Abstract
The lack of bacteriophages capable of infecting the Listeria species, Listeria grayi, is academically intriguing and presents an obstacle to the development of bacteriophage-based technologies for Listeria. We describe the isolation and engineering of a novel L. grayi bacteriophage, LPJP1, isolated from farm silage. With a genome over 200,000 base pairs, LPJP1 is the first and only reported jumbo bacteriophage infecting the Listeria genus. Similar to other Gram-positive jumbo phages, LPJP1 appeared to contain modified base pairs, which complicated initial attempts to obtain genomic sequence using standard methods. Following successful sequencing with a modified approach, a recombinant of LPJP1 encoding the NanoLuc luciferase was engineered using homologous recombination. This luciferase reporter bacteriophage successfully detected 100 stationary phase colony forming units of both subspecies of L. grayi in four hours. A single log phase colony forming unit was also sufficient for positive detection in the same time period. The recombinant demonstrated complete specificity for this particular Listeria species and did not infect 150 non-L. grayi Listeria strains nor any other bacterial genus. LPJP1 is believed to be the first reported lytic bacteriophage of L. grayi as well as the only jumbo bacteriophage to be successfully engineered into a luciferase reporter.
Similar content being viewed by others
Introduction
Listeria grayi includes two genetically related subspecies, L. grayi subsp. grayi and L. grayi subsp. murrayi. Although typically classified as non-pathogenic, L. grayi has been associated with severe disease in rare cases involving immunocompromised individuals1,2,3. Despite lacking clinical significance to the general population, L. grayi shares a number of important characteristics with the food-borne pathogen L. monocytogenes, including motility and growth at refrigerated temperatures4. These shared features have allowed this and other non-pathogenic Listeria species to serve as useful tools in food safety. One particular example of this is seen in Listeria environmental monitoring programs5. Detection of any viable Listeria species during sampling leads to preventative intervention to eliminate growth conditions and preempt contamination with pathogenic species, particularly L. monocytogenes. Thus, studies on L. grayi and other non-pathogenic Listeria species are beneficial to both academic understanding and public health initiatives.
To our knowledge, no bacteriophage has been reported to infect and lyse L. grayi. This is unexpected as the number of Listeria species has grown from six in 1984 to 21 in 2020, and L. grayi was described over 50 years ago, providing ample time for phage discovery4. In addition, hundreds of Listeria phages have been described over the years, and the lack of lytic phages appears to be unique to L. grayi. None of 16 phages comprising a Listeria typing system could lyse L. grayi6. Further, isolation of Listeria-specific phage from food processing plants found phage capable of lysing all tested Listeria species except L. grayi7. The lack of reported lytic phages for this species could have indicated a unique phage resistance mechanism or imply that such a phage was rare or challenging to isolate. Given the use of L. monocytogenes for isolation of phage in these previously described studies, Listeria phage specific to L. grayi would also remain undetected.
Engineered bacteriophages have great potential as diagnostic tools. The use of engineered recombinant phages encoding a reporter enzyme, such as a luciferase, is particularly promising in facilitating detection of bacteria. When viable hosts are present in a sample, recombinant phages will infect these cells, leading to production of the reporter enzyme and a detectable signal8. Phages have been recently engineered in this fashion to detect a variety of bacteria, ranging from food-borne contaminants such as E. coli O157:H7, Salmonella and L. monocytogenes to clinical pathogens such as S. aureus and M. tuberculosis9,10,11,12,13. The viability of phage-based reporters is linked to successful isolation and engineering of suitable phage cocktails14. Despite growing phage collections, bacterial species susceptible to limited or no known phages present a challenge to developing this technology across diverse niches.
Luciferase reporter phages have been previously engineered in the pursuit of specific and sensitive detection of Listeria species in food products11,15. Initial assays utilized a lytic phage, A511, that was engineered to encode the LuxAB luciferase from Vibrio harveyi11. Among known phages, A511 is an excellent choice for a reporter, as it broadly recognizes the Listeria genus, covering most serotypes and species. This method has recently been further improved by incorporating NanoLuc, an engineered luciferase evolved from the Oplophorus gracilirostris luciferase15. Using NanoLuc-encoding A511 recombinants, reliable detection of a single CFU of L. monocytogenes in a 25 g portion of various food products was possible after a 20 h enrichment. Despite the successes and proven viability of this approach, L. grayi continues to be absent in the host range of these reporters and a blind spot in phage-based technologies.
The primary objectives of this study were to: (1) isolate a lytic bacteriophage recognizing L. grayi and (2) engineer and characterize a NanoLuc-encoding recombinant of said phage. Using silage, a common medium for isolating Listeria phages, this report describes the isolation and characterization of the first phage, to our knowledge, uniquely capable of lysing L. grayi. Further, homologous recombination was used to create a NanoLuc-encoding recombinant, which ultimately proved capable of sensitive and accurate detection of L. grayi, the first in phage-based technologies.
Results
Isolation and basic characterization of LPJP1
Silage has been recognized as an excellent source of diverse Listeria bacteriophages16. Silage obtained from a Wisconsin farm was probed for the presence of phages capable of lysing L. grayi. From this material, a virulent phage referred to as LPJP1 was isolated on L. grayi lawns and subsequently purified by serial plaque isolation. LPJP1 formed small clear plaques at 30 °C on the L. grayi strain ATCC 19,120 (Supplementary Fig. 1). Interestingly, plaques were not observed at 37 °C (data not shown). The mechanism behind this phenotype is not fully understood but is consistent with temperature-dependent plaque formation observed with other Listeria phages17. A one-step growth curve of LPJP1 revealed a burst size of approximately 50 to 60 pfu/cell with a latent period of roughly 70 min. To our knowledge, LPJP1 is the first published phage capable of lysing and forming plaques on L. grayi.
Transmission electron microscopy was used to confirm phage presence and determine the morphology of LPJP1. Microscopy revealed the presence of a large icosahedral head, non-flexible long straight tail, and contractile outer tail sheath (Fig. 1a,b). These characteristics match the previously described myovirid morphotype of tailed phages (caudoviruses)18,19.
Genome sequencing and engineering of LPJP1
The possibility of engineering the first L. grayi luciferase reporter phage from LPJP1 was intriguing. Previous success has been achieved by inserting a reporter downstream of a major capsid protein10,12,15. In order to replicate this approach with LPJP1, this location first had to be identified through sequencing. Surprisingly, initial attempts to sequence the genome of this phage were unsuccessful despite using a previously successful approach10,12. Despite obtaining sufficient DNA quantity and quality, library preparation using bead-lined transposomes (Illumina, San Diego, CA, USA) could not be achieved, failing at DNA amplification. This library preparation method is generally considered to be quite robust, demonstrating resistance to poor DNA quality and productivity with various DNA quantities20. One possible explanation for this failure is the presence of modified DNA base pairs, which have been observed to serve a number of functions in some bacteriophages21. In particular, the presence of deaminated base pairs, such as uracil or hypoxanthine, in DNA templates can inhibit commercial high-fidelity proof-reading archaeal polymerases22,23. Unfortunately, the polymerase used in the above library preparation kit remains undisclosed to our knowledge. To circumvent this possibility, DNA preparations of LPJP1 were amplified using the TempliPhi DNA amplification kit (Cytiva, Marlborough, MA, USA), which utilizes the Phi29 bacteriophage polymerase capable of reading past uracil-containing DNA24,25. PCR amplification is expected to convert uracil to thymine, as done typically with bisulfite-modified DNA26. As expected, library preparation was successfully completed using PCR-amplified DNA of LPJP1. Further support of both the presence of modified base pairs in LPJP1 and the ability to overcome this with the TempliPhi kit was obtained using the assembled sequence. Using this sequence, primers were designed to amplify approximately 550 bp of the LPJP1 genome within a predicted major capsid protein. PCR amplification was performed using equivalent amounts of either native LPJP1 DNA or TempliPhi-treated LPJP1 DNA. Additionally, the reaction was performed using either the Q5 polymerase or the uracil-tolerant Q5U polymerase (New England BioLabs, Ipswich, MA, USA). When these reactions were analyzed by gel electrophoresis, a distinct band of the expected size was observed with native DNA and the Q5U polymerase and TempliPhi-treated DNA with both polymerases (Fig. 2). A clear difference was observed between the uracil-sensitive Q5 polymerase and the uracil-tolerant Q5U polymerase on native phage DNA. Importantly, the failure of the Q5 polymerase in PCR was specific to native DNA and TempliPhi-treated DNA could be effectively amplified by both polymerases. These results support the hypothesis that LPJP1 contains modified base pairs and affirm the value of using an alternative DNA amplification method prior to traditional work flows if sequencing issues arise with novel phage.
Gel electrophoresis of PCR-amplified LPJP1 DNA. Flanking lanes 1 and 6 contain O’GeneRuler 1 kb plus DNA ladder (Thermo Fisher Scientific, Waltham, MA, USA). Lanes 2 through 5 contain the PCR amplification of either native LPJP1 DNA (lanes 2 and 4) or TempliPhi-treated LPJP1 DNA (lanes 3 and 5) using either the Q5 DNA polymerase (lanes 2 and 3) or the uracil-tolerant Q5U DNA polymerase (lanes 4 and 5). Gel images were cropped to remove unrelated experimental data. Full-length gel image is presented in Supplementary Fig. 2.
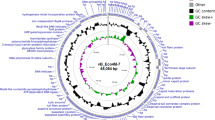
Assembly of LPJP1 sequences revealed a large genome, 223,580 bp in length. This crosses the threshold of 200,000 bp to be considered a jumbo phage27. As reviewed previously, jumbo phages are rarely described and the vast majority (greater than 95%) of these phages recognize Gram-negative bacteria28. Among the few jumbo phages infecting Gram-positive bacteria, most recognize species within the genus Bacillus. Similar to previously described uracil-containing phages, the G + C content of LPJP1 was low (25.9%)29. Annotation of the assembled genome was performed, revealing 229 open reading frames (ORFs) and four tRNA genes. Unsurprisingly, the majority of ORFs encoded hypothetical proteins, which is in line with other recently annotated jumbo phages30,31. The sequence and annotation of LPJP1 was submitted to GenBank (accession number: MZ422438).
Manual examination of predicted ORFs in LPJP1 revealed a candidate major capsid protein (Supplementary Table 1). BLAST analysis of this protein sequence indicated homology with the precursor of the major head subunit protein of several Gram-positive jumbo phages. Top hits in this category were two recently sequenced Staphylococcus jumbo phage, MarsHill and Machias, followed by the Bacillus jumbo phage AR9. Amino acid homology was also observed with the structural protein sp46 precursor of both the Bacillus jumbo phage vB_BpuM-BpSP and the Yersinia jumbo phage phiR1-37. Sp46 has previously been identified as the probable major capsid protein in phiR1-37, strengthening the support for this prediction32. Homologous recombination was used then to mediate insertion of a late gene promoter and the NanoLuc luciferase sequence immediately downstream of the coding sequence for this candidate major capsid protein. Sequences used in engineering are provided (Supplementary Table 1). A recombinant NanoLuc-encoding LPJP1, referred hereafter as LPJP1.NL, was isolated, purified, and confirmed using genome sequencing.
Limit of detection of LPJP1.NL
NanoLuc-encoding bacteriophages typically yield robust signal following infection of even a few bacterial cells10,12,15. In order to evaluate the sensitivity of LPJP1.NL for L. grayi, the limit of detection of this recombinant reporter was determined. LPJP1.NL was allowed to infect 0 to 10,000 colony forming units (CFU) of L. grayi for 4 h at 30 °C. After addition of substrate, NanoLuc production in each sample was quantified using a luminometer. Infection of L. grayi with LPJP1.NL yielded an easily detectable signal over medium background and increased proportionally with CFU (Table 1). Background from medium and reporter alone was a minimal 96 relative light units (RLU), while a single CFU of L. grayi was sufficient to achieve an average RLU signal of twice this amount. A threshold of approximately twice the background signal (190 RLU) was thus chosen to distinguish positive and negative samples. Using this cutoff, no positive signal was obtained in the absence of detectable CFU and all replicate wells were positive at burdens of 5 CFU or higher. As expected, variation between replicate wells and partial positives were noted when low bacterial burdens (1 or 2 CFU) were tested. Overall, these results indicate that LPJP1.NL is a sensitive reporter capable of producing a signal over background from a single CFU of L. grayi after a 4 h infection.
Previous concerns over the phenomenon of temperature-dependent plaque formation with Listeria phages and the implications on phage reporters have been raised17. Since LPJP1 shared this phenotype, an experiment was carried out to determine the role of temperature during infection on the signal production of LPJP1.NL. Minimal differences were noted between the RLU obtained from a 4 h infection at 30 °C or 37 °C and both temperatures yielded comparable results across all burdens (Supplementary Table 2). These results suggest that increased temperatures do not impede the production of signal from Listeria phage reporters.
Inclusivity of LPJP1.NL detection
Based upon plaque formation and the limit of detection, LPJP1.NL was clearly capable of infecting the L. grayi subsp. grayi strain ATCC 19,120. However, it was not known whether or not this bacteriophage could broadly infect other L. grayi strains. Although the number of strains commercially available for testing is limited, four additional strains of L. grayi were obtained, including members of the only other subspecies L. grayi subsp. murrayi. Stationary phase cultures of each strain were diluted to a low burden (approximately 100 CFU) or a high burden (OD600 of 0.2) and infected with LPJP1.NL for 4 h. The cutoff of 190 RLU, approximately two times background, was used to distinguish between positive and negative detection for all inclusivity and exclusivity testing. Similar thresholds have proven useful in defining the host range of other NanoLuc bacteriophage reporters9,10,12. Using this method, all five L. grayi strains were successfully detected at both low and high burdens, indicating infection and subsequent NanoLuc production (Table 2). As expected, the use of stationary phase bacterial cells resulted in reduced signal compared to the limit of detection assay results for 100 CFU. Despite this decrease, LPJP1.NL was capable of infecting and detecting low burdens of all tested L. grayi strains, including representatives of both known subspecies.
Specificity of LPJP1.NL detection across other Listeria species
Other characterized Listeria phages of the myovirid morphotype, such as P100 and A511, are capable of infecting multiple Listeria species, dependent on serovar33,34. While LPJP1.NL had demonstrated coverage across several L. grayi strains, the ability of this phage to infect other Listeria species was of significant interest. An exclusivity panel of 150 Listeria strains was assembled, consisting of 13 species and 15 different serovars. Due to commercial availability and the public health importance of L. monocytogenes, 106 strains of this species were included. Of 66 L. monocytogenes with source-provided serovar information, 29 strains were serovar 1/2 (including fifteen 1/2a, nine 1/2b, and two 1/2c), five were serovar 3 (including two 3a, one 3b, and one 3c), and 32 were serovar 4 (including four 4a, nineteen 4b, one 4bx, three 4c, three 4d, and one 4e). In order to assess exclusivity, overnight stationary phase cultures of each strain were directly infected with LPJP1.NL for 4 h. This was expected to represent a significant bacterial burden and allow detection of even limited infection. Despite this burden, no strain in this Listeria panel yielded a positive result for NanoLuc production, as defined by the 190 RLU threshold (Table 3). RLU data and source of each strain are provided (Supplementary Table 3). The lack of any substantial signal over background from these strains suggests that LPJP1.NL is incapable of infecting other Listeria species besides L. grayi.
Specificity of LPJP1.NL detection across other genera
To evaluate the specificity of LPJP1.NL for Listeria, an exclusivity panel of 26 Gram-negative and 19 Gram-positive strains was assembled. Representatives of 19 unique genera and 42 species were included. As done with Listeria species, overnight stationary phase cultures of each strain were infected with LPJP1.NL for 4 h. Unsurprisingly, no positive signal was detected from any of the 45 members of the exclusivity panel (Table 4). The absence of NanoLuc production in these strains further supports the notion that LPJP1.NL is highly specific for L. grayi.
Discussion
L. grayi is one of several Listeria species often encountered in food-related environments35,36,37,38. Monitoring of Listeria species in these environments is often used by the food industry to identify and preemptively eliminate conditions supporting the growth of L. monocytogenes5. For these reasons, coverage of L. grayi is desirable and frequently incorporated into newly developed Listeria species detection assays39,40,41. Bacteriophage reporter assays represent one promising methodology capable of facilitating sensitive and specific detection of target organisms. Despite prior development of phage reporters for Listeria, phage-based detection of L. grayi has not been previously reported.
Although phages have been found for many Listeria species, phages targeting L. grayi have thus far remained elusive6,33. The absence of such phages prior to this study was both intriguing and potentially restrictive. As phage-based applications are often reliant on the engineering of naturally occurring phages, bacteria that cannot be infected by known phages are excluded from these otherwise promising technologies. In this study, the L. grayi phage LPJP1 was isolated from farm silage and formed clear plaques on lawns of L. grayi (Supplementary Fig. 1). Plaque formation only occurred at 30 °C and was not observed at 37 °C, a phenotype also observed in other Listeria phages (data not shown)17. TEM revealed that LPJP1 was a tailed phage (caudovirus) of the myovirid morphotype, with a large icosahedral head, a long straight tail, and a contractile outer tail sheath (Fig. 1a,b)18,19. At 223,580 base pairs, LPJP1 is, to our knowledge, the only Listeria phage to meet the “jumbo phage” designation. Annotation of LPJP1 revealed 229 ORFs, consisting largely of hypothetical proteins, and 4 tRNA genes. Analysis of predicted ORFs was conducted to identify a predicted major capsid protein (Supplementary Table 1). Homology was found between this identified protein and the major capsid proteins of other jumbo phages, particularly those infecting Gram-positive bacteria. LPJP1 thus appears to not only be the first L. grayi phage, but also the largest phage infecting the Listeria genus reported to date.
NanoLuc-encoding phages have repeatedly been found to exhibit impressive diagnostic sensitivity and robust signal production across multiple genera9,10,12,13,15. To our knowledge, however, no reporter has previously been generated from a jumbo phage. Despite this lack of precedent, a NanoLuc-encoding recombinant, LPJP1.NL was successfully generated using standard homologous recombination methods. Infection with LPJP1.NL produced substantial RLU from limited bacterial burdens without enrichment, requiring only a single log phase L. grayi bacterium to yield a detectable signal after a 4 h infection (Table 1). As expected, signal increased proportionally with bacterial burden. Sensitive detection of L. grayi by LPJP1.NL may have practical value as a member of a phage cocktail covering all Listeria species. Such a cocktail could facilitate phage-based monitoring of non-pathogenic species and allow for remediation of pro-Listeria environments prior to growth of pathogenic species. Additionally, the success of LPJP1.NL also highlights the untapped potential of jumbo phages in phage-based diagnostics as a whole.
Interestingly, comparable NanoLuc signal could be obtained from infections carried out at either 30 °C or 37 °C (Supplementary Table 2). Although plaques were not readily visualized at 37 °C, the robust signal from LPJP1.NL at this temperature suggests that phage DNA is injected successfully and expressed. Previous NanoLuc-encoding Listeria phage studies have also observed signal generation in strains that did not support plaque formation15. These observations demonstrate that some phage reporters may find success even in strains and conditions where visible plaque formation is not apparent. Although further studies are needed to elucidate the effects of temperature on this process, phage reporters such as LPJP1.NL may prove to be useful tools in understanding phage-host interactions in Listeria.
In addition to the parent L. grayi strain (ATCC 19,120), LPJP1.NL could mediate detection of low and high burdens of all five tested L. grayi strains in 4 h (Table 2). This panel was limited due to commercial availability of L. grayi, but included members of both subspecies, L. grayi subsp. grayi and L. grayi subsp. murrayi. Among these strains, detectable signal ranged from 1129 RLU to 5755 RLU with 100 stationary phase CFU to 58,170,464 to 278,885,856 RLU with higher bacterial burdens. Strain to strain differences, while minor, may be the result of several variables, including differences in growth rate, receptor density, or protein expression. LPJP1.NL was highly specific for L. grayi, demonstrating no cross-reactivity with other Listeria species or bacterial genera (Tables 3 and 4). The narrow host range of LPJP1 may indicate a cell receptor unique to L. grayi. Many Listeria phages are not species-dependent but are specific for one or more serovars33. Broad host range Listeria phages, such as A511, can infect multiple serovars by binding to rhamnose and N-acetylglucosamine, substituents of cell wall teichoic acids (WTAs)42,43. Unfortunately, L. grayi is rarely investigated, and to our knowledge the composition of the cell wall for these strains remains largely uncharacterized, including the presence of similar WTA decorations. Prior studies have noted that L. grayi is the only Listeria species with a homolog to TarM, a glycosyltransferase mediating WTA modification in S. aureus44. It is thus plausible that LPJP1 recognizes a WTA modification specific to L. grayi. Additionally, serological studies have revealed antigenic structures unique to L. grayi, which could participate in species-specific phage recognition45. Overall, the receptor and host range determinants of LPJP1 are unknown, but future studies may benefit from examination of WTA modifications or L. grayi-specific surface structures.
The sequencing issues encountered with LPJP1 may interfere with the discovery and engineering of similar jumbo phages. The success of library preparation only after DNA amplification with the phi29 bacteriophage polymerase suggests the presence of modified base pairs, such as uracil, in the genome of LPJP1. This hypothesis was further supported with PCR. In particular, DNA isolated from LPJP1 could only be directly amplified when an uracil-tolerant polymerase, such as Q5U, was used (Fig. 2). Importantly, modified base pairs are also found in several other Gram-positive jumbo phages46,47,48. In particular, the genomes of the Bacillus jumbo phages AR9 and PBS1 and the Staphylococcus jumbo phage S6 were all found to contain deoxyuridine in place of thymidine46,47,48. Alternative DNA base pairs are thought to primarily provide resistance against host defense systems, as DNA sequences containing modified base pairs are often resistant to various restriction enzymes49,50. Less commonly, however, modified base pairs have also been shown to contribute to other elements of phage biology, such as DNA packaging51. While the benefit of DNA amplification using an uracil-tolerant polymerase is clear, future studies will be necessary to confirm the presence, identity, and frequency of modified base pairs in LPJP1. The methods described in this study may thus be useful in facilitating the sequencing and engineering of other phages containing similar DNA modifications.
In this study, the isolation and engineering of a L. grayi phage, LPJP1, is described. NanoLuc-encoding recombinants were generated and found to be capable of sensitive and accurate detection of L. grayi in only 4 h. Practically, this reporter could prove to be a critical member of cocktails seeking to detect the presence of environmental Listeria species. Furthermore, this work highlights the value and diversity of naturally occurring phages as a foundation for genetic engineering and assay development. Using the methods described, the discovery and engineering of additional jumbo phages may prove beneficial in further expanding phage-based technologies.
Methods
Bacterial strains
Bacterial strains were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA), the Food Safety Laboratory of Cornell University (Ithaca, NY, USA), the University of Georgia (Athens, GA, USA) and Q Laboratories (Cincinnati, OH, USA). Cultures were routinely grown at 37 °C in brain heart infusion (BHI) broth (Becton Dickinson and Company, Sparks, MD, USA) with shaking at 225 revolutions per minute (rpm).
Bacteriophage isolation, purification, and high titer stock preparation
The L. grayi bacteriophage LPJP1 was isolated from silage collected from a Wisconsin farm in the fall of 2018. Silage was provided by a Wisconsin farmer along with permission to use the material without restriction or limitation. Three g of silage was added to 30 mL of BHI media and allowed to diffuse at 2–8 °C for 72 h. To remove bacteria and debris, this solution was centrifuged at 4700×g for 10 min and the supernatant passed through a 0.45 µm polyethersulfone (PES) filter followed by a 0.2 µm PES filter (Cytiva, Marlborough, MA, USA). As an initial assessment to determine phage presence, a spot test was performed using a standard overlay method52. Briefly, 100 µL of a log phase culture of the L. grayi strain ATCC 19,120 was mixed with 3 mL of melted 0.5% (w/v) semi-solid BHI agar and then poured evenly atop BHI agar plates to form a lawn. Once the semi-solid agar solidified, plates were spotted with 5 µL of the filtered supernatant and incubated overnight at 30 °C. As spot testing yielded promising results, single plaques were isolated using a modification of the above overlay method. Log phase culture (100 µL), diluted filtered supernatant (100 µL), and melted semi-solid (3 mL) was poured atop a BHI agar plate and incubated overnight at 30 °C. Individual plaques were then picked and resuspended in 1 mL BHI media. Single plaque isolation, dilution, and plating was serially repeated five times.
High titer phage stocks were generated from broth lysates as described previously10. Briefly, a log phase culture of L. grayi (ATCC 19,120) was infected at a multiplicity of infection (MOI) of 0.05 and incubated statically for 5 min to facilitate bacteriophage adsorption. Infected cells were diluted into fresh BHI media and incubated at 30 °C with shaking at 225 rpm until lysis was visually apparent. To remove debris, the lysate was clarified by centrifugation. The supernatant was centrifuged to pellet phage and the pellet was resuspended in TMS Buffer (50 mM Tris–HCl, pH 7.8, 10 mM MgCl2, and 300 mM NaCl). Resuspended phage were treated with RNase and DNase I before being further purified using a sucrose density gradient (10–30%). The phages were resuspended in SM buffer (50 mM Tris–HCl, pH 7.5, 8 mM MgSO4·7H2O, 100 mM NaCl, and 0.01% (w/v) gelatin). This method yielded stock solutions with a titer of about 1.5 × 1011 plaque forming units (pfu) per mL.
Bacteriophage characterization
To calculate the burst size and latent period of LPJP1, a standard one-step growth curve was performed using the L. grayi strain ATCC 19,120, as described previously53.
Transmission electron microscopy (TEM) of LPJP1 was performed as described previously10. Briefly, 400 mesh grids coated with a thin carbon film were glow discharged, floated on purified high titer phage stock, and subsequently stained with 2% uranyl acetate. A Tecnai G2 Spirit BioTWIN (FEI Company, Hillsboro, OR, USA) with an accelerating voltage set at 120 kV was used for image capture.
Bacteriophage DNA isolation, sequencing, and PCR
To isolate phage DNA, 6 × 1010 pfu/mL were first heated at 95 °C for 2 min and then allowed to cool to room temperature. Initial heating is anticipated to induce ejection of capsid-contained DNA54. Sodium dodecyl sulfate (SDS), ethylenediaminetetraacetic acid (EDTA), and proteinase K were added to a final concentration of 0.1% SDS, 5 mM EDTA, and 53 µg/mL proteinase K. This mixture was incubated for 1 h at 50 °C before performing three rounds of phenol/chloroform extractions, serially separating the aqueous phase. After the addition of 0.1 volumes of 3 M sodium acetate and 2.5 volumes of ethanol to this phase, DNA was precipitated at −80 °C. Precipitated DNA was pelleted and washed twice with 70% ethanol. Washed DNA was pelleted once again, air dried, and finally re-suspended in 10 mM Tris–HCL, pH 8.0.
Phage DNA was quantified using absorbance at 260 nm on a NanoDrop (Thermo Fisher Scientific, Waltham, MA, USA) and sent to Laragen Inc. (Los Angeles, CA, USA) for sequencing. Laragen Inc. quantified dsDNA using a Qubit dsDNA HS assay kit (Thermo Fisher Scientific, Waltham, MA, USA) and performed library preparation using the Nextera DNA Flex library prep (Illumina, San Diego, CA, USA). When successful library preparation was achieved, samples underwent MiSeq whole genome sequencing (Illumina, San Diego, CA, USA) and contig assembly using SPAdes genome assembler app in Illumina’s BaseSpace. When indicated, DNA was amplified prior to sequencing using a Illustra TempliPhi DNA amplification kit (Cytiva, Marlborough, MA, USA) according to manufacturer’s instructions. Amplified DNA was quantified and submitted for sequencing, as described above. Phage annotation was performed using a database for Caudovirales phages available from the Millard Lab (http://millardlab.org/bioinformatics/lab_server/phage-genome-annotation/) and Prokka (v1.14.6)55. The annotated genome was deposited in GenBank (accession number: MZ422438).
Polymerase chain reaction (PCR) was performed using either the Q5 hot start high-fidelity DNA polymerase or the Q5U hot start high-fidelity DNA polymerase (New England BioLabs, Ipswich, MA, USA). DNA templates were either native phage DNA or TempliPhi-amplified DNA prepared as described above. To ensure elements of the TempliPhi reaction did not interfere with PCR, DNA was purified using a Monarch PCR and DNA cleanup kit (New England BioLabs, Ipswich, MA, USA). PCR was performed using 2 ng of DNA, either amplified or native, and either the Q5 or Q5U polymerase, as per manufacturer’s instructions. Primers were (5′-AGGAAACAGCTATGACATGATTACGTTATCATAAAACTTTCGATGTAC-3′) and (5′-ATTTATACTCTATTTAACGAGCGTATTGAGTTG-3′) and were obtained from Eurofins Genomics (Louisville, KY, USA). PCR reactions were assessed using gel electrophoresis. One-half of each reaction was mixed with GelPilot DNA loading dye (Qiagen, Hilden, Germany) and loaded onto a 1% agarose gel (Bio-Rad Laboratories, Hercules, CA, USA) in standard Tris–acetate-EDTA (TAE) buffer. For size comparison, O’GeneRuler 1 kb plus DNA ladder (Thermo Fisher Scientific, Waltham, MA, USA) was added to flanking wells. SYBR Safe DNA Gel Strain (Invitrogen, Carlsbad, CA, USA) was included in the gel to allow visualization of results. The gel was electrophoresed for 100 Vh while submerged in TAE buffer. Gel imaging was performed using the Gel Doc EZ system and ImageLab (version 6.0.1) software (Bio-Rad, Hercules, CA, USA).
Design and engineering of luciferase reporter phage recombinants
Homologous recombination was used to insert a late gene promoter and NanoLuc downstream of the predicted major capsid protein to create a NanoLuc-encoding recombinant of LPJP1. No predicted genes are anticipated to be disrupted following recombination at this site. A similar approach has proven successful in other bacteriophages and previously yielded promising phage reporters10,12. A predicted major capsid protein was identified in LPJP1 by manual screening of possible open reading frames in the LPJP1 genome assembly (Supplementary Table 1). This prediction was supported by homology observed using BLAST (BLASTP)56,57. A shuttle vector containing homology flanks to mediate recombination, a late gene promoter, and the NanoLuc sequence was designed as follows. The upstream flank begins with a KpnI restriction site followed by 500 bp of homology upstream of the insertion site. The insertion site was immediately after the predicted major capsid protein of LPJP1. Following this flank, a promoter was added containing −10 and −35 elements from A511’s major capsid protein (a late gene) sequence and an A511 consensus ribosome binding site sequence42,58. The NanoLuc coding sequence was added following this promoter. The downstream flank contained 500 bp of homology downstream of the target insertion site and was followed by a SalI restriction site. Sequences for both flanks and the promoter are provided (Supplementary Table 1). The sequence of NanoLuc has been previously described59. These sequences were synthesized and inserted between the KpnI and SalI restriction sites of the multiple cloning site in the previously described shuttle vector pCE10460. All cloning was performed by Blue Heron Biotech (Bothell, WA, USA).
ATCC 19,120, the L. grayi strain used during isolation of LPJP1, was also selected as a host for recombination. Electrocompetent ATCC 19,120 was generated using a modification of a previously described method61. 10 µg/mL of Penicillin G (Thermo Fisher Scientific, Waltham, MA, USA) was added to a log phase culture of ATCC 19,120 and incubated for 1 h at 37 °C with shaking (225 rpm). Treated cultures were then chilled on ice for 30 min, pelleted, and washed three times with cold SMP (272 mM sucrose, 1 mM MgCl2, 7 mM sodium phosphate pH 6.8). Cells were pelleted a final time and resuspended in 1 mL of cold SMP. 100 µL aliquots were frozen and stored at −80 °C until use. 100 ng of homologous recombination plasmid DNA was added to thawed electrocompetent cells, incubated on ice for 15 min, and transferred to a 0.2 cm cuvette. Electroporation was carried out using a MicroPulser electroporation apparatus (Bio-Rad Laboratories, Hercules, CA, USA) with a voltage of 2.5 kV and a 2.8 ms time constant. Electroporated cells were recovered in fresh BHI for 2 h at 30 °C before being plated on BHI agar plates containing 5 µg/mL erythromycin sulfate (Alfa Aesar, Haverhill, MA, USA). Plates were incubated at 37 °C for 24 h before being examined for transformants.
Erythromycin-resistant colonies were picked, grown up in BHI containing 5 µg/mL erythromycin sulfate, and infected with varying amounts of LPJP1 equivalent to MOIs of 0.01 to 0.1. Infections were incubated for 4.5 h at 30 °C with 225 rpm shaking. To remove debris and intact bacteria, samples were centrifuged for 2 min at 6800×g and the supernatant passed through a 0.45 µm PES filter. This solution was transferred to a 100 kDa pore protein concentrator PES column (Pierce Biotechnology, Rockford, IL) and buffer exchanged with TMS. Potential phage recombinants were identified from this reaction as previously described10,12. Briefly, enrichment of limiting dilutions followed by screening for luciferase production was used to improve the likelihood of finding a recombination event. When found to be positive, these enrichments were plated to isolate single plaques on semi-solid agar, as described above. Candidate plaques were picked, mixed with diluted cultures of ATCC 19,120, and evaluated for NanoLuc production. Single plaque isolation, dilution, and plating was serially performed five times to establish a pure NanoLuc-encoding recombinant, called LPJP1.NL. Using the same preparation method described above, high titer stocks of LPJP1.NL were made and sent for genome sequencing. Analysis of the assembled genome of LPJP1.NL verified that the homologous recombination occurred as desired, inserting the promoter and NanoLuc downstream of the predicted major capsid protein.
Limit of detection of LPJP1.NL
The limit of detection of LPJP1.NL in L. grayi was determined as follows. A log phase culture (OD600 of 0.1 to 0.5) of the L. grayi strain ATCC 19,120 was diluted in BHI media to achieve 10 to 100,000 CFU/mL. 100 µL of each bacterial dilution or BHI media was added to at least six replicate wells on a 96-well plate. Each well was infected with 10 µL of LPJP1.NL at 1.2 × 107 pfu/mL and the infection was allowed to proceed for 4 h at 30 °C. At the end of the infection, 65 µL of a luciferase detection solution was added to each well. The luciferase detection solution consisted of 50 µL of NanoGlo buffer, 15 µL of Renilla lysis buffer, and 1 µL of NanoGlo substrate (Promega Corp., Madison, WI, USA). A GloMax Navigator luminometer (Promega Corp., Madison, WI, USA), set to a 3 min wait time and 1 s integration, was used to detect light production. Using this method, relative light units (RLU) values were obtained for each well and averaged among replicates.
When indicated, the above protocol was modified to determine the effect of infection temperature on signal production. Bacteria were prepared and infected as described above with the following exception. For each burden, two wells were infected with LPJP1.NL. One set of wells was infected for 4 h at 30 °C, while the other set was infected for 4 h at 37 °C. RLU values were obtained using a luciferase detection solution as described above.
Determining the host range of LPJP1.NL
The inclusivity of LPJP1.NL was determined using several commercially available L. grayi strains. Overnight stationary phase cultures of each strain were diluted in BHI media to either a high burden (OD600 of 0.2) or a low burden (1000 CFU/mL). 100 µL of each dilution was added to a well of a 96-well plate and infected with 10 µL of LPJP1.NL at 1.2 × 107 pfu/mL. As done previously, infected wells were incubated for 4 h at 30 °C before the addition of 65 µL of luciferase detection solution. RLU values were obtained for each sample on the GloMax Navigator as described above. Samples were evaluated using a threshold of 190 RLU, which was approximately twice the signal over background (BHI media control).
The exclusivity of LPJP1.NL was determined using both other Listeria species and a multitude of Gram-positive and Gram-negative genera. To assess the possibility of even minute signal production, a high bacterial burden was selected for testing. To this end, 100 µL of overnight culture was directly added to a 96-well plate and infected with 10 µL of LPJP1.NL at 1.2 × 107 pfu/mL. As done previously, infected wells were incubated for 4 h at 30 °C before the addition of 65 µL of luciferase detection solution. Luminescence was measured and analyzed as described above for inclusivity assays.
Data availability
Data supporting the reported results can be found in the manuscript and supplementary materials with the following exception. Sequence and annotation of LPJP1 was deposited to GenBank (accession number: MZ422438). Researchers receiving resources generated in this study may be asked to sign a Materials Transfer Agreement that covers potential commercial use.
References
Todeschini, G. et al. A case of Listeria murray/grayi bacteremia in a patient with advanced Hodgkin’s disease. Eur. J. Clin. Microbiol. Infect. Dis. 17, 808–810. https://doi.org/10.1007/s100960050193 (1998).
Rapose, A., Lick, S. D. & Ismail, N. Listeria grayi bacteremia in a heart transplant recipient. Transpl. Infect. Dis. 10, 434–436. https://doi.org/10.1111/j.1399-3062.2008.00333.x (2008).
Salimnia, H., Patel, D., Lephart, P. R., Fairfax, M. R. & Chandrasekar, P. H. Listeria grayi: vancomycin-resistant, gram-positive rod causing bacteremia in a stem cell transplant recipient. Transpl. Infect. Dis. 12, 526–528. https://doi.org/10.1111/j.1399-3062.2010.00539.x (2010).
Orsi, R. H. & Wiedmann, M. Characteristics and distribution of Listeria spp., including Listeria species newly described since. Appl. Microbiol. Biotechnol. 100, 5273–5287. https://doi.org/10.1007/s00253-016-7552-2 (2016).
Tompkin, R. B. Control of Listeria monocytogenes in the food-processing environment. J. Food Prot. 65, 709–725. https://doi.org/10.4315/0362-028x-65.4.709 (2002).
Loessner, M. J. & Busse, M. Bacteriophage typing of Listeria species. Appl. Environ. Microbiol. 56, 1912–1918. https://doi.org/10.1128/AEM.56.6.1912-1918.1990 (1990).
Kim, J. W., Siletzky, R. M. & Kathariou, S. Host ranges of Listeria-specific bacteriophages from the turkey processing plant environment in the United States. Appl. Environ. Microbiol. 74, 6623–6630. https://doi.org/10.1128/AEM.01282-08 (2008).
Smartt, A. E. & Ripp, S. Bacteriophage reporter technology for sensing and detecting microbial targets. Anal. Bioanal. Chem. 400, 991–1007. https://doi.org/10.1007/s00216-010-4561-3 (2011).
Zhang, D. et al. The use of a novel NanoLuc-based reporter phage for the detection of Escherichia coli O157:H7. Sci. Rep. 6, 33235. https://doi.org/10.1038/srep33235 (2016).
Nguyen, M. M. et al. Accurate and sensitive detection of Salmonella in foods by engineered bacteriophages. Sci. Rep. 10, 17463. https://doi.org/10.1038/s41598-020-74587-8 (2020).
Loessner, M. J., Rees, C. E., Stewart, G. S. & Scherer, S. Construction of luciferase reporter bacteriophage A511::luxAB for rapid and sensitive detection of viable Listeria cells. Appl. Environ. Microbiol. 62, 1133–1140. https://doi.org/10.1128/AEM.62.4.1133-1140.1996 (1996).
Brown, M. et al. Development and evaluation of a sensitive bacteriophage-based MRSA diagnostic screen. Viruses 12, 631. https://doi.org/10.3390/v12060631 (2020).
Jain, P. et al. Nanoluciferase Reporter Mycobacteriophage for Sensitive and Rapid Detection of Mycobacterium tuberculosis Drug Susceptibility. J Bacteriol 202, doi:https://doi.org/10.1128/JB.00411-20 (2020).
Meile, S., Kilcher, S., Loessner, M. J. & Dunne, M. Reporter phage-based detection of bacterial pathogens: Design guidelines and recent developments. Viruses https://doi.org/10.3390/v12090944 (2020).
Meile, S. et al. Engineered reporter phages for rapid bioluminescence-based detection and differentiation of viable Listeria cells. Appl. Environ. Microbiol. https://doi.org/10.1128/AEM.00442-20 (2020).
Vongkamjan, K., Switt, A. M., den Bakker, H. C., Fortes, E. D. & Wiedmann, M. Silage collected from dairy farms harbors an abundance of Listeria phages with considerable host range and genome size diversity. Appl. Environ. Microbiol. 78, 8666–8675. https://doi.org/10.1128/AEM.01859-12 (2012).
Tokman, J. I., Kent, D. J., Wiedmann, M. & Denes, T. Temperature significantly affects the plaquing and adsorption efficiencies of listeria phages. Front. Microbiol. 7, 631. https://doi.org/10.3389/fmicb.2016.00631 (2016).
Fokine, A. & Rossmann, M. G. Molecular architecture of tailed double-stranded DNA phages. Bacteriophage 4, e28281. https://doi.org/10.4161/bact.28281 (2014).
Barylski, J. et al. Analysis of spounaviruses as a case study for the overdue reclassification of tailed phages. Syst. Biol. 69, 110–123. https://doi.org/10.1093/sysbio/syz036 (2020).
Bruinsma, S. et al. Bead-linked transposomes enable a normalization-free workflow for NGS library preparation. BMC Genomics 19, 722. https://doi.org/10.1186/s12864-018-5096-9 (2018).
Weigele, P. & Raleigh, E. A. Biosynthesis and function of modified bases in bacteria and their viruses. Chem. Rev. 116, 12655–12687. https://doi.org/10.1021/acs.chemrev.6b00114 (2016).
Fogg, M. J., Pearl, L. H. & Connolly, B. A. Structural basis for uracil recognition by archaeal family B DNA polymerases. Nat. Struct. Biol. 9, 922–927. https://doi.org/10.1038/nsb867 (2002).
Russell, H. J., Richardson, T. T., Emptage, K. & Connolly, B. A. The 3’-5’ proofreading exonuclease of archaeal family-B DNA polymerase hinders the copying of template strand deaminated bases. Nucleic Acids Res. 37, 7603–7611. https://doi.org/10.1093/nar/gkp800 (2009).
Reagin, M. J. et al. TempliPhi: A sequencing template preparation procedure that eliminates overnight cultures and DNA purification. J. Biomol. Tech. 14, 143–148 (2003).
Serrano-Heras, G., Bravo, A. & Salas, M. Phage phi29 protein p56 prevents viral DNA replication impairment caused by uracil excision activity of uracil-DNA glycosylase. Proc. Natl. Acad. Sci. U. S. A. 105, 19044–19049. https://doi.org/10.1073/pnas.0808797105 (2008).
Frommer, M. et al. A genomic sequencing protocol that yields a positive display of 5-methylcytosine residues in individual DNA strands. Proc. Natl. Acad. Sci. U. S. A. 89, 1827–1831. https://doi.org/10.1073/pnas.89.5.1827 (1992).
Hendrix, R. W. Jumbo bacteriophages. Curr. Top. Microbiol. Immunol. 328, 229–240. https://doi.org/10.1007/978-3-540-68618-7_7 (2009).
Yuan, Y. & Gao, M. Jumbo bacteriophages: An overview. Front. Microbiol. 8, 403. https://doi.org/10.3389/fmicb.2017.00403 (2017).
Nagy, K. K., Skurnik, M. & Vertessy, B. G. Viruses with U-DNA: New avenues for biotechnology. Viruses 13, 875. https://doi.org/10.3390/v13050875 (2021).
Imam, M. et al. vB_PaeM_MIJ3, a novel jumbo phage infecting Pseudomonas aeruginosa, possesses unusual genomic features. Front. Microbiol. 10, 2772. https://doi.org/10.3389/fmicb.2019.02772 (2019).
Lee, Y., Son, B., Cha, Y. & Ryu, S. Characterization and genomic analysis of PALS2, a novel staphylococcus jumbo bacteriophage. Front. Microbiol. 12, 622755. https://doi.org/10.3389/fmicb.2021.622755 (2021).
Kiljunen, S. et al. Yersiniophage phiR1-37 is a tailed bacteriophage having a 270 kb DNA genome with thymidine replaced by deoxyuridine. Microbiology (Reading) 151, 4093–4102. https://doi.org/10.1099/mic.0.28265-0 (2005).
Klumpp, J. & Loessner, M. J. Listeria phages: Genomes, evolution, and application. Bacteriophage 3, e26861. https://doi.org/10.4161/bact.26861 (2013).
Habann, M. et al. Listeria phage A511, a model for the contractile tail machineries of SPO1-related bacteriophages. Mol. Microbiol. 92, 84–99. https://doi.org/10.1111/mmi.12539 (2014).
Soriano, J. M., Rico, H., Molto, J. C. & Manes, J. Listeria species in raw and ready-to-eat foods from restaurants. J. Food Prot. 64, 551–553. https://doi.org/10.4315/0362-028x-64.4.551 (2001).
O’Connor, L. et al. The characterization of Listeria spp. isolated from food products and the food-processing environment. Lett. Appl. Microbiol. 51, 490–498. https://doi.org/10.1111/j.1472-765X.2010.02928.x (2010).
Silva, I. M., Almeida, R. C., Alves, M. A. & Almeida, P. F. Occurrence of Listeria spp. in critical control points and the environment of Minas Frescal cheese processing. Int. J. Food Microbiol. 81, 241–248. https://doi.org/10.1016/s0168-1605(02)00223-4 (2003).
Prazak, A. M., Murano, E. A., Mercado, I. & Acuff, G. R. Prevalence of Listeria monocytogenes during production and postharvest processing of cabbage. J. Food Prot. 65, 1728–1734. https://doi.org/10.4315/0362-028x-65.11.1728 (2002).
Tonner, E. et al. Evaluation of the solus one listeria method for the detection of listeria species on environmental surfaces. J. AOAC Int. 102, 570–579. https://doi.org/10.5740/jaoacint.18-0199 (2019).
Bodner, J. et al. Evaluation of the CERTUS environmental listeria species detection kit for the detection of listeria species on environmental surfaces: AOAC performance tested MethodSM 101802. J AOAC Int 103, 833–842. https://doi.org/10.1093/jaocint/qsz021 (2020).
Joelsson, A. C. et al. Comparative evaluation of veriflow((R))Listeria species to USDA culture-based method for the detection of Listeria spp. in food and environmental samples. J. AOAC Int. 100, 1434–1444. https://doi.org/10.5740/jaoacint.17-0080 (2017).
Klumpp, J. et al. The terminally redundant, nonpermuted genome of Listeria bacteriophage A511: A model for the SPO1-like myoviruses of gram-positive bacteria. J. Bacteriol. 190, 5753–5765. https://doi.org/10.1128/JB.00461-08 (2008).
Eugster, M. R. et al. Bacteriophage predation promotes serovar diversification in Listeria monocytogenes. Mol. Microbiol. 97, 33–46. https://doi.org/10.1111/mmi.13009 (2015).
Xia, G. et al. Glycosylation of wall teichoic acid in Staphylococcus aureus by TarM. J. Biol. Chem. 285, 13405–13415. https://doi.org/10.1074/jbc.M109.096172 (2010).
Garcia, J. A. et al. Revision of the antigenic structure of genus Listeria. FEMS Microbiol. Lett. 55, 113–119. https://doi.org/10.1016/0378-1097(90)90178-s (1990).
Takahashi, I. & Marmur, J. Replacement of thymidylic acid by deoxyuridylic acid in the deoxyribonucleic acid of a transducing phage for Bacillus subtilis. Nature 197, 794–795. https://doi.org/10.1038/197794a0 (1963).
Lavysh, D. et al. The genome of AR9, a giant transducing Bacillus phage encoding two multisubunit RNA polymerases. Virology 495, 185–196. https://doi.org/10.1016/j.virol.2016.04.030 (2016).
Uchiyama, J. et al. Intragenus generalized transduction in Staphylococcus spp. by a novel giant phage. ISME J. 8, 1949–1952. https://doi.org/10.1038/ismej.2014.29 (2014).
Zhou, Y. et al. A widespread pathway for substitution of adenine by diaminopurine in phage genomes. Science 372, 512–516. https://doi.org/10.1126/science.abe4882 (2021).
Hutinet, G. et al. 7-Deazaguanine modifications protect phage DNA from host restriction systems. Nat. Commun. 10, 5442. https://doi.org/10.1038/s41467-019-13384-y (2019).
Scraba, D. G., Bradley, R. D., Leyritz-Wills, M. & Warren, R. A. Bacteriophage phi W-14: The contribution of covalently bound putrescine to DNA packing in the phage head. Virology 124, 152–160. https://doi.org/10.1016/0042-6822(83)90298-2 (1983).
Gratia, A. Des relations numeriques entre bacteries lysogenes et particules de bacteriophage. Ann. Inst. Pasteur 57, 652–676 (1936).
Ellis, E. L. & Delbrück, M. The Growth of Bacteriophage. J. Gen. Physiol. 22, 365–384. https://doi.org/10.1085/jgp.22.3.365 (1939).
Qiu, X. Heat induced capsid disassembly and DNA release of bacteriophage lambda. PLoS ONE 7, e39793. https://doi.org/10.1371/journal.pone.0039793 (2012).
Seemann, T. Prokka: rapid prokaryotic genome annotation. Bioinformatics 30, 2068–2069. https://doi.org/10.1093/bioinformatics/btu153 (2014).
Altschul, S. F. et al. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res 25, 3389–3402. https://doi.org/10.1093/nar/25.17.3389 (1997).
Altschul, S. F. et al. Protein database searches using compositionally adjusted substitution matrices. FEBS J. 272, 5101–5109. https://doi.org/10.1111/j.1742-4658.2005.04945.x (2005).
Loessner, M. J. & Scherer, S. Organization and transcriptional analysis of the Listeria phage A511 late gene region comprising the major capsid and tail sheath protein genes cps and tsh. J. Bacteriol. 177, 6601–6609. https://doi.org/10.1128/jb.177.22.6601-6609.1995 (1995).
Hall, M. P. et al. Engineered luciferase reporter from a deep sea shrimp utilizing a novel imidazopyrazinone substrate. ACS Chem. Biol. 7, 1848–1857. https://doi.org/10.1021/cb3002478 (2012).
Murray, D. L. et al. Localization of biologically important regions on toxic shock syndrome toxin 1. Infect. Immun. 64, 371–374. https://doi.org/10.1128/IAI.64.1.371-374.1996 (1996).
Park, S. F. & Stewart, G. S. High-efficiency transformation of Listeria monocytogenes by electroporation of penicillin-treated cells. Gene 94, 129–132. https://doi.org/10.1016/0378-1119(90)90479-b (1990).
Acknowledgements
The authors wish to express their gratitude to Brinna Zimmer, Henriett Zahn, Thai Dong, and Dustin Stevens for their help in exclusivity testing. Also, the authors wish to express their gratitude to Dwight Anderson for his expert review and feedback regarding this manuscript.
Author information
Authors and Affiliations
Contributions
Study was conceived by J.G., M.B., M.M.N., S.E. and W.H. Methods were developed by J.G., M.M.N., S.E. and W.H. Investigation was performed by A.I.M., J.G., J.P., M.M.N., R.B., S.E. and W.H. The original draft was written by M.B. and S.E. Review and editing were performed by all authors. Figures were prepared by J.P., M.B., S.E. and W.H. Supervision was provided by A.I.M., M.M.N. and S.E. The project was conducted under the direction of S.E. and M.E. All authors have read and agreed to this version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
Several authors (J.G., J.P., M.B., M.E., M.M.N., S.E., and W.H.) are employees of Laboratory Corporation of America Holdings (Labcorp). Labcorp provided all funding for this study. S.E. and J.G. are inventors of United States patents (US9482668B2 and US10519483B2), which are relevant to the phage engineering protocol utilized in this work. Additionally, a provisional patent has been filed regarding the results of this study. A.I.M. and R.B. declare no potential conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Erickson, S., Paulson, J., Brown, M. et al. Isolation and engineering of a Listeria grayi bacteriophage. Sci Rep 11, 18947 (2021). https://doi.org/10.1038/s41598-021-98134-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-98134-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.