Abstract
Pachychoroid neovasculopathy (PNV) is a new concept of macular disorder. Some cases diagnosed as age-related macular degeneration (AMD) have been re-diagnosed as PNV. However, the biological features of PNV are still uncertain. The purpose of this study was to compare PNV and AMD by analyses focusing on von Willebrand factor (VWF) and complement factor H (CFH). Ninety-seven patients who were previously diagnosed with treatment naïve AMD were enrolled in this study. They were re-classified as either PNV or AMD based on the clinical criteria and 33 patients were classified as PNV and 64 patients as AMD. We examined the clinical data, analyzed VWF multimer and two genetic polymorphisms (I62V and Y402H) in the CFH. PNV group was significantly younger than AMD group (P = 0.001). In both I62V and Y402H, there were no significant differences between PNV and AMD while the recessive homozygous (AA) was found only in PNV group in I62V. The presence of unusually large VWF multimers (UL-VWFMs) and subretinal hemorrhages were significantly higher in PNV than in AMD (P = 0.045, P = 0.020, respectively). Thus, the residual UL-VWFMs may result in platelet thrombosis and hemorrhages in the choriocapillaris of PNV. In conclusion, our results suggest the biological differences between PNV and AMD.
Similar content being viewed by others
Introduction
Age-related macular degeneration (AMD) is a major retinal disease in the elderly1. AMD can be categorized by the stage of the disease. In the early stage of AMD, the abnormalities of retinal pigment epithelium (RPE) and drusen (yellowish extracellular material between Bruch's membrane and RPE) are the clinical signs2. The late stage of AMD can be classified into neovascular (exudative) AMD and atrophic (dry) AMD2. Choroidal neovascularization (CNV) is pathognomonic for neovascular AMD. Typical neovascular AMD is commonly classified according to the location of the CNV, beneath (Type 1) or above the RPE (Type 2). There are also two specific types in neovascular AMD: polypoidal choroidal vasculopathy (PCV) and retinal angiomatous proliferation (RAP)1. PCV is characterized by polypoidal lesions in indocyanine green angiography (ICGA) and has recently been considered a subtype of Type 1 AMD3. As for atrophic AMD, atrophied RPE and visible choroidal vessels (geographic atrophy) are distinctive.
AMD is a multifactorial disease, and several causes have been suggested such as the sex of the individual, smoking, inflammation, and genetic factors2,4. Complement factor H (CFH) is also a well-known risk factor for AMD5,6, and it is activated by various pathways to initiate immune responses. This activation may then cause cell damage7. CFH also promotes the degradation of the von Willebrand factor (VWF)8,9,10. VWF, a large glycoprotein with a multimeric mass structure, is exclusively produced by vascular endothelial cells as unusually large VWF multimers (UL-VWFMs) and is secreted into the plasma11,12. The UL-VWFMs are cleaved by a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMTS13). Although VWF plays an important role in pertinent coagulation, the presence of UL-VWFMs in the plasma is a risk factor for arterial thrombosis13,14. Some researchers have reported an elevation of the plasma levels of VWF in treatment naïve AMD patients15,16, and we also have demonstrated the higher percentages of existence of UL-VWFMs in patients with AMD compared to that in controls17.
Pang et al. have proposed a new disease concept called ‘pachychoroid neovasculopathy’ (PNV)3,18,19. PNV is a disorder which is characterized by the development of Type1 CNV secondary to central serous chorioretinopathy (CSC) or pachychoroid pigment epitheliopathy (PPE). CSC occurs mostly in middle aged males and is characterized by choroidal thickening and dysfunction of the RPE which results in the presence of subretinal fluid (SRF) and RPE atrophy19,20. PPE is characterized by hyperplasia of the RPE and choroidal thickening without SRF, and it was suggested as an incomplete state or a pre-stage of CSC18. In PNV, several clinical features have been noted: choroidal thickening (pachychoroid), dilated choroidal vessels (pachyvessels), increased choroidal vascular permeability, and absence of drusen3,19. However, the biological features of PNV are still uncertain.
We consider that many cases diagnosed as AMD may in fact be PNV and that comparisons of the biological characteristics of AMD and PNV have not been well done.
Thus, the purpose of this study is to compare the characteristics of AMD to that of PNV. To accomplish this, we re-examined previously diagnosed AMD cases and separated them into PNV and AMD. We then performed VWF-based clinical and hematological analyses and genetic polymorphisms in the CFH to investigate the differences between two ocular disorders.
Results
The baseline characteristics of patients are available in Table 1. The median age was 77.0 years old and seventy were male (72%). There were 51 past or current smokers (53%). The median central choroidal thickness (CCT) was 217 μm and the median choroidal vessel diameter (CVD) was 144 μm. There was a strong positive correlation between CCT and CVD (r = 0.854, P < 0.001, Pearson's correlation coefficient). We also measured the blood type of all patients because it has been reported that VWF in individuals with blood type O is more likely to be cleaved by ADAMTS1321,22.
Of the 97 total patients, 33 patients (34%) were diagnosed with PNV. The comparisons of patients based on PNV and AMD are summarized in Table 2. The patients in PNV group were significantly younger than those in AMD group (P = 0.001), and CCT was significantly thicker in PNV group (P < 0.001). CVD was also significantly greater in PNV than in AMD (P < 0.001). The rate of positive UL-VWFMs was significantly higher in PNV group than that in AMD group (36% vs 17%, P = 0.045), and the rate of subretinal hemorrhage was also significantly higher in PNV group than in AMD group (24% vs 6%, P = 0.020). The presence of SRF was significantly higher in PNV group than in AMD group (85% vs 52%, P = 0.001). There was no difference in the distribution of the blood type between the two groups.
The significant differences were not found in the levels of plasma VWF Antigen (VWF: Ag), the activity of ADAMTS13 (ADAMTS13: AC), and the plasma vascular endothelial growth factor A (VEGF-A) between PNV group and AMD group (Fig. 1).
VWF: Ag, ADAMTS13:AC, and VEGF-A in PNV and AMD. Each of the values in the graph is the median with the interquartile range. VWF: Ag, plasma VWF: Antigen. ADAMTS13: AC, plasma ADAMTS13 activity. VEGF-A, vascular endothelial growth factor A. MOD, minimum detectable dose. the MDD of VEGF-A defined by the manufacturer was 9 pg/ml.
The comparisons of patients based on the presence of UL-VWFMs are summarized in Table 3. The rate of past or current smokers was significantly higher in UL-VWFM positive group than that in UL-VWFM negative group (P = 0.032). The frequency of PNV diagnosis was significantly higher in UL-VWFM positive group than that in UL-VWFM negative group (P = 0.045). The rate of subretinal hemorrhage (P < 0.001) and PCV (P = 0.001) was also significantly higher in UL-VWFM positive group.
ADAMTS13: AC was significantly lower in UL-VWFM positive group (P = 0.023). In contrast, there were no significant differences in VWF: Ag and VEGF-A between two groups (Fig. 2).
VWF: Ag, ADAMTS13:AC, and VEGF-A in UL-VWFM (+) and UL-VWFM (−). Each of the values in the graph is the median with the interquartile range. The ADAMTS13:AC was significantly lower in UL-VWFM (+) than in UL-VWFM (−) (*P < 0.05). VWF: Ag, plasma VWF: Antigen. ADAMTS13: AC, plasma ADAMTS13 activity. VEGF-A, vascular endothelial growth factor A.
The distribution of gene polymorphisms in the CFH was showed in Tables 4 and 5. In the I62V, although there was no significant difference in the allele A frequency between PNV group and AMD group (OR 1.41; 95% CI 0.69–2.94, P = 0.44), the recessive homozygous (AA) was found only in PNV group. In the Y402H, the recessive homozygous (CC) did not exist in both groups and there was no significant difference in allele C frequency (OR 0.20; 95% CI 0.03–1.28, P = 0.17).
Discussion
Recently, Hosoda et al. used machine learning to review Japanese patients with AMD and suggested that 46% of cases were reclassified as PNV23. However, the diagnosis criteria for PNV have not been clearly determined. Thus, we employed the definition criteria of previous reports19,24 and found a strong correlation between CCT and CVD. This suggests that the combination of both parameters in the diagnosis of PNV may lead to a more accurate assessment. In our study, 34% of cases were reclassified as PNV. This percentage was relatively lower than Hosoda’s report but still demonstrated that many cases previously diagnosed as AMD would be PNV by clinical characteristics.
Interestingly, our results showed the differences in clinical and biological characteristics between PNV and AMD. The rate of SRF was significantly higher in PNV group than in AMD group. This seems reasonable considering previous reports of PNV secondary to CSC18,19. The rate in the presence of UL-VWFMs was significantly higher in PNV group than in AMD group.
We also showed that subretinal hemorrhages were significantly higher in PNV group than in AMD group. Tagawa et al. reported that subretinal hemorrhages were found in 20% of patients with PNV25. Nagai et al. also revealed that the pachyvessels (CVD ≥ 180 μm) and pachychoroid (CCT ≥ 220 μm) are risk factors for macular exudative changes after the induction phase of anti-VEGF injections24. These findings suggested that the CNVs were more likely to proceed hemorrhage in eyes in PNV group than in AMD group. Our results also showed that subretinal hemorrhages and PCV were significantly higher in the UL-VWFM positive group. The increased incidence of other thrombotic disorders with bleeding in UL-VWFM-positive patients has been shown in previous studies11,13,14.
A recent study reported that the density of the choriocapillaris was decreased in the PNV, and the decreased density areas were corresponding to the presence of pachyvessels26. These results suggest that the pachyvessels may compress the choriocapillaris, then this leads to ischemia in the choroidal surface layer. Gelfand et al. have proposed a hemodynamic model of high shear stress acting on the choriocapillaris in eyes with AMD27. Matsumoto et al. reported the presence of anastomosis and formation of collateral blood vessels in the pachyvessels, suggesting a long-term blood stasis28. Thus, the vessels of the choriocapillaris in eyes with PNV would have especially higher shear stress than that in eyes with AMD.
It is known that the UL-VWFMs under low shear stress in large vessels take globular forms and are difficult to bind to platelets. However, in the micro vessels, UL-VWFMs are exposed to high shear stress and become extended forms that easily bind to platelets11,29. Normally, ADAMTS13 has an antithrombotic function by promptly cleaving UL-VWFMs which maintains the proper hemostasis. We found that ADAMTS13: AC was significantly lower in UL-VWFM positive group than that in UL-VWFM negative group. That means remained UL-VWFMs existed although ADAMTS13 was consumed. Notably, the results of recent studies indicated that CFH promotes the cleavage of UL-VWFMs8,9,10. That is, CFH specifically binds to the UL-VWFM A2 domain where located the cleavage site of ADAMTS13, then the UL-VWFM cleavage by ADAMTS13 is enhanced8. Together with these results, genetic alterations of CFH in PNV may lead to the persistence of UL-VWFMs and may induce platelet thrombosis.
Recently, Yamashiro et al. revealed that in CFH I62V, the G allele is a risk gene for developing AMD and a protective gene for developing PNV, while the A allele is a risk gene for developing PNV and a protective gene for developing AMD3,20. However, there was no significant difference in allele A frequency in CFH in our study, while the recessive homozygous (AA) was found only in PNV group. Because the number of patients was limited, the difference might not be significant. We also found that there is no significant difference between PNV and AMD in the Y402H.
The choriocapillaris in patients with PNV is supposed to be under high shear stress suppressed by the pachyvessels. This situation may further activate UL-VWFM leading to thrombus formation and the further deterioration of blood flow in the choriocapillaris. The deterioration of blood flow may promote CNV formation and make it more likely to bleeding, subretinal hemorrhage.
There are several limitations in this study. First, we used a relatively small sample size for the comparisons. Second, the long-term clinical course is still unknown because we evaluated only at the first examination. Further long-term follow-up is desired to determine the clinical differences of the two groups.
Conclusions
The residual UL-VWFMs in the plasma may result in the formation of platelet thrombosis and hemorrhages in the choriocapillaris of PNV. Our findings suggest biological differences between PNV and AMD.
Methods
This was a cross-sectional study of 97 treatment naïve patients who had been diagnosed with exudative AMD based on the definition of the Japanese AMD Study Group30. In more detail, exudative AMD was diagnosed in patients who were over 50 years old with at least one following abnormalities (CNV, serous pigment epithelium detachment (PED), subretinal hemorrhage, and fibrous scars) within an area of 6000 μm in diameter centered at the fovea by using fundus photography, optical coherence tomography (OCT), fluorescein angiography (FA), and indocyanine green angiography (ICGA). Severe myopia (over − 6.0 diopter), uveitis, trauma and other degenerative diseases were excluded. All participants were patients of the Nara Medical University Hospital, Kashihara City, Nara Prefecture, Japan from 2014 to 2019. This study protocol was approved by the Institutional Review Board of the Nara Medical University and a signed informed consent was obtained from all the participants before the examinations. This study was performed in accordance with the Declaration of Helsinki. We confirmed that all methods were performed in accordance with the relevant guidelines and regulations.
All patients underwent conventional ophthalmological examinations including slit-lamp examinations, fundus examinations, OCT (Spectralis, Heidelberg Engineering, Dossenheim, Germany), fundus photography, FA, and ICGA at the initial examination. All of the abnormalities, such as subretinal hemorrhages, intra- or subretinal fluid, PED, and polypoidal lesion were detected at that examination. The fundus photographs, OCT and FA images were used to determine the presence of subretinal hemorrhages by two retinal specialists. We used enhanced-depth imaging (EDI) OCT to increase the visibility of choroidal structures. Central choroidal thickness (CCT) and choroidal vessel diameter (CVD) were determined by EDI-OCT images. Type 1 CNV was defined as early leakage in FA and highly reflective lesions beneath the RPE by EDI-OCT. After re-examination of all of the images, the patients were diagnosed with PNV or AMD.
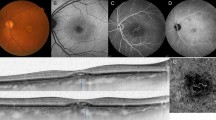
According to the recent reports on PNV analysis3,19,23,24,25,31, we diagnosed a patient as PNV when all of the following criteria were satisfied: Type1 CNV (including PCV), CVD ≥ 180 μm, CCT ≥ 220 μm, absence of drusen in the fundus photographs, and increased choroidal vascular permeability in the late phase of the ICGA images (Fig. 3). PCV was characterized by polypoidal lesions in IGCA. CVD was defined as the vertical diameter of the largest vessel in the Haller's layer. CVD and CCT were measured independently by two different researchers using a scale bar contained within the OCT system24. The judgments were made independently by two retinal specialists, and in cases when the judgments were different, the final decision was made by a macular disease specialist (N.O.).
Fundus photograph of 76-year-old man with pachychoroid neovasuculopathy (PNV). (A) Drusen are not present in the fundus photograph. (B) Indocyanine green angiography (ICGA) showing increased choroidal vascular permeability. The dilated blood vessels pass through the areas close to the macula. (C) The central choroidal thickness (CCT) is 335 μm and the choroidal vessel diameter (CVD) is 201 μm. Choroidal vessels in Haller's layer are dilated.
We collected blood samples by venipuncture at the initial examination. The whole blood samples were stored in tubes containing a 1:9 volume of 3.8% trisodium citrate. The plasma was separated and saved at – 80 °C. For the measurements, the plasma was thawed and maintained at 37 °C before the measurements. The level of plasma VWF Antigen (VWF: Ag) was measured by sandwich enzyme-linked immunosorbent assay (ELISA) using a rabbit anti-human VWF polyclonal antiserum (DAKO, Glostrup, Denmark)14. We determined the activity of ADAMTS13 (ADAMTS13: AC) by a chromogenic ADAMTS13 activity ELISA kit (Kainos, Tokyo, Japan)32. The 100% reference value was defined as the amount of VWF: Ag and ADAMTS13: AC in the plasma of 20 normal volunteers (10 men and 10 women ages 20–40 years). We also measured the plasma vascular endothelial growth factor A (VEGF-A) by ELISA (Quantikine VEGF ELISA kit; R&D Systems, Minneapolis, Minnesota, USA). The VWF multimers were analyzed by the method of Ruggeri and Zimmerman33, modified by Warren et al.34. The measurements were made under the conditions described by Budde et al.35 and we defined the high molecular weight bands that were not detected in normal plasma as UL-VWFMs. Then, we performed densitometric analysis using ImageJ (National Institute of Health, Bethesda, Maryland, USA). UL-VWFMs were defined as being positive when the ratio of UL-VWFMs to total VWF was > 1% (Fig. 4)36.
von Willebrand factor (VWF) multimer analysis in representative cases. VWF multimer of normal pool plasma from 40 healthy subjects (NP) is shown in the far left column, and the plasma of representative 10 cases are the next 10 columns. The VWF multimers above the dotted line are defined as unusually large VWF multimers (UL-VWFMs). AMD, age-related macular degeneration; PNV, pachychoroid neovasculopathy. VWF: Ag, plasma VWF antigen. ADAMTS13: AC, plasma ADAMTS13 activity.
We extracted genomic DNA from the leukocytes by a genomic DNA kit (QIAmpDNA; Qiagen, Valencia, California, USA) and genotyped the SNPs of p.I62V (rs800292) and p.Y402H (rs1061170) in CFH. Polymerase chain reaction (PCR) with specific primers was used to amplify the polymorphic sites37. The PCR products was used as the templates for direct DNA sequencing (Applied Biosystems, Foster City, California, USA) on an automated sequencer (3730xl DNA analyzer; Applied Biosystems). The genotypes in the CFH were classified based on the previous studies38,39.
Statistical analyses
Two-tailed Mann–Whitney U tests were used to compare the averages of continuous variables (such as age) and Fisher’s exact tests to compare the proportions of categorical variables (such as sex) between groups. Odds ratios and 95% confidence intervals were calculated by using Fisher’s exact tests. All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria)40. Since both CCT and CVD followed a normal distribution, the correlation was evaluated at Pearson's correlation coefficient. On the other hand, the measured values of VEGF-A were not normally distributed, and the exact values were not partially shown because the values were below the minimum detectable dose of 9 pg/ml. Therefore, a non-parametric test was applied, and a conservative value of 8 pg/ml was input for each value below the MDD when performing the calculations. The threshold for statistically significance was P < 0.05.
Data availability
The datasets generated or analyzed during the current study are available from the corresponding author on reasonable request.
References
Ricci, F. et al. Neovascular age-related macular degeneration: Therapeutic management and new-upcoming approaches. Int. J. Mol. Sci. https://doi.org/10.3390/ijms21218242 (2020).
Heesterbeek, T. J., Lorés-Motta, L., Hoyng, C. B., Lechanteur, Y. T. E. & den Hollander, A. I. Risk factors for progression of age-related macular degeneration. Ophthalmic Physiol. Opt. 40, 140–170. https://doi.org/10.1111/opo.12675 (2020).
Yamashiro, K., Hosoda, Y., Miyake, M., Ooto, S. & Tsujikawa, A. Characteristics of pachychoroid diseases and age-related macular degeneration: Multimodal imaging and genetic backgrounds. J. Clin. Med. https://doi.org/10.3390/jcm9072034 (2020).
Smith, W. et al. Risk factors for age-related macular degeneration: Pooled findings from three continents. Ophthalmology 108, 697–704. https://doi.org/10.1016/s0161-6420(00)00580-7 (2001).
Klein, R. J. et al. Complement factor H polymorphism in age-related macular degeneration. Science 308, 385–389. https://doi.org/10.1126/science.1109557 (2005).
Toomey, C. B., Johnson, L. V. & Bowes Rickman, C. Complement factor H in AMD: Bridging genetic associations and pathobiology. Prog. Retin Eye Res. 62, 38–57. https://doi.org/10.1016/j.preteyeres.2017.09.001 (2018).
Morgan, B. P. & Harris, C. L. Complement, a target for therapy in inflammatory and degenerative diseases. Nat. Rev. Drug Discov. 14, 857–877. https://doi.org/10.1038/nrd4657 (2015).
Feng, S. et al. The interaction between factor H and Von Willebrand factor. PLoS One 8, e73715. https://doi.org/10.1371/journal.pone.0073715 (2013).
Nolasco, L., Nolasco, J., Feng, S., Afshar-Kharghan, V. & Moake, J. Human complement factor H is a reductase for large soluble von Willebrand factor multimers—Brief report. Arterioscler. Thromb. Vasc. Biol. 33, 2524–2528. https://doi.org/10.1161/ATVBAHA.113.302280 (2013).
Rayes, J. et al. The interaction between factor H and VWF increases factor H cofactor activity and regulates VWF prothrombotic status. Blood 123, 121–125. https://doi.org/10.1182/blood-2013-04-495853 (2014).
Crawley, J. T., de Groot, R., Xiang, Y., Luken, B. M. & Lane, D. A. Unraveling the scissile bond: How ADAMTS13 recognizes and cleaves von Willebrand factor. Blood 118, 3212–3221. https://doi.org/10.1182/blood-2011-02-306597 (2011).
Matsushita, K. et al. Vascular endothelial growth factor regulation of Weibel–Palade-body exocytosis. Blood 105, 207–214. https://doi.org/10.1182/blood-2004-04-1519 (2005).
Buchtele, N., Schwameis, M., Gilbert, J. C., Schörgenhofer, C. & Jilma, B. Targeting von Willebrand factor in ischaemic stroke: Focus on clinical evidence. Thromb. Haemost. 118, 959–978. https://doi.org/10.1055/s-0038-1648251 (2018).
Matsumoto, M. et al. Platelets treated with ticlopidine are less reactive to unusually large von Willebrand factor multimers than are those treated with aspirin under high shear stress. Pathophysiol. Haemost. Thromb. 34, 35–40. https://doi.org/10.1159/000088546 (2005).
Lip, P. L., Blann, A. D., Hope-Ross, M., Gibson, J. M. & Lip, G. Y. Age-related macular degeneration is associated with increased vascular endothelial growth factor, hemorheology and endothelial dysfunction. Ophthalmology 108, 705–710. https://doi.org/10.1016/s0161-6420(00)00663-1 (2001).
Malukiewicz-Wiśniewska, G., Rość, D., Kałuzny, B. & Kałuzny, J. J. Von Willebrand factor in plasma of patients with age related macular degeneration. Klin Oczna 107, 70–72 (2005).
Yamashita, M. et al. Intravitreal injection of aflibercept, an anti-VEGF antagonist, down-regulates plasma von Willebrand factor in patients with age-related macular degeneration. Sci. Rep. 8, 1491. https://doi.org/10.1038/s41598-018-19473-0 (2018).
Pang, C. E. & Freund, K. B. Pachychoroid neovasculopathy. Retina 35, 1–9. https://doi.org/10.1097/IAE.0000000000000331 (2015).
Miyake, M. et al. Pachychoroid neovasculopathy and age-related macular degeneration. Sci. Rep. 5, 16204. https://doi.org/10.1038/srep16204 (2015).
Hosoda, Y. et al. CFH and VIPR2 as susceptibility loci in choroidal thickness and pachychoroid disease central serous chorioretinopathy. Proc. Natl. Acad. Sci. U. S. A. 115, 6261–6266. https://doi.org/10.1073/pnas.1802212115 (2018).
Bowen, D. J. An influence of ABO blood group on the rate of proteolysis of von Willebrand factor by ADAMTS13. J. Thromb. Haemost. 1, 33–40. https://doi.org/10.1046/j.1538-7836.2003.00007.x (2003).
Gallinaro, L. et al. A shorter von Willebrand factor survival in O blood group subjects explains how ABO determinants influence plasma von Willebrand factor. Blood 111, 3540–3545. https://doi.org/10.1182/blood-2007-11-122945 (2008).
Hosoda, Y. et al. Deep phenotype unsupervised machine learning revealed the significance of pachychoroid features in etiology and visual prognosis of age-related macular degeneration. Sci. Rep. 10, 18423. https://doi.org/10.1038/s41598-020-75451-5 (2020).
Nagai, N. et al. Dynamic changes in choroidal conditions during anti-vascular endothelial growth factor therapy in polypoidal choroidal vasculopathy. Sci. Rep. 9, 11389. https://doi.org/10.1038/s41598-019-47738-9 (2019).
Tagawa, M. et al. Characteristics of pachychoroid neovasculopathy. Sci. Rep. 10, 16248. https://doi.org/10.1038/s41598-020-73303-w (2020).
Baek, J., Kook, L. & Lee, W. K. Choriocapillaris flow impairments in association with pachyvessel in early stages of pachychoroid. Sci. Rep. 9, 5565. https://doi.org/10.1038/s41598-019-42052-w (2019).
Gelfand, B. D. & Ambati, J. A revised hemodynamic theory of age-related macular degeneration. Trends Mol. Med. 22, 656–670. https://doi.org/10.1016/j.molmed.2016.06.009 (2016).
Matsumoto, H., Kishi, S., Mukai, R. & Akiyama, H. Remodeling of macular vortex veins in pachychoroid neovasculopathy. Sci. Rep. 9, 14689. https://doi.org/10.1038/s41598-019-51268-9 (2019).
Kroll, M. H., Hellums, J. D., McIntire, L. V., Schafer, A. I. & Moake, J. L. Platelets and shear stress. Blood 88, 1525–1541 (1996).
Takahashi, K., Ishibashi, T., Ogur, Y. & Yuzawa, M. Classification and diagnostic criteria of age-related macular degeneration. Nippon Ganka Gakkai Zasshi 112, 1076–1084 (2008).
Biçer, Ö., Demirel, S., Yavuz, Z., Batioğlu, F. & Özmert, E. Comparison of morphological features of type 1 CNV in AMD and pachychoroid neovasculopathy: An OCTA study. Ophthalmic Surg. Lasers Imaging Retina 51, 640–647. https://doi.org/10.3928/23258160-20201104-06 (2020).
Kato, S. et al. Novel monoclonal antibody-based enzyme immunoassay for determining plasma levels of ADAMTS13 activity. Transfusion 46, 1444–1452. https://doi.org/10.1111/j.1537-2995.2006.00914.x (2006).
Ruggeri, Z. M. & Zimmerman, T. S. Variant von Willebrand’s disease: Characterization of two subtypes by analysis of multimeric composition of factor VIII/von Willebrand factor in plasma and platelets. J. Clin. Investig. 65, 1318–1325. https://doi.org/10.1172/jci109795 (1980).
Warren, C. M., Krzesinski, P. R. & Greaser, M. L. Vertical agarose gel electrophoresis and electroblotting of high-molecular-weight proteins. Electrophoresis 24, 1695–1702. https://doi.org/10.1002/elps.200305392 (2003).
Budde, U. et al. Luminographic detection of von Willebrand factor multimers in agarose gels and on nitrocellulose membranes. Thromb. Haemost. 63, 312–315 (1990).
Yoshikawa, T. et al. Ischaemia–reperfusion injury with Pringle’s maneuver induces unusually large von Willebrand factor multimers after hepatectomy. Thromb. Res. 183, 20–27. https://doi.org/10.1016/j.thromres.2019.09.005 (2019).
Ohkuma, Y. et al. Retinal angiomatous proliferation associated with risk alleles of ARMS2/HTRA1 gene polymorphisms in Japanese patients. Clin. Ophthalmol. 8, 143–148. https://doi.org/10.2147/OPTH.S56483 (2014).
Wang, Z. Y. et al. Systematic review and meta-analysis of the association between complement factor H I62V polymorphism and risk of polypoidal choroidal vasculopathy in Asian populations. PLoS One 9, e88324. https://doi.org/10.1371/journal.pone.0088324 (2014).
Chen, G. et al. Pharmacogenetics of complement factor H Y402H polymorphism and treatment of neovascular AMD with anti-VEGF agents: A meta-analysis. Sci. Rep. 5, 14517. https://doi.org/10.1038/srep14517 (2015).
Kanda, Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 48, 452–458. https://doi.org/10.1038/bmt.2012.244 (2013).
Acknowledgements
The authors thank Hiroki Tsujinaka for useful discussions, Yutaro Mizusawa and Hironobu Jimura for the data collection.
Author information
Authors and Affiliations
Contributions
Conception and design: M.M., T.U., and N.O., Writing the article: H.H., M.Y., M.M., N.O., Analysis: H.H., M.Y., M.H., K.S. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hirai, H., Yamashita, M., Matsumoto, M. et al. Analysis focusing on plasma von Willebrand factor in pachychoroid neovasculopathy and age-related macular degeneration. Sci Rep 11, 19987 (2021). https://doi.org/10.1038/s41598-021-99557-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-99557-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.