Abstract
During lateral lumbar interbody fusion (LLIF), unintended intraoperative endplate injury (IEPI) can occur and thereafter lead cage subsidence. The aim of this study was to investigate the incidence of IEPI during LLIF, and its predisposing factors. A retrospective review was conducted on consecutive patients (n = 186; mean age, 70.0 ± 7.6 years) who underwent LLIF at 372 levels. Patient’s demographic and surgical data were compared between patients with and without IEPI. Also, the radiographic data of each level were compared between intact and IEPI segments. IEPI was identified at 76 levels (20.4%) in 65 patients. The incidences of IEPI at every 100 consecutive segments were not different. When 372 segments were analyzed independently, sagittal disc angle (DA) in the extended position (4.3° ± 3.6° at IEPI segments vs. 6.4° ± 4.0° at intact segments), the difference between sagittal DA in the extended position and cage angle (− 2.2° ± 4.0° vs. 0.0° ± 3.9°), and the difference between preoperative disc height and cage height (− 5.4 mm ± 2.4 mm vs. − 4.7 mm ± 2.0 mm) were different significantly. Also, endplate sclerosis was more common at intact segments than IEPI segments (33.2% vs. 17.3%). Multivariate analysis showed that male sex (odds ratio [OR] 0.160; 95% confidence interval [CI] 0.036–0.704), endplate sclerosis (OR 3.307; 95% CI 1.450–8.480), and sagittal DA in the extended position (OR 0.674; 95% CI 0.541–0.840) were significant associated factors for IEPI. IEPI was correlated not with surgeon’s experience, but with patient factors, such as sex, preoperative disc angle, and endplate sclerosis. Careful surgical procedures should be employed for patients with these predisposing factors.
Similar content being viewed by others
Introduction
Lumbar interbody fusion (LIF) has been widely used to treat degenerative diseases presenting with low back pain and neurological symptoms. LIF may increase fusion rate and restore ideal segmental lordosis. Traditionally, LIF has been performed via several open approaches; anterior, posterior, or transforaminal. Among these options, anterior LIF (ALIF) has some advantages. It can provide greater bone-graft contact surface and the possibility to insert more lordotic cages, improving sagittal balance1,2,3,4. However, some pitfalls of conventional ALIF exit: great vessel injury, abdominal organ injury, and incisional hernia5.
Several minimally invasive spine surgical techniques have been developed to achieve better clinical outcomes and reduce postoperative complications compared to conventional open procedures. Minimally invasive surgery (MIS) has been shown to reduce postoperative pain; perioperative complications including hospital stay, blood loss, and need for analgesics; and lead to earlier recovery compared to conventional open techniques6,7,8. Recently, MIS-lateral LIF (LLIF) has gained popularity because it can provide similar effects as ALIF using tubular retractors. It can be performed by two approaches, (1) extreme LIF (XLIF), which accesses the intervertebral disc via transpsoas and (2) oblique LIF (OLIF), which is accessed via the oblique corridor between the aorta and the psoas muscle. The processes, in common, consist of discectomy, endplate preparation, and cage placement.
However, these LLIF procedures can result in several perioperative complications, including nerve injury, vascular injury or endplate injury9,10,11. Among these, endplate injury often occurs during endplate preparation and cage placement. Once it occurs, it can result in cage subsidence, leading to the loss of segmental lordosis and foraminal height. This type of endplate injury has features different from spontaneous cage subsidence in the postoperative course. Because the former is considered iatrogenic, careful surgical skills are indispensable. However, patient-related factors may also affect the development of intraoperative endplate injury (IEPI)12. The purpose of this study was to investigate the incidence of IEPI and identify its risk factors in our consecutive MIS-LLIF series.
Results
A total of 186 patients were eligible for inclusion in this study. The mean age at the time of surgery was 70.0 ± 7.6 years (range 31–85) and 79.6% (148/186) were female. In those patients, 372 levels were treated using MIS-LLIF. The most common segment was L2-3 (170 segments, 45.7%), followed by L3-4 (130 segments, 34.9%), L1-2 (52 segments, 14.0%), and L4-5 (20 segments, 5.4%). Forty-two patients underwent single-level LLIF and 144 patients underwent multiple level LLIF. The patient demographic details are shown in Table 1.
Incidence of IEPI
The interobserver intra-class coefficients (ICC) for the measurement of IEPI was 0.842. The intraobserver ICC were 0.933 and 0.886 for each examiner. IEPI was identified at 76 levels (20.4%) in 65 patients. Unilateral endplate injury, indicated by damage of either endplate in an intervertebral disc, was noted at 67 levels and bilateral injury, indicated by damage of both endplates in an intervertebral disc, was noted at nine levels. Injury at the endplate cranial and caudal to the disc was noted at 33 and 52 levels, respectively (Table 2). The incidence of IEPI at each level ranged from 19.4% (33/170) to 35% (7/20). However, significant differences between the incidence at each segment were not found (Table 2). The type of IEPI had no association with the disc level. The incidence of IEPI every 100 segments is shown in Fig. 1. There was no significant difference between the incidence in each group.
Characteristics of IEPI segments and risk factors
Age, BMI, BMD of the lumbar spine, and whether single- or multiple-level LLIF were not different between the patients with IEPI and the patients without IEPI (Table 3). Sex distribution was significantly different between the two groups (Table 3). When 372 LLIF segments were analyzed independently, coronal DA and sagittal DA in the neural and flexed positions were not different between the segments with and without IEPI (Table 4). The degree of facet arthrosis and disc height were not different between the two groups. However, sagittal DA in the extended position was significantly smaller at the IEPI segments than the intact segments (4.3° ± 3.6° vs. 6.4° ± 4.0°, P = 0.001). The difference between sagittal DAs in the extended position and cage angle was significantly different (− 2.2° ± 4.0° at IEPI segments vs. 0.0° ± 3.9° at intact segments, P < 0.001). Also, the difference between the preoperative disc height and the cage height was greater in the IEPI segments than in the intact segments (− 5.4 mm ± 2.4 mm vs. − 4.7 mm ± 2.0 mm, P = 0.042). The presence of endplate sclerosis was more frequent at the intact segments compared to the IEPI segments (33.2% [67/296] vs. 17.3% [9/76], P = 0.028). The incidence of IEPI did not differ significantly according to the cage angle and height (data not shown).
Multivariate logistic regression analysis revealed that sex, sagittal DA in the extended position, and endplate sclerosis were risk factors for IEPI (Table 5). Male sex (odds ratio [OR] 0.160; 95% confidence interval [CI] 0.036–0.704; P = 0.015) and endplate sclerosis (OR 3.307; 95% CI 1.450–8.480; P = 0.005) were negatively associated with the development of IEPI. Smaller sagittal DA in the extended position was a risk factor for IEPI (OR 0.674; 95% CI 0.541–0.840; P < 0.001).
Discussion
The incidence of IEPI was 20.4% (76/372), which was higher than that in the previous reports. This might have resulted from our definition of IEPI, which was as at least 1 mm of cage settling, compared to the study by Satake et al.12 that defined IEPI was defined as at least 2 mm of cage settling. Vertebral endplate thickness has been reported to range from 0.35 to 1.03 mm13,14,15,16,17. Therefore, the authors decided to use at least 1 mm as the criteria for IEPI in this study.
The vertebral endplate is a thin cortical bone located at the cranial and caudal surfaces of the vertebral bodies. In their histologic study, Hou et al.18 showed that the endplate was not genuine cortical bone but a porous structure with the involvement of trabeculae. The significance of the endplate had been already demonstrated in many reports. Removal of the endplate can significantly decrease the structural properties of the lumbar vertebral bodies18,19,20,21. Interbody cages in the lumbar spine are commonly used to increase mechanical stability and promote fusion, however, lumbar vertebrae with endplate damage have a higher risk of cage subsidence.
The factors associated with cage subsidence following intervertebral fusions have been reported to involve BMD22, cage geometry23,24, cage material25, cage location26,27,28,29, and the use of osteobiologics30. Cage subsidence is thought to result from biological remodeling at the cage-bone interface in a chronic fashion31. In contrast, IEPI develops in an acute fashion during the surgical procedure and the development of IEPI may have the different pathomechanisms and risk factors. There were little studies about IEPI after MIS-LLIF. Two risk factors for IEPI after MIS-LLIF had been reported in the study by Satake et al.12: reduced BMD and cage height.
Although our results that female sex was a significant risk factor for IEPI in this study could suggest a possible correlation between BMD and IEPI, the direct measurement of BMD in each vertebra was not correlated with IEPI, unlike the previous report12. BMD, which is usually assessed by DEXA, shows trabecular bone quality. Hou et al.26 and Patel et al.32 conducted biomechanical tests on human cadaveric lumbar vertebrae and their results indicated that reduced BMD was positively associated with the failure load of the endplate, which ranged from 20 to 50%. They concluded that the lumbar vertebrae with reduced BMD had a higher risk of cage subsidence26,32. However, IEPI actually means cortical bone injury. Thus, the authors concluded that IEPI might be affected by cortical bone strength. Some parameters for cortical bone status of the endplate have been reported in previous studies. The endplate cranial to the intervertebral disc was thicker and had a higher density than the caudal one13,14,15,16,17,33,34. Based on this biomechanical property, IEPI could be expected to occur at the weak endplate. Our findings that the endplate injury was more common at the caudal endplate and one of the associated factors was endplate sclerosis (as a protective factor) supported the previous data.
Also, the characteristics of the intervertebral disc should be considered as factors affecting the development of IEPI. Previous reports showed that over-distraction of the intervertebral height by a tall cage could damage the endplate either intraoperatively or postoperatively12,34. However, there was no effect of segmental disc height and its difference from cage height on the development of IEPI. In the current study, the degree of facet arthrosis was not associated with the development of IEPI. However, a smaller sagittal disc angle in the extended position was found to be positively correlated with IEPI. It is plausible that the extent of disc motion could affect the development of IEPI; the more mobile disc, the less IEPI, and vice versa. We believe our results support this hypothesis. When planning LLIF followed by posterior spinal fixation in patients with less mobile discs, a different surgical strategy, for example, 3-stage surgery (posterior facetectomy-to-LLIF-to-posterior fixation) should be considered.
IEPI was considered somewhat iatrogenic in the previous reports. However, the conclusions were not an evidence-based, but empirical conclusions10,12. To the best of our knowledge, no study has analyzed the learning curve for IEPI after MIS-LLIF. In our study, there was no difference in the incidences of IEPI during MIS-LLIF at every 100 discs. This suggests that the iatrogenic factor for IEPI was minimal or none.
There were some limitations in our study. First, this was a retrospective study. Therefore, the factor of surgeon’s caution in osteoporotic patients could not be considered. Second, the radiographic measurement of the endplate injury was not verified because it could be missed on plain radiographs, especially in patients with scoliosis. Third, we did not evaluate the radiographic findings and clinical prognosis during the late period. IEPI was a radiographic finding and its clinical significance was not analyzed. However, some patients with IEPI experienced progressive cage subsidence and even vertebral body fracture. We are preparing the next article about clinical outcomes of IEPI. Fourth, the quantification of IEPI was not included in this study. The authors defined IEPI as > 1 mm of endplate damage. However, the surgical prognosis could be different according to the severity of the IEPI.
In conclusion, this study showed that the development of IEPI after MIS-LLIF was significantly correlated with some patient-related factors, including gender, sagittal disc angle in the extended position, and endplate sclerosis, whereas the surgeon’s experience did not affect the development of IEPI. Therefore, patients who have these risk factors are at risk of IEPI after MIS-LLIF. Thorough preoperative evaluation is needed to avoid IEPI when considering MIS-LLIF surgery and careful surgical procedures should be performed in patients with an elevated risk.
Methods
Patients
This retrospective study was approved by The Catholic University of Korea Catholic Medical Center’s Institutional Review Board before study initiation and all methods were performed in accordance with the relevant guidelines (approval no. KC20RISI0169). Informed consents were waived by The Catholic University of Korea Catholic Medical Center’s Institutional Review Boar because of the retrospective study design. All consecutive patients who underwent MIS-LLIF for degenerative lumbar disc diseases (from L1-2 to L4-5) between May 2012 and December 2017 were reviewed and the operative data in the medical records were investigated. To minimize bias, patients who underwent operations by surgeons other than the single senior author (KYH) were excluded. The patients included in this study were the first 186 patients who underwent MIS-LLIF performed by this surgeon. Clinical data, including age, sex, body mass index (BMI), and bone mineral density (BMD) were reviewed in the medical records. BMD at the L1-4 levels of the posteroanterior spine was measured using dual-energy X-ray absorptiometry (DEXA) bone densitometry (Lunar Prodigy Advance, GE Healthcare, Waukesha, WI, USA). T-scores of the lumbar spine (L1-4) were recorded and BMD in each vertebra was recorded as g/m2.
Surgical procedures
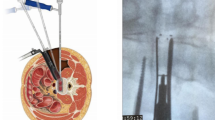
MIS-LLIF was performed, according to XLIF manner, by splitting the psoas muscle using tubular retractors and intraoperative neuromonitoring5. All procedures were performed in a right lateral decubitus position with the hip and knee joints flexed. A surgical incision was made through the skin after identification of the position of the target disc using a C-arm. A single 5-cm skin incision was made in patients who underwent LLIF in one or two segments, whereas two separate incisions were made in patients who underwent LLIF in three or more segments. The retroperitoneal space was reached via blunt dissection and tubular retractors were placed onto the disc. After that, removal of the disc materials and endplate preparation was conducted. For endplate preparation, a Cobb elevator and ring curette were used. None of the shavers was used at any step. The cage size was determined using trial cages and then the real cage was inserted with two containment blades. The dimensions of each cage were recorded in the medical charts. All procedures were performed under fluoroscopic guidance. Poly-etherether-ketone (PEEK) cages (Clydesdale, Medtronic Sofamor Danek, Memphis, TN, USA) were used in all patients. After anterior surgery, posterior fusion with pedicle screws was performed in a single or staged manner.
Radiographic measurements
IEPI was identified on the immediate postoperative lateral X-ray compared to the preoperative lateral X-ray. It was defined as cage sinking of more than 1 mm from the bony endplate (Fig. 2). Two spine fellows, who had not been participate in the surgery, independently measured the extent of endplate injury and the same measurements were repeated by these two examiners. The average values were obtained and used in the definition of IEPI. IEPI was classified into two groups based on involvement: unilateral or bilateral, superior or inferior. The profiles of the inserted cage, such as its height and lordotic angle, were recorded. The following parameters of each intervertebral disc were measured: (1) segmental disc angle (DA) on the sagittal plane in the neural, flexed, and extended positions and (2) disc height (DH) in the anterior and posterior corners. We calculated the differences in their height and angle between the disc and the cage. Because endplate sclerosis could affect endplate injury, the existence of sclerosis was investigated. Four grades of facet joint arthrosis were identified on the computed tomography (CT) and magnetic resonance imaging (MRI) using the criteria proposed by Weishaupt et al.35.
Overall incidence of intraoperative endplate injury and its distribution according to experience
The incidence of IEPI in each segment (from L1–2 to L4–5) was calculated. Because the development of IEPI could be affected by surgical skills, the authors divided the cohort into four arbitrary groups, one every 100 segments, and analyzed the IEPI incidence of each group to evaluate learning-curve effects.
Statistical analysis
SPSS 21.0 (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses. The demographic, surgical, and radiographic data were compared between the IEPI group and the no-IEPI group. The Chi-squared test was used for categorical data and the Student’s t-test was used for continuous data. To identify the risk factors for IEPI, univariate analysis for each parameter of demographic, surgical, and radiographic data was performed using logistic regression analysis. Parameters with a P-value ≤ 0.10 were included in multivariate logistic regression analysis. Inter- and intraobserver reliability for the measurement of IEPI were analyzed using intra-class coefficients (ICC). Statistical significance was considered at P < 0.05.
References
Watkins, R. G. 4th., Hanna, R., Chang, D. & Watkins, R. G. 3rd. Sagittal alignment after lumbar interbody fusion: Comparing anterior, lateral, and transforaminal approaches. J. Spinal Disord. Tech. 27, 253–256. https://doi.org/10.1097/BSD.0b013e31828a8447 (2014).
Kapustka, B. et al. Anterior lumbar interbody fusion (ALIF): Biometrical results and own experiences. Neurosurg. Rev. https://doi.org/10.1007/s10143-019-01108-1 (2019).
Hsieh, P. C. et al. Anterior lumbar interbody fusion in comparison with transforaminal lumbar interbody fusion: Implications for the restoration of foraminal height, local disc angle, lumbar lordosis, and sagittal balance. J. Neurosurg. Spine 7, 379–386. https://doi.org/10.3171/spi-07/10/379 (2007).
Kim, H. J. et al. Adult spinal deformity: Current concepts and decision-making strategies for management. Asian Spine J. 14, 886–897. https://doi.org/10.31616/asj.2020.0568 (2020).
Kim, Y. H. et al. Lumbar interbody fusion: Techniques pearls and pitfalls. Asian Spine J. 14, 730–741. https://doi.org/10.31616/asj.2020.0485 (2020).
Goldstein, C. L., Phillips, F. M. & Rampersaud, Y. R. Comparative effectiveness and economic evaluations of open versus minimally invasive posterior or transforaminal lumbar interbody fusion: A systematic review. Spine 41(Suppl 8), S74-89. https://doi.org/10.1097/brs.0000000000001462 (2016).
Goldstein, C. L., Macwan, K., Sundararajan, K. & Rampersaud, Y. R. Perioperative outcomes and adverse events of minimally invasive versus open posterior lumbar fusion: Meta-analysis and systematic review. J. Neurosurg. Spine 24, 416–427. https://doi.org/10.3171/2015.2.Spine14973 (2016).
Park, J. et al. Minimally invasive spine surgery: Techniques, technologies, and indications. Asian Spine J. 14, 694–701. https://doi.org/10.31616/asj.2020.0384 (2020).
Walker, C. T. et al. Complications for minimally invasive lateral interbody arthrodesis: A systematic review and meta-analysis comparing prepsoas and transpsoas approaches. J. Neurosurg. Spine https://doi.org/10.3171/2018.9.Spine18800 (2019).
Zeng, Z. Y. et al. Complications and prevention strategies of oblique lateral interbody fusion technique. Orthop. Surg. 10, 98–106. https://doi.org/10.1111/os.12380 (2018).
Abe, K. et al. Perioperative complications in 155 patients who underwent oblique lateral interbody fusion surgery: perspectives and indications from a retrospective, multicenter survey. Spine 42, 55–62. https://doi.org/10.1097/brs.0000000000001650 (2017).
Satake, K., Kanemura, T., Yamaguchi, H., Segi, N. & Ouchida, J. Predisposing factors for intraoperative endplate injury of extreme lateral interbody fusion. Asian Spine J. 10, 907–914. https://doi.org/10.4184/asj.2016.10.5.907 (2016).
Edwards, W. T., Zheng, Y., Ferrara, L. A. & Yuan, H. A. Structural features and thickness of the vertebral cortex in the thoracolumbar spine. Spine 26, 218–225. https://doi.org/10.1097/00007632-200101150-00019 (2001).
Roberts, S., McCall, I. W., Menage, J., Haddaway, M. J. & Eisenstein, S. M. Does the thickness of the vertebral subchondral bone reflect the composition of the intervertebral disc?. Eur. Spine J. 6, 385–389. https://doi.org/10.1007/bf01834064 (1997).
Silva, M. J., Wang, C., Keaveny, T. M. & Hayes, W. C. Direct and computed tomography thickness measurements of the human, lumbar vertebral shell and endplate. Bone 15, 409–414. https://doi.org/10.1016/8756-3282(94)90817-6 (1994).
Zhao, F. D., Pollintine, P., Hole, B. D., Adams, M. A. & Dolan, P. Vertebral fractures usually affect the cranial endplate because it is thinner and supported by less-dense trabecular bone. Bone 44, 372–379. https://doi.org/10.1016/j.bone.2008.10.048 (2009).
Wang, Y., Battie, M. C., Boyd, S. K. & Videman, T. The osseous endplates in lumbar vertebrae: Thickness, bone mineral density and their associations with age and disk degeneration. Bone 48, 804–809. https://doi.org/10.1016/j.bone.2010.12.005 (2011).
Hou, Y., Yuan, W., Kang, J. & Liu, Y. Influences of endplate removal and bone mineral density on the biomechanical properties of lumbar spine. PLoS ONE 8, e76843. https://doi.org/10.1371/journal.pone.0076843 (2013).
Flamme, C. H., von der Heide, N., Heymann, C. & Hurschler, C. Primary stability of anterior lumbar stabilization: Interdependence of implant type and endplate retention or removal. Eur. Spine J. 15, 807–818. https://doi.org/10.1007/s00586-005-0993-4 (2006).
Polikeit, A., Ferguson, S. J., Nolte, L. P. & Orr, T. E. The importance of the endplate for interbody cages in the lumbar spine. Eur. Spine J. 12, 556–561. https://doi.org/10.1007/s00586-003-0556-5 (2003).
Oxland, T. R., Grant, J. P., Dvorak, M. F. & Fisher, C. G. Effects of endplate removal on the structural properties of the lower lumbar vertebral bodies. Spine 28, 771–777 (2003).
Oh, K. W., Lee, J. H., Lee, J. H., Lee, D. Y. & Shim, H. J. The correlation between cage subsidence, bone mineral density, and clinical results in posterior lumbar interbody fusion. Clin. Spine Surg. 30, E683-e689. https://doi.org/10.1097/bsd.0000000000000315 (2017).
Alkalay, R. N., Adamson, R. & Groff, M. W. The effect of interbody fusion cage design on the stability of the instrumented spine in response to cyclic loading: An experimental study. Spine J. 18, 1867–1876. https://doi.org/10.1016/j.spinee.2018.03.003 (2018).
Yuan, W., Kaliya-Perumal, A. K., Chou, S. M. & Oh, J. Y. Does lumbar interbody cage size influence subsidence? A biomechanical study. Spine https://doi.org/10.1097/brs.0000000000003194 (2019).
Seaman, S., Kerezoudis, P., Bydon, M., Torner, J. C. & Hitchon, P. W. Titanium vs polyetheretherketone (PEEK) interbody fusion: Meta-analysis and review of the literature. J. Clin. Neurosci. 44, 23–29. https://doi.org/10.1016/j.jocn.2017.06.062 (2017).
Hou, Y. & Luo, Z. A study on the structural properties of the lumbar endplate: Histological structure, the effect of bone density, and spinal level. Spine 34, E427-433. https://doi.org/10.1097/BRS.0b013e3181a2ea0a (2009).
Alimi, M. et al. The impact of cage dimensions, positioning, and side of approach in extreme lateral interbody fusion. Clin. Spine Surg. 31, E42-e49. https://doi.org/10.1097/bsd.0000000000000507 (2018).
Lang, G. et al. Elimination of subsidence with 26-mm-wide cages in extreme lateral interbody fusion. World Neurosurg. 104, 644–652. https://doi.org/10.1016/j.wneu.2017.05.035 (2017).
Briski, D. C. et al. Does spanning a lateral lumbar interbody cage across the vertebral ring apophysis increase loads required for failure and mitigate endplate violation. Spine 42, E1158-e1164. https://doi.org/10.1097/brs.0000000000002158 (2017).
Vaidya, R. et al. Complications in the use of rhBMP-2 in PEEK cages for interbody spinal fusions. J. Spinal Disord. Tech. 21, 557–562. https://doi.org/10.1097/BSD.0b013e31815ea897 (2008).
Fyhrie, D. P. & Schaffler, M. B. Failure mechanisms in human vertebral cancellous bone. Bone 15, 105–109. https://doi.org/10.1016/8756-3282(94)90900-8 (1994).
Patel, R. R. et al. Evaluation and prediction of human lumbar vertebrae endplate mechanical properties using indentation and computed tomography. J. Biomech. Eng. https://doi.org/10.1115/1.4040252 (2018).
Grant, J. P., Oxland, T. R. & Dvorak, M. F. Mapping the structural properties of the lumbosacral vertebral endplates. Spine 26, 889–896. https://doi.org/10.1097/00007632-200104150-00012 (2001).
Tohmeh, A. G., Khorsand, D., Watson, B. & Zielinski, X. Radiographical and clinical evaluation of extreme lateral interbody fusion: Effects of cage size and instrumentation type with a minimum of 1-year follow-up. Spine 39, E1582-1591. https://doi.org/10.1097/brs.0000000000000645 (2014).
Weishaupt, D., Zanetti, M., Boos, N. & Hodler, J. MR imaging and CT in osteoarthritis of the lumbar facet joints. Skeletal. Radiol. 28, 215–219. https://doi.org/10.1007/s002560050503 (1999).
Acknowledgements
This work was supported by Small Grant for Exploratory Research (SGER) through the Ministry of Education of the Republic of Korea (Grant Number: NRF-2018R1D1A1A02086045).
Author information
Authors and Affiliations
Contributions
Y.H.K., K.Y.H. and S.I.K. contributed to the conception or design of the work. Y.H.K. and S.I.K. contributed to drafting the work and revising it critically for important intellectual content. D.G.C, H.Y.P., E.J.Y. and K.T.K. contributed to interpret and analyse data for the work. Y.H.K. and S.I.K. wrote the first draft of the manuscript and all authors contributed to the review, approval of the final manuscript, and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, YH., Ha, KY., Kim, KT. et al. Risk factors for intraoperative endplate injury during minimally-invasive lateral lumbar interbody fusion. Sci Rep 11, 20149 (2021). https://doi.org/10.1038/s41598-021-99751-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-99751-6
This article is cited by
-
Effectiveness of a two-stage posterior-anterior–posterior surgery using subcutaneously preserved autologous bone grafts for adult spinal deformity: a retrospective observational study
Journal of Orthopaedic Surgery and Research (2024)
-
Coronal vertical fracture of vertebral body following minimally invasive lateral lumbar interbody fusion: risk factor analysis in consecutive case series
Acta Neurochirurgica (2024)
-
Biomechanical properties of lumbar vertebral ring apophysis cage under endplate injury: a finite element analysis
BMC Musculoskeletal Disorders (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.