ratio, serum aspartate transaminase (AST), alanine transaminase (ALT), admitting hematocrit, total white blood cells, lymphocytes, neutrophils, platelets, admitting NIHSS, NIHSS at 2 h and 24 h, systolic blood pressure on admission, 2 h and 24 h, diastolic blood pressure on admission, 2 h and 24 h, baseline mRS, mRS at discharge, 90-day mRS, TOAST classification, SICH, onset-to-treatment time for tPA administration, and LVO status. 30 imputed datasets were created and estimates from the multivariate analysis were pooled.
Ethics approval and consent to participate
Ethics approval was obtained from National Healthcare Group—Domain Specific Review Board (NHG DSRB Reference: 2010/00509) where approval for informed consent was waived as this was patient level data.
Results
Baseline characteristics
A total of 698 patients satisfying the eligibility criteria were included in the final analysis. The baseline characteristics of the study population is summarised in Table 1. The patient cohort had a median age of 65 years (IQR: 55–76), was predominantly male (n = 420, 61.5%) and of Chinese ethnicity (n = 424, 68.7%), and the median admitting NIHSS was 14 (IQR: 8–21). With respect to stroke subtypes, there were large-vessel atherosclerotic strokes (n = 157, 30.4%), cardioembolic strokes (n = 198, 38.3%), small-vessel occlusion (n = 71, 13.7%), strokes of other determined etiology (n = 10, 1.9%) and stroke of unknown etiology (n = 81, 15.7%). A large proportion of patients had hypertension (n = 428, 80.8%) and hyperlipidemia (n = 326, 70.7%), and a smaller proportion of patients had DM (n = 214, 30.7%), were smokers (n = 108, 34.6%) and had AF (n = 124, 38.9%). The median TyG index was 8.62 (IQR: 8.29–9.01). There were n = 63 deaths (9.1%) and n = 308 patients (44.6%) experienced poor functional outcome (mRS 3–6) ninety days after stroke onset. ENI was experienced by n = 389 patients (59.7%) and n = 32 patients (4.6%) suffered a SICH. Median TyG index was significantly lower in survivors at 90-days than those who died (8.61 [IQR: 8.27–8.99] vs 8.76 [IQR: 8.39–9.40], p = 0.007) (Table 1).
The median TyG index was significantly lower in those who experienced ENI (8.56 [IQR: 8.26–8.92] vs 8.69 [IQR: 8.31–9.07], p = 0.007), but there was no significant difference in median TyG index observed between those with good and poor functional outcomes (8.62 [IQR: 8.29–8.98] vs 8.62 [IQR: 8.28–9.08], p = 0.623). There was no significant difference in median TyG index between those who experienced SICH compared to those who had not (8.59 [IQR: 8.22–8.97] vs 8.62 [IQR: 8.29–9.01], p = 0.823) (Table 2).
Multivariate analysis of association between TyG index and mortality, neurological outcomes and intracranial hemorrhage
Results of the logistic regression analysis are shown in Table 3. Age, LVO status, hypertension and admitting NIHSS was significantly associated with mortality in univariate analysis and these variables were adjusted for in the multivariate analysis. On adjustment with these factors, the TyG index remained significantly associated with mortality (OR: 2.12, 95% CI: 1.39–3.23, p = 0.001).
With respect to neurological outcomes, TyG index was significantly associated with poor functional outcomes (OR: 1.41, 95% CI: 1.05–1.90, p = 0.022) as well as poorer ENI (OR: 0.68, 95% CI: 0.52–0.89, p = 0.004). No significant association was observed between TyG index and SICH (OR: 0.90, 95% CI: 0.50–1.64, p = 0.738) (Table 3).
Comparing ‘High TyG’ and ‘Low TyG’ patients using a TyG index cut-off point
Patients with a TyG index of < 9.15 were considered to have ‘Low TyG’ (n = 563) and patients with TyG index of ≥ 9.15 were considered to have ‘High TyG’ (n = 135), utilizing literature cut-offs for triglyceride (150 mg/dL or 1.7 mmol/L) and fasting glucose (7.0 mmol/L or 126 mg/dL)30,31,34. ROC curve analysis was employed to evaluate the association between the TyG index and the primary outcome of mortality, demonstrating an Area Under the Curve (AUC) of 0.604 (95% CI: 0.530–0.678, p = 0.007) (Fig. 1). The value of 9.15 derived as the cut-off for TyG index served as a possible optimal cut-off point on the ROC curve, predicting mortality with 33.3% sensitivity and 81.7% specificity. There were a higher proportion of deaths among ‘High TyG’ (n = 21, 15.6%) patients than ‘Low TyG’ patients (n = 42, 7.6%, p = 0.006). (Supplementary Table 1 [see Additional File 1]).
ROC curve for TyG index predicting mortality. TyG index of 9.15 was found to be a possible optimal cut-off point on the ROC curve (AUC: 0.604, 95% CI: 0.530–0.678, p = 0.007), predicting mortality with 33.3% sensitivity and 81.7% specificity. ROC receiver operating characteristic, TyG index triglyceride-glucose index, AUC area under the curve, CI confidence interval (De Long), p p-value calculated using Mann–Whitney U test.
On multivariate logistic regression, after adjusting for clinical parameters (age, hypertension and LVO status) and stroke severity (admitting NIHSS), ‘High TyG’ index remained independently associated with mortality at 3 months (OR: 2.63, 95% CI: 1.39–4.99, p = 0.003), poor functional outcome (OR: 1.83, 95% CI: 1.17–2.85, p = 0.008) but not ENI (OR: 0.68, 95% CI: 0.45–1.02, p = 0.064), or SICH (OR: 0.95, 95% CI: 0.37–2.39, p = 0.909) (Table 4).
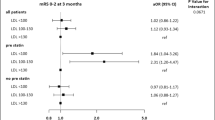
On ordinal shift analysis, patients with ‘High TyG’ were associated with having an unfavourable shift in ordinal mRS (OR: 2.00, 95% CI: 1.42–2.83, p < 0.001) after adjusting for clinical parameters (age, hypertension and LVO status), and stroke severity (admitting NIHSS) (Fig. 2).
Ordinal shift analysis—comparison of mRS distribution between ‘low TyG’ and ‘high TyG’ patients. OR represents adjusted multivariate ordinal logistic regression of TyG index in model against ordinal mRS, adjusting for hypertension, age, admitting NIHSS and LVO status. Legend (right) demonstrates how mRS is represented by colours from 0 (in light blue) to 6 (in dark blue). Values with missing mRS values have been excluded from diagram. OR odds ratio, 95% CI 95% confidence interval, p p-value calculated from ordinal logistic regression, TyG index triglyceride-glucose index, mRS modified Rankin scale, NIHSS National Institutes of Health Stroke Scale, LVO large vessel occlusion.
Subgroup analysis of TyG index and outcomes in patients with and without diabetes
Patients with DM had significantly higher median TyG index than patients without diabetes (8.96 [IQR: 8.55–9.41] vs 8.52 [IQR: 8.19–8.84], p < 0.001). (Supplementary Table 2 [see Additional File 1]) In a subgroup analysis of patients with diabetes (n = 214), the TyG index was not significantly associated with mortality (OR: 1.82, 95% CI: 0.95–3.49, p = 0.069), poor functional outcome (OR: 1.65, 95% CI: 0.99–2.75, p = 0.056), ENI (OR: 0.84, 95% CI: 0.54–1.30, p = 0.436) or SICH (OR: 0.46, 95% CI: 0.14–1.51, p = 0.201) in a multivariate model after adjustment for age, hypertension, admitting NIHSS and LVO status. In comparison, in patients without diabetes (n = 484), the TyG index was significantly associated with mortality (OR: 2.27, 95% CI: 1.15–4.47, p = 0.018), but not significantly associated with poor functional outcome (OR: 0.95, 95% CI: 0.63–1.43, p = 0.805), poor ENI (OR: 0.79, 95% CI: 0.54–1.17, p = 0.239) or SICH (OR: 1.20, 95% CI: 0.54–2.67, p = 0.649) in multivariate analyses (Table 5).
Discussion
In this study, we observed an association between increased TyG index and mortality, poor functional outcome and poor ENI among post-thrombolysis AIS patients. Furthermore, on subgroup analyses, the TyG index was associated with an increased odds of mortality in patients without diabetes.
The TyG index was first introduced as a marker of insulin resistance in 2008 by Simental-Mendia to find a reliable alternative to HOMA-IR, with high sensitivity in detecting IR amidst non-diabetic subjects32. Compared to the HOMA-IR which utilizes glucose and insulin levels, the TyG index uses glucose and triglycerides values. Insulin is not routinely measured in clinical practice and the insulin assays are variable across platforms35,36. In comparison, serum triglyceride and plasma glucose levels are routinely performed in clinical practice and are well-calibrated across platforms14. The gold standard for evaluating IR is the hyperinsulinemic-euglycemic clamp (HEC), however, this labour-intensive and time-consuming procedure is should be performed in specialized metabolic research units requiring trained staff37,38. In 2010, it was then found that TyG index was comparable to and as reliable as the HEC test in assessing IR in a generic patient population including both diabetics and non-diabetics14. The TyG index has been shown to be a good predictor of metabolic health and early identification of IR, by identifying individuals with metabolic derangements39, and indexes involving the TyG index performed better than anthropometric measurements and traditional lipid markers to identify metabolic disease12,40.
The role of the TyG index in various cardiovascular and cerebrovascular diseases has also been increasingly studied. It is associated with arterial stiffness41, increased risk of cardiovascular disease and worse post-percutaneous coronary intervention outcomes19. In stroke, the TyG index has been associated with increased risk of stroke22, in-hospital mortality in critically-ill stroke24, and early recurrent ischemic lesions25. Baseline TyG index has been associated with increased mortality and neurologic deterioration in ischemic stroke23. However, studies have not been conducted to investigate the role of TyG index in patients post-thrombolysis, a key component of stroke management.
Calculation of the TyG index involves fasting glucose and triglycerides, both of which have been shown to have some association with stroke independently. Hyperglycemia in stroke is well known to be associated with poor survival and outcomes, increased infarct volume, increased risk of hemorrhagic conversion and reduced recanalization after IV-tPA42,43. While studies have not been conducted on the associations of post-thrombolysis outcomes with fasting triglycerides, the Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial (REDUCE-IT) study suggested that triglyceride lowering via the use of icosapent ethyl, a purified eicosapentaenoic acid (EPA) ethyl ester, significantly reduces the risk of composite cardiovascular outcomes and stroke44. However, there is evidence to suggest the TyG index is more strongly associated with cardiovascular and cerebrovascular outcomes than fasting glucose and triglycerides alone18,45. A large-scale prospective cohort study comprising 273,368 individuals found a significant association of the TyG index in cerebrovascular and ischemic stroke events, compared to the relationship of joint exposure of glucose and triglycerides scores with cerebrovascular disease which did not reach significance, recommending the TyG index to be a sensitive pre-diagnostic indicator for disease18. Furthermore, the TyG index is shown to be superior to fasting glucose or triglycerides in diagnosing metabolic syndrome and for diabetes prediction17,46. These argue for the TyG index’s utility as an effective way to assess outcomes in cerebrovascular disease as an appropriate measure of IR.
We observed worse mortality, neurological and functional outcomes in patients with high TyG index. A possible mechanism is that IR causes an increase in chronic proinflammatory cytokines and prothrombotic responses and endothelial dysfunction, exacerbating brain damage post stroke47,48,49. It has been suggested that the highly related concept of metabolic syndrome could increase resistance to thrombolysis due to an impaired fibrinolytic system or increased clot density50, and those with more metabolic syndrome components could increase the density of the clot structure and make it more resistant to lysis51. This suggests that identifying and targeting IR for patients to prevent future poor response to thrombolysis may be beneficial, but further studies will be required.
As there is a high prevalence of stroke patients who have concomitant diabetes in our local population (43.2%)52, and patients with diabetes are known to have poorer post-thrombolysis stroke outcomes compared to those without diabetes53, we proceeded to perform a subgroup analysis comparing the outcomes of patients with and without diabetes. Our study observed no association between TyG index and stroke outcomes in patients with diabetes, although the association with mortality and poorer functional outcomes was approaching statistical significance. There are several possible mechanisms for poor response to thrombolysis in diabetic patients—including vascular changes such as cerebral vascular endothelial dysfunction, arterial stiffness and thickening of capillary basal membrane54, hyperglycemia-induced overproduction of reactive oxygen species55, and increased and deregulated neuroinflammation, which could lead to worsened cerebral ischemia and incomplete recanalization after thrombolysis in a patient with diabetes56,57. These affect neurological outcomes through processes that may not be related to IR. Furthermore, in our study, subjects with diabetes were older, and had more cardiovascular risk factors including hypertension and hyperlipidemia. (Supplementary Table 2 [see Additional File 1]) Hence, after adjustment for these comorbidities in multivariate analysis, and accounting for other mechanisms that influence neurological outcomes in diabetes, the TyG index could exert less predictive effect on mortality and functional outcomes in a patient with established diabetes. Nonetheless, the associations of TyG index with mortality and functional outcomes were approaching statistical significance, which also may be related to the relatively small sample size of patients with diabetes in this study (n = 214).
Meanwhile, we found that higher TyG index was significantly associated with mortality but not functional outcomes, ENI or SICH after thrombolysis in patients without diabetes. IR has been associated with poor outcomes after ischemic stroke in patients without diabetes58,59, including in those who received thrombolysis27. The TyG index was also shown to be a good predictor of neurological worsening and stroke recurrence in AIS patients without diabetes23. Although our study did not replicate previous results in demonstrating an association of the TyG index with functional outcomes or ENI, this could be due to differing study populations and sample size. Nonetheless, our results imply that identifying and treating elevated IR even among AIS patients without diabetes could be important to improving post-thrombolysis stroke outcomes including mortality.
Emerging studies have suggested targeting IR as a therapeutic strategy to improve post-stroke outcomes using anti-diabetic drugs in both patients with and without diabetes. The Insulin Resistance Intervention after Stroke (IRIS) trial which involved 3876 patients, demonstrated that pioglitazone, an insulin-sensitizer, reduced the risk of recurrent stroke and myocardial infarction in patients without diabetes who had recent ischemic stroke or TIA11. Others observed associations between prior metformin use and better neurological outcomes in patients with AIS and those who received thrombolytic therapy60. A meta-analysis on glucagon-like peptide-1 (GLP-1) receptor agonists like liraglutide, which work by glucose-dependent insulin release, have shown benefits for primary stroke prevention61. Experimental studies also showed how GLP-1 agonists may exert neuroprotective effects including through mechanisms that decrease insulin resistance in the brain62. These results suggest that trials targeting IR using anti-hyperglycemic insulin-sensitising agents could be explored to further target IR in a post-thrombolysis population, possibly even in patients without diabetes with high levels of IR.
With respect to limitations, this was a retrospective cohort study where causality could not be established. We were not able to compare the TyG index with other markers of insulin resistance, such as the HOMA-IR or hyperinsulinemic-euglycemic clamp, as only data which had been collected on admission as part of routine clinical practice was used. Furthermore, we did not have sufficient data on height, weight, or waist circumference (WC) to compare other related TyG indices such as TyG-BMI (Body Mass Index) or TyG-WC Index. The retrospective nature of our cohort study performed in a single centre warrants future prospective studies to validate the role of the TyG index in predicting thrombolysis outcomes, and could include comparison with HOMA-IR or between patients with and without diabetes. Further studies could also investigate the effect of anti-diabetic agents which affect IR on post-thrombolysis stroke outcomes.
Conclusion
In AIS patients who received tPA, the TyG index, a measure of insulin resistance, was significantly associated with increased 90-day mortality and poorer neurological and functional outcomes.
Data availability
The data that support the findings of this study are available from National University Hospital but restrictions apply to the availability of these data, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of National University Hospital.
References
George, B. P. et al. United States trends in thrombolysis for older adults with acute ischemic stroke. Clin. Neurol. Neurosurg. 139, 16–23. https://doi.org/10.1016/j.clineuro.2015.08.031 (2015).
Fargen, K. M., Meyers, P. M., Khatri, P. & Mocco, J. Improvements in recanalization with modern stroke therapy: A review of prospective ischemic stroke trials during the last two decades. J. NeuroIntervent. Surg. 5, 506. https://doi.org/10.1136/neurintsurg-2012-010541 (2013).
The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the third international stroke trial [IST-3]): A randomised controlled trial. Lancet 379, 2352–2363. https://doi.org/10.1016/S0140-6736(12)60768-5 (2012).
Balami, J. S., Hadley, G., Sutherland, B. A., Karbalai, H. & Buchan, A. M. The exact science of stroke thrombolysis and the quiet art of patient selection. Brain J. Neurol. 136, 3528–3553. https://doi.org/10.1093/brain/awt201 (2013).
Saklayen, M. G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 20, 12. https://doi.org/10.1007/s11906-018-0812-z (2018).
Powers, W. J. et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 50, e344–e418. https://doi.org/10.1161/str.0000000000000211 (2019).
Liu, L. et al. Metabolic syndrome and the short-term prognosis of acute ischemic stroke: A hospital-based retrospective study. Lipids Health Dis. 14, 76–76. https://doi.org/10.1186/s12944-015-0080-8 (2015).
Dorado, L. et al. Metabolic syndrome predicts refractoriness to intravenous thrombolysis in acute ischemic stroke. J. Stroke Cerebrovasc. Dis. 24, 2605–2612. https://doi.org/10.1016/j.jstrokecerebrovasdis.2015.07.015 (2015).
Bas, D. F. & Ozdemir, A. O. The effect of metabolic syndrome and obesity on outcomes of acute ischemic stroke patients treated with systemic thrombolysis. J. Neurol. Sci. 383, 1–4. https://doi.org/10.1016/j.jns.2017.10.012 (2017).
Moller, D. E. & Flier, J. S. Insulin resistance–mechanisms, syndromes, and implications. N. Engl. J. Med. 325, 938–948. https://doi.org/10.1056/nejm199109263251307 (1991).
Kernan, W. N. et al. Pioglitazone after ischemic stroke or transient ischemic attack. N. Engl. J. Med. 374, 1321–1331. https://doi.org/10.1056/NEJMoa1506930 (2016).
Du, T. et al. Clinical usefulness of lipid ratios, visceral adiposity indicators, and the triglycerides and glucose index as risk markers of insulin resistance. Cardiovasc. Diabetol. 13, 146. https://doi.org/10.1186/s12933-014-0146-3 (2014).
Lee, S. H. et al. Predicting the development of diabetes using the product of triglycerides and glucose: The Chungju Metabolic Disease Cohort (CMC) study. PLoS ONE 9, e90430. https://doi.org/10.1371/journal.pone.0090430 (2014).
Guerrero-Romero, F. et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J. Clin. Endocrinol. Metab. 95, 3347–3351. https://doi.org/10.1210/jc.2010-0288 (2010).
Kang, B. et al. Triglycerides/glucose index is a useful surrogate marker of insulin resistance among adolescents. Int. J. Obes. (Lond.) 41, 789–792. https://doi.org/10.1038/ijo.2017.14 (2017).
Vasques, A. C. et al. TyG index performs better than HOMA in a Brazilian population: A hyperglycemic clamp validated study. Diabetes Res. Clin. Pract. 93, e98–e100. https://doi.org/10.1016/j.diabres.2011.05.030 (2011).
Navarro-González, D., Sánchez-Íñigo, L., Pastrana-Delgado, J., Fernández-Montero, A. & Martinez, J. A. Triglyceride-glucose index (TyG index) in comparison with fasting plasma glucose improved diabetes prediction in patients with normal fasting glucose: The vascular-metabolic CUN cohort. Prevent. Med. 86, 99–105. https://doi.org/10.1016/j.ypmed.2016.01.022 (2016).
Si, S. et al. Causal effect of the triglyceride-glucose index and the joint exposure of higher glucose and triglyceride with extensive cardio-cerebrovascular metabolic outcomes in the UK Biobank: A Mendelian randomization study. Front. Cardiovasc. Med. 7, 583473. https://doi.org/10.3389/fcvm.2020.583473 (2020).
Ma, X. et al. Triglyceride glucose index for predicting cardiovascular outcomes after percutaneous coronary intervention in patients with type 2 diabetes mellitus and acute coronary syndrome. Cardiovasc. Diabetol. 19, 31. https://doi.org/10.1186/s12933-020-01006-7 (2020).
Hu, C. et al. Discordance between the triglyceride glucose index and fasting plasma glucose or HbA1C in patients with acute coronary syndrome undergoing percutaneous coronary intervention predicts cardiovascular events: a cohort study from China. Cardiovasc. Diabetol. 19, 116. https://doi.org/10.1186/s12933-020-01091-8 (2020).
Wang, A. et al. Triglyceride-glucose index and the risk of stroke and its subtypes in the general population: An 11-year follow-up. Cardiovasc. Diabetol. 20, 46. https://doi.org/10.1186/s12933-021-01238-1 (2021).
Shi, W. et al. Value of triglyceride-glucose index for the estimation of ischemic stroke risk: Insights from a general population. Nutr. Metab. Cardiovasc. Dis. 30, 245–253. https://doi.org/10.1016/j.numecd.2019.09.015 (2020).
Zhou, Y. et al. Triglyceride glucose index and prognosis of patients with ischemic stroke. Front. Neurol. 11, 456. https://doi.org/10.3389/fneur.2020.00456 (2020).
Zhang, B. et al. Triglyceride-glucose index linked to hospital mortality in critically ill stroke: An observational multicentre study on eICU database. Front. Med. Lausanne 7, 591036. https://doi.org/10.3389/fmed.2020.591036 (2020).
Nam, K.-W., Kwon, H.-M. & Lee, Y.-S. High triglyceride-glucose index is associated with early recurrent ischemic lesion in acute ischemic stroke. Sci. Rep. 11, 15335. https://doi.org/10.1038/s41598-021-94631-5 (2021).
Calleja, A. I. et al. Insulin resistance is associated with a poor response to intravenous thrombolysis in acute ischemic stroke. Diabetes Care 34, 2413–2417. https://doi.org/10.2337/dc11-1242 (2011).
Bas, D. F., Ozdemir, A. O., Colak, E. & Kebapci, N. Higher insulin resistance level is associated with worse clinical response in acute ischemic stroke patients treated with intravenous thrombolysis. Transl. Stroke Res. 7, 167–171. https://doi.org/10.1007/s12975-016-0453-y (2016).
Lyden, P. et al. Improved reliability of the NIH Stroke Scale using video training. NINDS TPA Stroke Study Group. Stroke 25, 2220–2226. https://doi.org/10.1161/01.str.25.11.2220 (1994).
Adams, H. P., Jr. et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of org 10172 in acute stroke treatment. Stroke 24, 35–41. https://doi.org/10.1161/01.str.24.1.35 (1993).
Grundy, S. M. et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 139, e1082–e1143. https://doi.org/10.1161/CIR.0000000000000625 (2019).
American Diabetes, A. 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes-2021. Diabetes Care 44, S15–S33. https://doi.org/10.2337/dc21-S002 (2021).
Simental-Mendia, L. E., Rodriguez-Moran, M. & Guerrero-Romero, F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab. Syndr. Relat. Disord. 6, 299–304. https://doi.org/10.1089/met.2008.0034 (2008).
Hacke, W. et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet (London, England) 352, 1245–1251. https://doi.org/10.1016/s0140-6736(98)08020-9 (1998).
Arnett, D. K. et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 140, e563–e595. https://doi.org/10.1161/CIR.0000000000000677 (2019).
Radziuk, J. Insulin sensitivity and its measurement: Structural commonalities among the methods. J. Clin. Endocrinol. Metab. 85, 4426–4433. https://doi.org/10.1210/jcem.85.12.7025 (2000).
Pacini, G. & Mari, A. Methods for clinical assessment of insulin sensitivity and β-cell function. Best Pract. Res. Clin. Endocrinol. Metab. 17, 305–322. https://doi.org/10.1016/S1521-690X(03)00042-3 (2003).
Tam, C. S. et al. Defining insulin resistance from hyperinsulinemic-euglycemic clamps. Diabetes Care 35, 1605–1610. https://doi.org/10.2337/dc11-2339 (2012).
Rudvik, A. & Månsson, M. Evaluation of surrogate measures of insulin sensitivity - Correlation with gold standard is not enough. BMC Med. Res. Methodol. 18, 64. https://doi.org/10.1186/s12874-018-0521-y (2018).
Lee, S. H. et al. A novel criterion for identifying metabolically obese but normal weight individuals using the product of triglycerides and glucose. Nutr. Diabetes 5, e149. https://doi.org/10.1038/nutd.2014.46 (2015).
Ramdas Nayak, V. K., Nayak, K. R., Vidyasagar, S. Predictive performance of traditional and novel lipid combined anthropometric indices to identify prediabetes. Diabetes Metab. Syndrome Clin. Res. Rev. 14, 1265–1272. https://doi.org/10.1016/j.dsx.2020.06.045 (2020).
Won, K. B. et al. Relationship of insulin resistance estimated by triglyceride glucose index to arterial stiffness. Lipids Health Dis. 17, 268. https://doi.org/10.1186/s12944-018-0914-2 (2018).
Mi, D. et al. Correlation of hyperglycemia with mortality after acute ischemic stroke. Ther. Adv. Neurol. Disord. 11, 1756285617731686. https://doi.org/10.1177/1756285617731686 (2018).
Bruno, A. et al. Admission glucose level and clinical outcomes in the NINDS rt-PA stroke trial. Neurology 59, 669–674. https://doi.org/10.1212/wnl.59.5.669 (2002).
Bhatt, D. L. et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N. Engl. J. Med. 380, 11–22. https://doi.org/10.1056/NEJMoa1812792 (2019).
Jin, J.-L. et al. Triglyceride glucose index for predicting cardiovascular outcomes in patients with coronary artery disease. J. Thorac. Dis. 10, 6137–6146. https://doi.org/10.21037/jtd.2018.10.79 (2018).
Khan, S. H. et al. Metabolic clustering of risk factors: Evaluation of Triglyceride-glucose index (TyG index) for evaluation of insulin resistance. Diabetol. Metab. Syndr. 10, 74. https://doi.org/10.1186/s13098-018-0376-8 (2018).
Ozkul, A. et al. The relationship between insulin resistance and hypercoagulability in acute ischemic stroke. Eur. Neurol. 64, 201–206. https://doi.org/10.1159/000319196 (2010).
de Luca, C. & Olefsky, J. M. Inflammation and insulin resistance. FEBS Lett. 582, 97–105. https://doi.org/10.1016/j.febslet.2007.11.057 (2008).
Kernan, W. N. et al. Insulin resistance and risk for stroke. Neurology 59, 809–815. https://doi.org/10.1212/wnl.59.6.809 (2002).
Mosimah, C. I., Murray, P. J. & Simpkins, J. W. Not all clots are created equal: A review of deficient thrombolysis with tissue plasminogen activator (tPA) in patients with metabolic syndrome. Int. J. Neurosci. 129, 612–618. https://doi.org/10.1080/00207454.2018.1550400 (2019).
Carter, A. M., Cymbalista, C. M., Spector, T. D. & Grant, P. J. Heritability of clot formation, morphology, and lysis: The EuroCLOT study. Arterioscler. Thromb. Vasc. Biol. 27, 2783–2789. https://doi.org/10.1161/atvbaha.107.153221 (2007).
Tan, B. Y. Q. et al. Long-term trends in ischemic stroke incidence and risk factors: Perspectives from an Asian Stroke Registry. J. Stroke 22, 396–399. https://doi.org/10.5853/jos.2020.00878 (2020).
Nikneshan, D. et al. Predicting clinical outcomes and response to thrombolysis in acute stroke patients with diabetes. Diabetes Care 36, 2041–2047. https://doi.org/10.2337/dc12-2095 (2013).
Chen, R., Ovbiagele, B. & Feng, W. Diabetes and stroke: Epidemiology, pathophysiology, pharmaceuticals and outcomes. Am. J. Med. Sci. 351, 380–386. https://doi.org/10.1016/j.amjms.2016.01.011 (2016).
Brownlee, M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes 54, 1615–1625. https://doi.org/10.2337/diabetes.54.6.1615 (2005).
Tang, H. et al. Unfavorable neurological outcome in diabetic patients with acute ischemic stroke is associated with incomplete recanalization after intravenous thrombolysis. J. Neurointervent. Surg. 8, 342–346. https://doi.org/10.1136/neurintsurg-2014-011643 (2016).
Shukla, V., Shakya, A. K., Perez-Pinzon, M. A. & Dave, K. R. Cerebral ischemic damage in diabetes: An inflammatory perspective. J. Neuroinflamm. 14, 21. https://doi.org/10.1186/s12974-016-0774-5 (2017).
Chang, Y., Kim, C. K., Kim, M.-K., Seo, W. K. & Oh, K. Insulin resistance is associated with poor functional outcome after acute ischemic stroke in non-diabetic patients. Sci. Rep. 11, 1229. https://doi.org/10.1038/s41598-020-80315-z (2021).
Jing, J. et al. Insulin resistance and prognosis of nondiabetic patients with ischemic stroke. Stroke 48, 887–893. https://doi.org/10.1161/STROKEAHA.116.015613 (2017).
Westphal, L. P. et al. Association of prestroke metformin use, stroke severity, and thrombolysis outcome. Neurology 95, e362–e373. https://doi.org/10.1212/wnl.0000000000009951 (2020).
Malhotra, K. et al. GLP-1 receptor agonists in diabetes for stroke prevention: A systematic review and meta-analysis. J. Neurol. 267, 2117–2122. https://doi.org/10.1007/s00415-020-09813-4 (2020).
Erbil, D. et al. GLP-1’s role in neuroprotection: a systematic review. Brain Inj. 33, 734–819. https://doi.org/10.1080/02699052.2019.1587000 (2019).
Funding
No source of funding for the research was obtained.
Author information
Authors and Affiliations
Contributions
E.M.S.T., A.Y.L.L., B.Y.Q.T. contributed to conception and design of the study. E.M.S.T., A.Y.L.L., B.Y.Q.T. analyzed and interpreted the patient data. E.M.S.T., A.Y.L.L., B.W.Q.T. and B.Y.Q.T. were major contributors in writing the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Toh, E.M.S., Lim, A.Y.L., Ming, C. et al. Association of triglyceride-glucose index with clinical outcomes in patients with acute ischemic stroke receiving intravenous thrombolysis. Sci Rep 12, 1596 (2022). https://doi.org/10.1038/s41598-022-05467-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-05467-6
This article is cited by
-
The impact of triglyceride-glucose index on ischemic stroke: a systematic review and meta-analysis
Cardiovascular Diabetology (2023)
-
The metabolic score for insulin resistance as a predictor of clinical outcome in stroke patients treated by intravenous thrombolysis
Neurological Sciences (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.