Abstract
Epidermal growth factor receptor (EGFR) exon 20 insertion mutations (Exon20ins) account for 4–12% of all EGFR mutations in non-small cell lung cancer (NSCLC) patients. Data on the differences in clinical characteristics between patients with Exon20ins and major mutations (M-mut) such as exon 19 deletion and L858R are limited. We retrospectively reviewed advanced NSCLC patients with EGFR mutations, who were treated with systemic therapy between January 2011 and December 2019. We identified 23 patients with Exon20ins and 534 patients with M-mut. In Exon20ins patients, the median age was 60 (range 27–88) years, and females and never-smokers were predominant. Clinical characteristics were similar in the two groups. In Exon20ins patients, 17 patients received platinum doublet as first-line therapy, and the overall response rate (ORR) and median progression-free survival (mPFS) were 11.8% and 8.9 months. Additionally, seven patients received conventional EGFR-tyrosine kinase inhibitors (TKIs), and eight patients anti-PD-1 antibodies in any-line therapy. ORR and mPFS of EGFR-TKIs and anti-PD-1 antibodies were 0%, 2.2 months and 25%, 3.1 months, respectively. Overall survival was significantly shorter in Exon20ins patients than in M-mut patients (29.3 vs. 43.4 months, p = 0.04). The clinical outcomes in Exon20ins patients were not satisfactory compared to M-mut patients.
Similar content being viewed by others
Introduction
Epidermal growth factor receptor (EGFR) mutations mainly occur between exons 18 and 21 in non-small cell lung cancer (NSCLC), and are commonly found in never smokers, women, and patients with lung adenocarcinoma1,2. The frequency of EGFR mutations has been reported to be 47.9% in adenocarcinoma and 4.6% in lung squamous cell carcinoma among East Asian populations, and 19.2% in lung adenocarcinoma and 3.3% in lung squamous cell carcinoma among Western populations3. The most common genetic mutation is the deletion of exon 19 and L858R in exon 21, which accounts for about 70–80% of all EGFR mutations4,5. Most advanced NSCLC patients with these EGFR mutations respond to treatment with EGFR-tyrosine kinase inhibitors (EGFR-TKIs) such as gefitinib, erlotinib, afatinib, and osimertinib, with median progression-free survivals (mPFS) of 9.2–18.9 months6,7,8,9,10,11.
Exon 20 insertion mutations are the third most common subtype of EGFR mutation, which accounts for about 4–12% of all EGFR mutations, and are mutually exclusive with other known driver mutations. Exon 20 insertion mutations are also associated with a lack of sensitivity to the aforementioned EGFR-TKIs4,12,13,14. The standard treatment for patients with exon 20 insertion is systemic chemotherapy, which is similar to the treatment of other NSCLC cases without driver mutations15,16. On the other hands, novel targeted therapies against NSCLC with EGFR exon 20 insertion mutations, such as poziotinib17, mobocertinib (TAK-788)18,19, and amivantamab (JNJ-61186372)20 have been developed in preclinical and early clinical trials. There has been a growing interest on this subgroup of EGFR-mutant NSCLC patients.
Few studies have focused on the differences in clinical characteristics between patients with EGFR exon 20 insertions and major mutations. Our study therefore aimed to clarify the clinical characteristics and outcomes, including the efficacy of systemic treatment in patients with EGFR exon 20 insertion mutations, compared with those with major mutations.
Patient and methods
Subjects
We retrospectively reviewed advanced NSCLC patients with EGFR exon 20 insertion mutations treated with systemic chemotherapy, and those with EGFR major mutations (e.g., deletion in exon 19 and L858R in exon 21) treated with EGFR-TKIs as initial treatment at the National Cancer Center Hospital in Japan between January 2011 and December 2019. We collected data on patient characteristics, variants of exon 20 insertion, and clinical outcomes from medical records.
Detection of EGFR mutation including exon 20 insertion mutations
The diagnosis of EGFR mutation including exon 20 insertion was performed based on PCR-based methods (therascreen EGFR RGQ PCR Kit [Scorpion-ARMS technology]; QIAGEN, Hilden, Germany, and Cobas EGFR Mutation Test v2; Roche Diagnostics, Basel, Switzerland)21,22 and next-generation sequencing (NGS) testing (OncoGuide NCC Oncopanel System, Sysmex, Kobe, Japan)23.
Statistical analysis
To evaluate the differences in clinical characteristics between the patients, Fisher’s exact test was performed. The treatment effect was evaluated based on the Response Evaluation Criteria in Solid Tumors (RECIST version 1.1)24. The overall response rate (ORR) was defined as the percentage of patients with the best overall response of complete response (CR) or partial response (PR). We also used the Kaplan–Meier method to investigate PFS and overall survival (OS). OS was defined as the time from the date of diagnosis of advanced disease to death. PFS was defined as the time from the start of treatment to disease progression or death and was censored on the date the patient was last known as progression-free. All statistical analyses were performed using the EZR ver. 1.4125. This study was approved by the Ethics Committee of the National Cancer Center Hospital (2015-355 and 2019-123).
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of National Cancer Center Hospital in Japan (2015-355 and 2019-123).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Patients has consented regarding publishing their data.
Results
Patient characteristics
We identified 23 patients with exon 20 insertions and 534 patients with major mutations, including 285 patients with an exon 19 deletion and 249 patients with an L858R mutation in exon 21. Patient characteristics according to EGFR mutation status are shown in Table 1. Patients with exon 20 insertions were significantly younger than those with major mutations (median age 60 vs. 66 years, p = 0.017). There were no significant differences in baseline characteristics between patients with exon 20 insertions and major mutations, except for age. Regarding the metastatic spread, bone (21.6%) was the most common metastatic site in patients with exon 20 insertions, followed by the central nervous system (CNS) (13.0%), liver (17.4%). Patients with intrathoracic metastases were more common in patients with exon 20 insertions (52.2%) than in those with major mutations (35.2%), although the differences were not significant. Of the 23 patients with exon 20 insertions, four were assessed for variants of exon 20 insertions by NGS.
Efficacy of platinum doublet chemotherapy in patients with exon 20 insertions
Of the 23 patients with exon 20 insertions, 17 received platinum doublet chemotherapy, including two patients who received platinum doublet chemotherapy in combination with anti-PD-1 antibody, and 1 in combination with EGFR-TKIs. Other first-line treatments were as follows: four pembrolizumab, one EGFR-TKI, and one pemetrexed monotherapy (Supplementary Table 1). The ORR and mPFS of first-line platinum doublet chemotherapy in patients with exon 20 insertions were 11.8% (95% CI 1.5–36.4), and 8.9 months (95% confidence interval [CI] 5.0–17.3), compared with ORR of 21.5% (95% CI 15.4–28.6) and PFS of 5.5 months (95% CI 4.6–6.2) in patients with major mutations (ORR: p = 0.75; PFS: p = 0.01, Table 2 and Fig. 1a).
Efficacy of EGFR-TKIs in patients with exon 20 insertions
Over the clinical course in patients with exon 20 insertions, 7 patients received EGFR-TKIs. The differences in the ORR and PFS between patients with exon 20 insertions and major mutation shown in Table 2 and Fig. 1b. The ORR and mPFS of EGFR-TKIs were 0%, 2.2 months (95% CI 1.1 to NA) in patients with exon 20 insertions and 57.9% (95% CI 53.5–62.1), 13.6 months (95% CI 12.6–14.9) in those with major mutation (ORR: p = 0.003 and, PFS: p = 0.08).
Efficacy of anti-PD-1 antibody in patients with exon 20 insertions
Eight patients received anti-PD-1 antibody monotherapy in patients with exon 20 insertions. The differences in the ORR and PFS between exon 20 and major mutation patients in the anti-PD-1 antibody monotherapy are shown in Table 2 and Fig. 1c. ORR and PFS of anti-PD-1 antibody monotherapy was 25% (95% CI 3.2–65.1), 3.1 months (95% CI 0.7–6.0) in patients with exon 20 insertions, and 15.8% (95% CI 6.0–31.3), 2.2 months (95% CI 1.5–3.4) in those with major mutation (ORR: p = 0.61 and, PFS: p = 0.80).
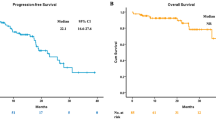
Overall survival in advanced NSCLC patients with exon 20 insertions
The median overall survival in patients with exon 20 insertions was 29.3 months (95% confidence interval [CI] 14.1). On the other hand, OS in patients with major mutations who received EGFR-TKIs was 43.4 months (95% CI 38.7–54.2). Patients with exon 20 insertions had a significantly shorter OS than those with major mutations (p = 0.04, Fig. 2). The clinical outcomes of the four patients with the identified variants are shown in Supplementary Table 2.
Discussion
We found that there were no significant differences in clinical characteristics, including the distribution of metastatic sites between patients with EGFR exon 20 insertion and major mutations. The OS of patients with exon 20 insertions was significantly shorter than in patients with major mutations who received EGFR-TKIs as initial treatment.
Few reports have focused on the differences in clinical characteristics between patients with exon 20 insertions and major mutations. Previous studies have shown that EGFR exon 20 insertion is more likely to occur in never or light smoking patients and those with lung adenocarcinomas26,27. In our study, however, there were no differences in sex, smoking history, histology, metastatic spread or stage at diagnosis between the two groups, while patients with exon 20 insertions were significantly younger than those with major mutations.
EGFR exon 20 insertions are related to the intrinsic resistance to conventional EGFR-TKIs compared with major mutations, such as exon 19 deletion and L858R in exon 214,12,28. Due to the limited efficacy of EGFR-TKIs, platinum combination chemotherapy is still the standard therapy for patients with exon 20 insertion. Previous studies have reported that mPFS was 4.2–6.4 months and OS was 16.4–29.4 months, which were similar to our data16,29,30. On the other hands, the clinical efficacy of EGFR-TKIs in patients with EGFR exon 20 insertion has been reported to differ according to the variant26. Some variants such as EGFR A763_Y764insFQEA mutation have been reported to associate with sensitivity to first generation EGFR TKIs in both preclinical and clinical setting26,31,32,33. However, in the current clinical practice, we did not necessarily obtain detailed variant information, and the frequency of sensitive variants seems quite low. Thus, our results strongly support that EGFR exon 20 insertions are not sensitive to the conventional EGFR-TKIs.
We also evaluated the efficacy of anti-PD-1/PD-L1 antibodies in NSCLC with EGFR exon 20 insertions. In general, anti-PD-1/PD-L1 antibodies are poorly effective in EGFR-mutated NSCLC compared with those without EGFR mutations34,35,36. In this study, the ORR and mPFS of the anti-PD-1 antibody in patients with EGFR exon 20 insertions were 25% and 3.1 months (95% CI 0.7–6.0). Recent studies have reported that patients with EGFR exon 20 insertions showed better clinical outcomes of anti-PD-1 antibody compared with those with EGFR major mutations37. However, the therapeutic effect is still limited in patients with EGFR exon 20 insertions, and more specific treatment for advanced NSCLC with EGFR exon 20 insertions is desirable.
Recently, novel targeted therapies against EGFR exon 20 insertion mutations, such as poziotinib, mobocertinib, and amivantmab have been developed17,18,19,20. Poziotinib, a potent TKI against EGFR and HER2 exon 20 insertion mutations, showed an ORR of 15–44% and PFS of 4.2–5.6 months in the phase II trial and results from the expanded access program38,39,40. Mobocertinib is an EGFR-TKI with potent and selective preclinical inhibitory activity against EGFR exon 20 insertions, with an ORR of 43% and PFS of 7.3 months in a phase II trial41. A phase III trial comparing mobocertinib with platinum-based chemotherapy as first-line therapy is currently ongoing (NCT04129502)42. Amivantamab is an anti-EGFR-MET bispecific antibody that can target diseases driven by both EGFR and MET, and has shown therapeutic efficacy in patients with a variety of mutations, including EGFR C797S, T790M, exon20 insertion mutation, and MET amplification. Amivantamab showed a response rate of 36% and a PFS of 8.3 months in a Phase II/III study20. A study is planned for advanced NSCLC patients with EGFR exon 20 insertion mutations, with carboplatin and pemetrexed with and without amivantamab (NCT04538664).
This study has some limitations. First, it is a single-center, retrospective study with a small sample size as patients with EGFR exon 20 mutations are rare. Additionally, genetic variants of exon 20 insertion were assessable in only four patients, as PCR-based testing showed only the presence of exon 20 insertion, not variant types, NGS was not approved for the detection of EGFR mutation at the testing time. EGFR exon 20 insertions are structurally and pharmacologically heterogeneous, with variability in their position and size having implications for response to conventional EGFR TKIs43,44,45. In this study, only four patients had detailed information on insertion variants, and we three different variants of EGFR exon 20 insertions. Indeed, preclinical studies showed A767_V769dupASV, A767_S768insTLA and D770_N771insSVD mutation which was similar variant to D770_N771insASV, are associated with resistance to first-generation EGFR TKIs, while showing a wide therapeutic window for osimertinib in preclinical studies46,47.
In conclusion, the OS of patients with exon 20 insertions was significantly shorter than those with major mutations due to the lack of targeted therapies, although clinical characteristics, including the distribution of metastatic sites was very similar between two groups. Additionally, the effectiveness of anti-PD-1 antibodies in patients with EGFR exon 20 insertion is limited as with those with EGFR major mutations. Therefore, the development of novel targeted therapies against NSCLC with EGFR exon 20 insertion mutations is warranted to improve the prognosis. On the other hands, EGFR exon 20 insertion is heterogeneous group of aberrations. Further investigation on association how the heterogeneous nature of EGFR exon 20 insertion mutations affect the clinical outcomes including the efficacy of these drugs will be warranted.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Lan, Q. et al. Genome-wide association analysis identifies new lung cancer susceptibility loci in never-smoking women in Asia. Nat. Genet. 44(12), 1330–1335 (2012).
Marchetti, A. et al. EGFR mutations in non-small-cell lung cancer: Analysis of a large series of cases and development of a rapid and sensitive method for diagnostic screening with potential implications on pharmacologic treatment. J. Clin. Oncol. 23(4), 857–865 (2005).
Dearden, S. et al. Mutation incidence and coincidence in non small-cell lung cancer: Meta-analyses by ethnicity and histology (mutMap). Ann. Oncol. 24(9), 2371–2376 (2013).
Yasuda, H., Kobayashi, S. & Costa, D. B. EGFR exon 20 insertion mutations in non-small-cell lung cancer: Preclinical data and clinical implications. Lancet Oncol. 13(1), e23-31 (2012).
Riess, J. W. et al. Diverse EGFR Exon 20 insertions and co-occurring molecular alterations identified by comprehensive genomic profiling of NSCLC. J. Thorac. Oncol. 13(10), 1560–1568 (2018).
Zhou, C. et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): A multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 12(8), 735–742 (2011).
Rosell, R. et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 13(3), 239–246 (2012).
Soria, J. C. et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N. Engl. J. Med. 378(2), 113–125 (2018).
Sequist, L. V. et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J. Clin. Oncol. 31(27), 3327–3334 (2013).
Mitsudomi, T. et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 11(2), 121–128 (2010).
Maemondo, M. et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N. Engl. J. Med. 362(25), 2380–2388 (2010).
Vyse, S. & Huang, P. H. Targeting EGFR exon 20 insertion mutations in non-small cell lung cancer. Signal Transduct. Target Ther. 4, 5 (2019).
van Veggel, B. et al. Osimertinib treatment for patients with EGFR exon 20 mutation positive non-small cell lung cancer. Lung Cancer 141, 9–13 (2020).
Yang, J. C. et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: A combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol. 16(7), 830–838 (2015).
Wu, J. Y., Yu, C. J. & Shih, J. Y. Effectiveness of treatments for advanced non-small-cell lung cancer with Exon 20 insertion epidermal growth factor receptor mutations. Clin. Lung Cancer 20(6), e620–e630 (2019).
Byeon, S. et al. Clinical outcomes of EGFR Exon 20 insertion mutations in advanced non-small cell lung cancer in Korea. Cancer Res. Treat. 51(2), 623–631 (2019).
Le, X. et al. Poziotinib shows activity and durability of responses in subgroups of previously treated EGFR exon 20 NSCLC patients. J. Clin. Oncol. 38(15_suppl), 9514–9514 (2020).
Horn, L. et al. Indirect comparison of TAK-788 vs real-world data outcomes in refractory non-small cell lung cancer (NSCLC) with EGFR exon 20 insertions. J. Clin. Oncol. 38(15_suppl), 9580–9580 (2020).
Gonzalvez, F. et al. Mobocertinib (TAK-788): A targeted inhibitor of EGFR Exon 20 insertion mutants in non-small cell lung cancer. Cancer Discov. 11(7), 1672–1687 (2021).
Park, K. et al. Amivantamab (JNJ-61186372), an anti-EGFR-MET bispecific antibody, in patients with EGFR exon 20 insertion (exon20ins)-mutated non-small cell lung cancer (NSCLC). J. Clin. Oncol. 38(15_suppl), 9512–9512 (2020).
Malapelle, U. et al. Profile of the Roche cobas(R) EGFR mutation test v2 for non-small cell lung cancer. Expert Rev. Mol. Diagn. 17(3), 209–215 (2017).
Hsiue, E. H. et al. Profile of the therascreen(R) EGFR RGQ PCR kit as a companion diagnostic for gefitinib in non-small cell lung cancer. Expert. Rev. Mol. Diagn. 16(12), 1251–1257 (2016).
Sunami, K. et al. Feasibility and utility of a panel testing for 114 cancer-associated genes in a clinical setting: A hospital-based study. Cancer Sci. 110(4), 1480–1490 (2019).
Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 45(2), 228–247 (2009).
Kanda, Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 48(3), 452–458 (2013).
Arcila, M. E. et al. EGFR exon 20 insertion mutations in lung adenocarcinomas: Prevalence, molecular heterogeneity, and clinicopathologic characteristics. Mol. Cancer Ther. 12(2), 220–229 (2013).
Choudhury, N. J. et al. Response to standard therapies and comprehensive genomic analysis for patients with lung adenocarcinoma with EGFR Exon 20 insertions. Clin. Cancer Res. 27(10), 2920–2927 (2021).
Remon, J. et al. EGFR exon 20 insertions in advanced non-small cell lung cancer: A new history begins. Cancer Treat. Rev. 90, 102105 (2020).
Cardona, A. F. et al. EGFR exon 20 insertion in lung adenocarcinomas among Hispanics (geno1.2-CLICaP). Lung Cancer 125, 265–272 (2018).
Wang, Y. et al. Real-world treatment outcome of advanced Chinese NSCLC EGFR exon 20 insertion patients. J. Clin. Oncol. 37(15_suppl), 9043–9043 (2019).
Klughammer, B. et al. Examining treatment outcomes with erlotinib in patients with advanced non-small cell lung cancer whose tumors harbor uncommon EGFR mutations. J. Thorac. Oncol. 11(4), 545–555 (2016).
Qin, Y. et al. Variability of EGFR exon 20 insertions in 24 468 Chinese lung cancer patients and their divergent responses to EGFR inhibitors. Mol. Oncol. 14(8), 1695–1704 (2020).
Yasuda, H. et al. Structural, biochemical, and clinical characterization of epidermal growth factor receptor (EGFR) exon 20 insertion mutations in lung cancer. Sci. Transl. Med. 5(216), 216ra177 (2013).
Socinski, M. A. et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N. Engl. J. Med. 378(24), 2288–2301 (2018).
Lee, C. K. et al. Checkpoint inhibitors in metastatic EGFR-mutated non-small cell lung cancer—A meta-analysis. J. Thorac. Oncol. 12(2), 403–407 (2017).
Gainor, J. F. et al. EGFR mutations and ALK rearrangements are associated with low response rates to PD-1 pathway blockade in non-small cell lung cancer: A retrospective analysis. Clin. Cancer Res. 22(18), 4585–4593 (2016).
Yamada, T. et al. Retrospective efficacy analysis of immune checkpoint inhibitors in patients with EGFR-mutated non-small cell lung cancer. Cancer Med. 8(4), 1521–1529 (2019).
Socinski, M. A. et al. LBA60 ZENITH20, a multinational, multi-cohort phase II study of poziotinib in NSCLC patients with EGFR or HER2 exon 20 insertion mutations. Ann. Oncol. 31, S1188 (2020).
Heymach, J. et al. OA02.06 A Phase II trial of poziotinib in EGFR and HER2 exon 20 mutant non-small cell lung cancer (NSCLC). J. Thorac. Oncol. 13(10), S323–S324 (2018).
Prelaj, A. et al. Poziotinib for EGFR and HER2 exon 20 insertion mutation in advanced NSCLC: Results from the expanded access program. Eur. J. Cancer 149, 235–248 (2021).
Riely, G. J. et al. Activity and safety of mobocertinib (TAK-788) in previously treated non-small cell lung cancer with EGFR Exon 20 insertion mutations from a phase I/II trial. Cancer Discov. 11(7), 1688–1699 (2021).
Jänne, P. A. et al. 1412TiP Mobocertinib (TAK-788) as first-line treatment vs platinum-based chemotherapy (CT) for NSCLC with EGFR exon 20 insertions (exon20ins). Ann. Oncol. 31, S892–S893 (2020).
Arcila, M. E. et al. Prevalence, clinicopathologic associations, and molecular spectrum of ERBB2 (HER2) tyrosine kinase mutations in lung adenocarcinomas. Clin. Cancer Res. 18(18), 4910–4918 (2012).
Russo, A. et al. Heterogeneous responses to epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) in patients with uncommon EGFR mutations: New insights and future perspectives in this complex clinical scenario. Int. J. Mol. Sci0 20(6), 1431 (2019).
Zochbauer-Muller, S. et al. Case report: Afatinib treatment in a patient with NSCLC harboring a rare EGFR Exon 20 mutation. Front. Oncol. 10, 593852 (2020).
Gristina, V. et al. The significance of epidermal growth factor receptor uncommon mutations in non-small cell lung cancer: A systematic review and critical appraisal. Cancer Treat. Rev. 85, 101994 (2020).
Hirose, T. et al. Extensive functional evaluation of exon 20 insertion mutations of EGFR. Lung Cancer 152, 135–142 (2021).
Funding
No funds, grants, or other support was received.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by C.M., M.S. and T.Y. The first draft of the manuscript was written by Chie Morita and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
Dr. Yoshida has received grants and personal fees from AstraZeneca, Bristol-Myers Squibb, grants from Abbvie, MSD, Ono Pharmaceutical, Takeda Pharmaceutical, and personal fees from Chugai, Novartis. Dr. Matsumoto has received grants from Grant-in-Aid for Scientific Research on Innovative Areas, Hitachi High-Technologies, Hitachi, Ltd., National Cancer Center Research and Development Fund, and personal fees from AMCO INC., AstraZeneca, COOK, Olympus. Dr. Okuma has received grants from Abbvie. Dr. Goto has received grants and personal fees from Bristol-Myers Squibb, Daiichi- Sankyo, Eli Lilly, Guardant Health, MSD, Novartis, Ono Pharmaceutical, Pfizer, Taiho Pharmaceutical, grants from Kyorin, and personal fees from AstraZeneca, Boehringer Ingelheim, Chugai, Illumina. Dr. Horinouchi has received grants and personal fees from AstraZeneca, BMS, Chugai, Eli Lilly, MSD, Taiho Pharmaceutical, Ono Pharmaceutical, and grants from Astellas, Genomic Health, Merck Serono. Dr. Yamamoto has received grants and personal fees from BMS, Boehringer Ingelheim, Chugai, Eisai, Eli Lilly, Ono Pharmaceutical, Pfizer, Takeda Pharmaceutical, grants from Astellas, Bayer, Chiome Bioscience Inc., Daiichi-Sankyo, GSK, Janssen Pharma, Kyowa-Hakko kirin, MSD, Merck, Novartis, Otsuka, Taiho Pharmaceutical, Quintiles, Sumitomo Dainippon, and personal fees from AstraZeneca, Otsuka, Cimic, Sysmex. Dr. Yatabe has received personal fees from Archer, AstraZeneca, Chugai, Dako-Agilent, MSD, Novartis, Pfizer, Thermo-Fisher Science, Ventana-Roche. Dr. Ohe has received grants and personal fees from AstraZeneca, Bristol-Myers Squibb, Chugai, Eli Lilly, Janssen Pharma, Kyorin, MSD, Nippon Kayaku, Novartis, Ono Pharmaceutical, Pfizer, Taiho Pharmaceutical, Takeda Pharmaceutical, grants from Kissei, personal fees from Boehringer Ingelheim, Celtrion. Dr. Motoi has received grants and personal fees from Ono Pharmaceutical, Roche Diagnostics, grants from NEC, personal fees from AstraZeneca, Beckton Dickinson Japan, Covidien Japan Inc, Miraca Life Sciences, MSD, Novartis, Taiho Pharmaceutical. The remaining authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Morita, C., Yoshida, T., Shirasawa, M. et al. Clinical characteristics of advanced non-small cell lung cancer patients with EGFR exon 20 insertions. Sci Rep 11, 18762 (2021). https://doi.org/10.1038/s41598-021-98275-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-98275-3
This article is cited by
-
Characteristics, treatment patterns, and clinical outcomes in patients with advanced non-small cell lung cancer harboring EGFR exon 20 insertions
Journal of Cancer Research and Clinical Oncology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.