Abstract
Diabetes has been established as a strong risk factor for chronic kidney disease (CKD). Sleep apnea, poor sleep quality (PSQ), and autonomic imbalance are also considered to be potential risk factors for decline in renal function, though no known study has examined their integrated predictive value in diabetic and non-diabetic patients without CKD. The present cohort consisted of 754 serial patients (diabetes; n = 231, non-diabetes; n = 523) without CKD registered in the Hyogo Sleep Cardio-Autonomic Atherosclerosis (HSCAA) study. Patients underwent examinations to determine respiratory event index and objective sleep quality using actigraphy, as well as heart rate variability (HRV). Renal outcome was defined as a decline in estimated glomerular filtration rate to less than 60 ml/min/1.73 m2 for more than 3 months. Kaplan–Meier analysis showed that diabetic patients with PSQ or low HRV, but not sleep apnea, had a significantly increased risk for renal outcome. Furthermore, Cox proportional hazards analysis revealed that PSQ was significantly associated with elevated risk of renal outcome (HR: 2.57; 95% CI: 1.01–6.53, p = 0.045) independent of sleep apnea and classical risk factors. Low HRV tended to be, but not significantly (p = 0.065), associated with the outcome. In non-diabetic patients, PSQ was also significantly and independently associated with renal outcome, whereas sleep apnea and low HRV were not. In conclusion, PSQ and low HRV appear to be important predictors of decline in renal function in diabetic patients without CKD.
Similar content being viewed by others
Introduction
Diabetes mellitus (DM) has become established as one of the major risk factors for reduced renal function and chronic kidney disease (CKD)1, while it is also known to be associated with glycemic abnormalities, which cause microvascular complications. Additionally, recent studies have noted that sleep problems, including obstructive sleep apnea and poor sleep quality (PSQ), are becoming increasingly recognized as important risk factors for progression of CKD and changes in estimated glomerular filtration rate (eGFR)2,3,4,5. Moreover, autonomic nervous function, which regulates the global health of the vasculature, such as hemodynamics, vascular tone, metabolism, and inflammation, has also been shown to be associated with progression of renal dysfunction and CKD-related hospitalization6. Autonomic imbalance has been proposed to be an important mechanistic factor underlying the association of sleep quality with renal function7,8,9, though their integrated mutual significance remains unknown. Of particular importance, the impact on renal function in diabetic as compared with non-diabetic patients has yet to be elucidated.
Using data obtained in the Hyogo Sleep Cardio-Autonomic Atherosclerosis (HSCAA) cohort study, the aim of the present prospective investigation was to examine and compare the mutual integrated impact of sleep apnea, PSQ, and autonomic dysfunction on future decline in renal function in diabetic and non-diabetic patients without CKD.
Methods
Study design and participants
The ongoing HSCAA cohort study was started in October 2010, with 1097 patients registered up to March 2019 included in the present investigation. The study was approved by the Ethics Committee of Hyogo College of Medicine (approval No. 2351). All methods were conducted in accordance with relevant guidelines and regulations after written informed consent was obtained from all subjects. Sleep apnea, sleep quality, and autonomic nervous function were collectively evaluated at the baseline, then enrolled patients were routinely followed until June 2019. Among the factors examined, serum creatinine concentration was determined every 3 months and eGFR was calculated at each examination. After excluding 185 patients with missing data for sleep quality or regular use of hypnotics for mental disorders, 45 patients with baseline eGFR < 60 ml/min/1.73 m2 or urinary albumin excretion ≥ 300 mg/day, and 113 lost to follow-up or with a follow-up period < 3 months, baseline and follow-up data of the remaining 754 (diabetes: n = 231, non-diabetes: n = 523) were used for the present analyses (Fig. 1). Among the enrolled patients, 722 were evaluated for respiratory event index (REI) (diabetes: n = 225, non-diabetes: n = 497) and 697 for heart rate variability (HRV) (diabetes: n = 213, non-diabetes: n = 484).
Classical cardiovascular risk factors
8 type 1 and 223 type 2 diabetes patients were included in the diabetic group. Type 2 diabetes was diagnosed based on fasting plasma glucose ≥ 126 mg/dl, causal plasma glucose ≥ 200 mg/dl, or 2-h plasma glucose ≥ 200 mg/dl shown by a 75-g oral glucose tolerance test, or history of therapy for diabetes10. Type 1 diabetes was defined by typical clinical history findings, such as rapid onset of ketoacidosis, absolute deficiency of insulin secretion following treatment with multiple doses of insulin, or positivity for the autoantibody against glutamic acid decarboxylase. We defined history of cardiovascular events as past experience with coronary heart disease (myocardial infarction, coronary intervention) or stroke. Hypertension was defined as systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg, or treatment for hypertension. Dyslipidemia was defined as low density lipoprotein cholesterol ≥ 140 mg/dl, high density lipoprotein cholesterol ≤ 40 mg/dl, triglyceride level ≥ 150 mg/dl, or presently under treatment for dyslipidemia11. Serum creatinine concentration was determined using an enzymatic method. eGFR in each patient was calculated using an equation for subjects in Japan, as follows: eGFR (ml/min/1.73 m2) = 194 × age(years)-0.287 × S-Cr-1.094 (if female, × 0.739)12. Urinary albumin was determined using an immunoturbidimetry method, with microalbuminuria defined as UAE ≥ 30 mg/day13.
Sleep and autonomic nervous function
To examine the presence of sleep apnea, we used an Apnomonitor (SAS-2100®, Teijin, Tokyo, Japan) to determine REI, as previously described14,15. An actigraphy device (Ambulatory Monitoring, Inc., Ardley, New York, USA), which senses motion as acceleration, was placed on the wrist of the non-dominant arm and used for quantitative analysis of sleep quality, as previously reported16,17,18,19. Actigraphy results have been shown to be as reliable as those obtained with polysomnography for determination of sleep disturbance20. According to published recommendations for clinical use of actigraphy results21, activity index as a parameter of sleep quality was calculated as total body motion during sleep time, with a higher activity index value considered to be related to lower sleep quality.
HRV using an Active Tracer device (AC-301A®, Arm Electronics, Tokyo, Japan) was used for noninvasive determination of cardiac modulation based on autonomic balance, as previously described14,22,23,24. The final 24-h series of data from the 48-h recording was analyzed using a MemCalc Chiram 3 system, version 2.0 (Suwa Trust, Tokyo, Japan). Ectopic beats, noise data, and artifacts were manually corrected or excluded from calculation. According to the recommendations for clinical use of HRV25, the coefficient of variation of the R–R interval (CVRR) within the time domain was calculated.
Study outcomes
For the present study, the primary renal outcome was defined as a decline in eGFR to less than 60 ml/min/1.73 m2 for more than 3 months, as previously recommended.
Statistical analysis
The enrolled subjects were divided into the diabetic (n = 231) and non-diabetic (n = 523) groups. According to previously published studies of clinical use of actigraphy21 and apnomonitor26 results, they were also divided into those with and without PSQ using an activity index cut-off value of 50, and with and without sleep apnea using an REI cut-off value of 5. For examining autonomic nervous function, the subjects were divided into those with low and normal or high HRV based on the median CVRR value. To compare variables between the diabetic and non-diabetic groups, a non-repeated t-test (continuous variables with normal distribution), Mann–Whitney test (continuous variables with skewed distribution), and chi-squared test (categorical variables) were utilized as appropriate. The outcome rates for the groups were compared using Kaplan–Meier analysis and a log-rank test. Prognostic variables for decline in renal function were examined using a univariate or multivariate Cox proportional hazards regression model. All statistical analyses were performed using the Statistical Package for the Social Sciences software platform (PASW Statistics, version 18.0). All reported p values are two-tailed and were considered statistically significant at < 0.05.
Results
Baseline characteristics of study participant
Baseline characteristics of the subjects after dividing into those with and without diabetes are shown in Table 1. As compared to the non-diabetic group, patients with diabetes were older and exhibited greater values for body mass index (BMI), systolic or diastolic blood pressure (BP), albuminuria, HbA1c, fasting plasma glucose, activity index (median 33.5), and REI (median 8.9), and also showed a greater percentage of male gender, current smokers, subjects with a past history of cardiovascular disease (CVD), hypertension, or dyslipidemia, users of an Angiotensin converting enzyme (ACE) inhibitor or Angiotensin II receptor blocker (ARB), as well as cases of sleep apnea (66.6%) and low heart rate variability (HRV) (56.3%). The percentage of subjects with PSQ was not significantly different between the groups, while there were also no significant differences between them for the variables renal function, blood urea nitrogen, creatinine, and baseline eGFR (mean; DM: 89.8 ml/min/1.73m2, non-DM: 87.3 ml/min/1.73m2).
Association of sleep quality, sleep apnea and HRV with renal outcome
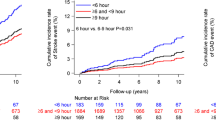
The median follow-up period of all analyzed subjects was 38.5 months. Kaplan–Meier analysis findings indicated that those with PSQ, sleep apnea, and low HRV exhibited a significantly greater risk for decline in renal function as compared to the others (Fig. 2A). Diabetic patients with PSQ and low HRV, but not sleep apnea, had a significantly greater risk for renal outcome as compared to the control group (log-rank test, PSQ; p < 0.001, low HRV; p = 0.024) (Fig. 2B). On the other hand, non-diabetic patients with PSQ and sleep apnea had a significantly greater risk for renal outcome, but not those with low HRV (Fig. 2C).
Kaplan–Meier analysis of associations of PSQ, sleep apnea, and autonomic nervous function with renal function decline in diabetic and non-diabetic patients. Subjects in the diabetic and non-diabetic groups were compared based on the presence or absence of PSQ, sleep apnea, and low HRV. Probability was analyzed using a log-rank test. PSQ poor sleep quality, HRV heart rate variability.
Results of univariate Cox proportional hazards regression analysis (Table 2) showed that age, baseline eGFR, and presence of albuminuria were significantly associated with renal outcome in both the diabetic and non-diabetic groups. In the patients with diabetes, use of anti-diuretics, and diabetes duration were significantly associated with higher risk for renal outcome, while past history of cardiovascular disease, hypertension, use of calcium channel blockers or α/β blockers, and use of statin were significantly associated with higher risk in the non-diabetic group. PSQ and low HRV were significantly associated with renal outcome in the diabetic group [PSQ: hazard ratio (HR) 2.90; 95% confidence interval (CI) 1.36–6.18, p = 0.005; low HRV: HR 2.43, 95% CI 1.09–5.41, p = 0.029], while sleep apnea was not. In the non-diabetic group, PSQ and sleep apnea, but not low HRV, were significantly associated with renal outcome (PSQ: HR 2.40, 95% CI 1.43–4.03, p < 0.001; sleep apnea: HR 2.54, 95% CI 1.56–4.13, p < 0.001).
To further examine whether sleep quality, sleep apnea, and low HRV had independent associations with renal outcome, multivariate Cox proportional analysis was performed (Table 3). In models that included age, gender, body mass index, smoking status, history of cardiovascular disease, hypertension, dyslipidemia, HbA1c, baseline eGFR, and albuminuria, PSQ showed a borderline significant association with decline in renal function in the diabetic group (HR 2.15, 95% CI 0.88–5.23, p = 0.091) (Model 1). As for the non-diabetic group, that model indicated that PSQ was significantly associated with renal outcome (HR 2.20, 95% CI 1.09–4.40, p = 0.025). Sleep apnea was not significantly associated with renal outcome after adjustment with other clinical factors in either the diabetic or non-diabetic group (Model 2). Low HRV was significantly and independently associated with renal function decline in patients with diabetes (HR 2.40, 95% CI 1.02–5.60, p = 0.043), but not in those without diabetes (Model 3). Of particular interest, the association of PSQ with renal outcome was significant after further adjustments for low HRV with (Model 7) or without (Model 5) sleep apnea in the diabetic group. In the non-diabetic group, the association of PSQ with renal outcome remained significant even after further adjustments for sleep apnea (Model 4), low HRV (Model 5), and both (Model 7). Also, the significant association of low HRV with renal outcome in the diabetic subjects was independent of PSQ (Model 5), while it was partially attenuated by sleep apnea (Models 6, 7).
Discussion
This is the first known study to examine the integrated impacts of sleep apnea, sleep quality, and autonomic function as predictors of decline in renal function in diabetes and non-diabetes patients without CKD. The results show that PSQ and low HRV are important predictors for decline in renal function in diabetic patients without CKD, with those relationships independent of known classical risk factors. In contrast, in the present non-diabetic subjects, PSQ, but not low HRV, was an independent predictor for decline in renal function.
In previous studies, sleep quality was shown to be associated with a higher risk for renal function decline2,27. However, no known investigation has been conducted to examine risk using quantitative measurements of sleep quality, and compare between diabetic and non-diabetic patients without CKD. The present results clearly showed that PSQ was indeed an essential predictor for decline in renal function in both groups. Among the causative factors for PSQ, sleep apnea has been shown to be strongly associated with renal dysfunction4,5,26, though a conflicting report has been presented28. Tahrani et al. reported that eGFR decreased faster in T2D patients with OSA compared to similar patients without OSA after an average of 2.5 years of follow-up26. In our diabetic subjects, although those with PSQ exhibited significantly higher REI values (median: 14.5) than those without PSQ (median: 8.1), sleep apnea was not independently associated with decline in renal function in the diabetic group in multivariate Cox proportional hazards analyses. This discrepancy may be the results of differences in lower BMI (25.5 vs. 35.4) and lower HbA1c (7.2 vs. 8.3) in our patients with sleep apnea, both of which are well-known risk factors related to renal dysfunction. Additionally, other covariates included only in our study, such as baseline eGFR and albuminuria values, or statistical methods, Cox proportional vs. logistic regression analyses may account for the differences. A retrospective study conducted in Taiwan found a relationship of sleep quality with incidence of CKD in subjects without apnea29. In the present study, the association of PSQ with renal outcome was shown to be unaffected after adjustment for sleep apnea in both diabetic and non-diabetic patients. Thus, the predictive impact of PSQ on progression of renal dysfunction appears necessarily not the result of potentially coexisting sleep apnea.
The present results are the first to show that low HRV is an important predictor for decline in renal function in pre-CKD patients with diabetes, but not in those without diabetes. Furthermore, that relationship was found to be independent of classical risk factors as well as sleep quality or presence of apnea. In the Atherosclerosis Risk in Communities (ARIC) study, which had a median follow-up period of 16 years, low HRV was demonstrated to be associated with occurrence of ESRD- and CKD-related hospitalization in CKD patients30, though they did not investigate the predictive value of low HRV in diabetic patients by subgroup analysis. The present findings are the first to show that the impact of autonomic function on renal function decline is dependent on the presence of diabetes. It is not clear at present why low HRV is associated with higher risk for renal dysfunction only in diabetic patients during the pre-CKD phase. Nevertheless, it is considered that the effects of low HRV may not be dependent on the presence of diabetic neuropathy, since that was not significantly associated with decline in renal function in the present subjects. Autonomic nervous imbalance has been speculated to have potential to mediate the effect of sleep on renal function7,31. In our previous investigations, sleep disturbance was shown to be associated with autonomic dysfunction and fluctuations in nocturnal blood pressure23,32. Uncontrolled nighttime sympathetic activation due to decreased autonomic function might be potentially involved, at least in part, in the relationship of PSQ with renal function decline. However, the present results clearly demonstrated a significant association of PSQ with decline in renal function, even after adjustments for low HRV along with well-established renal risk factors. Potential candidates include brain-derived neurotrophic factor (BDNF), which has been reported to have critical roles in survival, growth, and maintenance, as well as death of central and peripheral neurons33,34, while more recent studies including ours have also demonstrated associations of BDNF with sleep disturbance and autonomic function13,35,36. In that previous study conducted by our group, positive and significant associations of plasma BDNF with autonomic function and nighttime blood pressure fluctuation were found32, while our more recent report noted that low plasma BDNF level was independently associated with decline in renal function13.
The present study has some limitations. First, reduced eGFR (less than 60 ml/min/1.73 m2) for more than 3 months was used as the primary clinical endpoint for patients without CKD, whereas another well-used endpoint, 30% decrease in eGFR, was not, because the number of events during the study period were not adequate for analysis. Second, 24-h urinary albumin monitoring was not performed during the follow-up period, thus only decreased eGFR was used as the primary endpoint. Third, though our findings suggest that objective sleep quality and autonomic imbalance are important predictors of decline in renal function in DM patients in the pre-CKD phase, a well-controlled randomized study will be necessary to establish that causal relationship. Fourth, information of menopause age was not collected in our study, even though the relationship between menopause and sleep apnea has long been pointed out37. Indeed, REI was significantly higher in female over 50 years than those under 50. However, renal outcome was not significantly different between female with > 50 years and those with < 50 years of age (data not shown). Fifth, there may be misdiagnosed patients in the no sleep apnea group because REI values tend to be lower than AHI values measured by polysomnography15. Finally, while potential causative factors related to the association of PSQ with decline in renal function were extensively examined, the underlying detailed mechanisms for the relationship were not identified. Nevertheless, to the best of our knowledge, no previous study has explored the integrated impact of sleep quality, sleep apnea, and autonomic balance on renal function using comparisons of patients with and without diabetes.
In conclusion, PSQ and low HRV independent of sleep apnea are important risk factors for decline in renal function in pre-CKD diabetic patients.
References
Hesp, A. C. et al. The role of renal hypoxia in the pathogenesis of diabetic kidney disease: A promising target for newer renoprotective agents including SGLT2 inhibitors?. Kidney Int. 98, 579–589. https://doi.org/10.1016/j.kint.2020.02.041 (2020).
Yamamoto, R. et al. Sleep quality and sleep duration with CKD are associated with progression to ESKD. Clin. J. Am. Soc. Nephrol. 13, 1825–1832. https://doi.org/10.2215/CJN.01340118 (2018).
Park, S. et al. Short or long sleep duration and CKD: A Mendelian randomization study. J. Am. Soc. Nephrol. 31, 2937–2947. https://doi.org/10.1681/ASN.2020050666 (2020).
Full, K. M., Jackson, C. L., Rebholz, C. M., Matsushita, K. & Lutsey, P. L. Obstructive sleep apnea, other sleep characteristics, and risk of CKD in the atherosclerosis risk in communities sleep heart health study. J. Am. Soc. Nephrol. 31, 1859–1869. https://doi.org/10.1681/ASN.2020010024 (2020).
Marrone, O. et al. Effects of sleep apnea and kidney dysfunction on objective sleep quality in nondialyzed patients with chronic kidney disease: An ESADA study. J. Clin. Sleep Med. 16, 1475–1481. https://doi.org/10.5664/jcsm.8542 (2020).
Guyenet, P. G., Stornetta, R. L., Souza, G., Abbott, S. B. G. & Brooks, V. L. Neuronal networks in hypertension: Recent advances. Hypertension 76, 300–311. https://doi.org/10.1161/HYPERTENSIONAHA.120.14521 (2020).
Neumann, J., Ligtenberg, G., Klein, I. I., Koomans, H. A. & Blankestijn, P. J. Sympathetic hyperactivity in chronic kidney disease: Pathogenesis, clinical relevance, and treatment. Kidney Int. 65, 1568–1576. https://doi.org/10.1111/j.1523-1755.2004.00552.x (2004).
DiBona, G. F. Neural control of the kidney: Past, present, and future. Hypertension 41, 621–624. https://doi.org/10.1161/01.HYP.0000047205.52509.8A (2003).
DiBona, G. F. Physiology in perspective: The wisdom of the body: Neural control of the kidney. Am. J. Physiol. Regul. Integr. Comp. Physiol. 289, R633-641. https://doi.org/10.1152/ajpregu.00258.2005 (2005).
American Diabetes, A. Diagnosis and classification of diabetes mellitus. Diabetes Care 27(Suppl 1), S5–S10 (2004).
Teramoto, T. et al. Executive summary of the Japan Atherosclerosis Society (JAS) guidelines for the diagnosis and prevention of atherosclerotic cardiovascular diseases in Japan -2012 version. J. Atheroscler. Thromb. 20, 517–523 (2013).
Matsuo, S. et al. Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney Dis. 53, 982–992. https://doi.org/10.1053/j.ajkd.2008.12.034 (2009).
Kurajoh, M. et al. Plasma brain-derived neurotrophic factor concentration is a predictor of chronic kidney disease in patients with cardiovascular risk factors—Hyogo Sleep Cardio-Autonomic Atherosclerosis study. PLoS ONE 12, e0178686. https://doi.org/10.1371/journal.pone.0178686 (2017).
Kadoya, M. et al. Sleep, cardiac autonomic function, and carotid atherosclerosis in patients with cardiovascular risks: HSCAA study. Atherosclerosis 238, 409–414. https://doi.org/10.1016/j.atherosclerosis.2014.12.032 (2015).
Kapur, V. K. et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 13, 479–504. https://doi.org/10.5664/jcsm.6506 (2017).
Kadoya, M. et al. Serum macro TSH level is associated with sleep quality in patients with cardiovascular risks—HSCAA study. Sci. Rep. 7, 44387. https://doi.org/10.1038/srep44387 (2017).
Kadoya, M. et al. Low sleep quality is associated with progression of arterial stiffness in patients with cardiovascular risk factors: HSCAA study. Atherosclerosis 270, 95–101. https://doi.org/10.1016/j.atherosclerosis.2018.01.039 (2018).
Kadoya, M. & Koyama, H. Sleep, autonomic nervous function and atherosclerosis. Int. J. Mol. Sci. https://doi.org/10.3390/ijms20040794 (2019).
Kakutani-Hatayama, M. et al. Associations of sleep quality, sleep apnea and autonomic function with insulin secretion and sensitivity: HSCAA study. Metabol. Open 6, 100033. https://doi.org/10.1016/j.metop.2020.100033 (2020).
Sadeh, A. The role and validity of actigraphy in sleep medicine: An update. Sleep Med. Rev. 15, 259–267. https://doi.org/10.1016/j.smrv.2010.10.001 (2011).
Practice parameters for the use of actigraphy in the clinical assessment of sleep disorders. American Sleep Disorders Association. Sleep 18, 285–287 (1995).
Kurajoh, M. et al. Plasma leptin level is associated with cardiac autonomic dysfunction in patients with type 2 diabetes: HSCAA study. Cardiovasc. Diabetol. 14, 117. https://doi.org/10.1186/s12933-015-0280-6 (2015).
Kadoya, M. et al. Plasma brain-derived neurotrophic factor and reverse dipping pattern of nocturnal blood pressure in patients with cardiovascular risk factors. PLoS ONE 9, e105977. https://doi.org/10.1371/journal.pone.0105977 (2014).
Morimoto, A. et al. Subclinical decrease in cardiac autonomic and diastolic function in patients with metabolic disorders: HSCAA study. Metabol. Open 5, 100025. https://doi.org/10.1016/j.metop.2020.100025 (2020).
Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur. Heart J. 17, 354–381 (1996).
Tahrani, A. A. et al. Obstructive sleep apnea and diabetic nephropathy: A cohort study. Diabetes Care 36, 3718–3725. https://doi.org/10.2337/dc13-0450 (2013).
Li, J. et al. Sleep and CKD in Chinese adults: A cross-sectional study. Clin. J. Am. Soc. Nephrol. 12, 885–892. https://doi.org/10.2215/CJN.09270816 (2017).
Buyukaydin, B. et al. The effect of sleep apnea syndrome on the development of diabetic nephropathy in patients with type 2 diabetes. Diabetes Res. Clin. Pract. 98, 140–143. https://doi.org/10.1016/j.diabres.2012.07.007 (2012).
Huang, S. T., Lin, C. L., Yu, T. M., Yang, T. C. & Kao, C. H. Nonapnea sleep disorders and incident chronic kidney disease: A population-based retrospective cohort study. Medicine (Baltimore) 94, e429. https://doi.org/10.1097/MD.0000000000000429 (2015).
Brotman, D. J. et al. Heart rate variability predicts ESRD and CKD-related hospitalization. J. Am. Soc. Nephrol. 21, 1560–1570. https://doi.org/10.1681/ASN.2009111112 (2010).
Yamagata, K. et al. Risk factors for chronic kidney disease in a community-based population: A 10-year follow-up study. Kidney Int. 71, 159–166. https://doi.org/10.1038/sj.ki.5002017 (2007).
Kadoya, M. et al. Associations of sleep quality and awake physical activity with fluctuations in nocturnal blood pressure in patients with cardiovascular risk factors. PLoS ONE 11, e0155116. https://doi.org/10.1371/journal.pone.0155116 (2016).
Hofer, M. M. & Barde, Y. A. Brain-derived neurotrophic factor prevents neuronal death in vivo. Nature 331, 261–262. https://doi.org/10.1038/331261a0 (1988).
Pencea, V., Bingaman, K. D., Wiegand, S. J. & Luskin, M. B. Infusion of brain-derived neurotrophic factor into the lateral ventricle of the adult rat leads to new neurons in the parenchyma of the striatum, septum, thalamus, and hypothalamus. J. Neurosci. 21, 6706–6717 (2001).
Leenen, F. H. & Tuana, B. S. Cardioprotective brain mechanisms. Arterioscler. Thromb. Vasc. Biol. 32, 1749–1750. https://doi.org/10.1161/ATVBAHA.112.252627 (2012).
Okada, S. et al. Brain-derived neurotrophic factor protects against cardiac dysfunction after myocardial infarction via a central nervous system-mediated pathway. Arterioscler. Thromb. Vasc. Biol. 32, 1902–1909. https://doi.org/10.1161/ATVBAHA.112.248930 (2012).
Lee, J., Han, Y., Cho, H. H. & Kim, M. R. Sleep disorders and menopause. J. Menopausal Med. 25, 83–87. https://doi.org/10.6118/jmm.19192 (2019).
Acknowledgements
We thank all of the investigators and staff, as well as the participants in the Hyogo Sleep Cardio-Autonomic Atherosclerosis study for their valuable contributions.
Funding
This study was supported by JSPS KAKENHI grants (19K19421 to M. Kadoya, 18K08531 to H. Koyama), and a grant from Hyogo College of Medicine (Hyogo Innovative Challenge to H. Koyama).
Author information
Authors and Affiliations
Contributions
M.K. and H.K.: Conceptualization, Methodology, Software; M.K., Akik.M., Akio.M., M.K.-H. and K.K.-H.: Data curation; M.K. and H.K.: Writing- Original draft preparation; K.K., Y.K. and T.S.: Supervision; H.K.: Validation and Writing-Reviewing and Editing.All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kadoya, M., Morimoto, A., Miyoshi, A. et al. Sleep quality, autonomic dysfunction and renal function in diabetic patients with pre-CKD phase. Sci Rep 11, 19048 (2021). https://doi.org/10.1038/s41598-021-98505-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-98505-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.