Abstract
To identify the prevalence and risk factors for primary Epstein–Barr virus (EBV) infection in human immunodeficiency virus (HIV)-1-positive adult treatment-naïve patients between January 2018 and December 2019 in a state of the Brazilian Amazon region. A total of 268 HIV-1 positive patients and 65 blood donors participated in the study. Epidemiological data were obtained from medical records and through a designed questionnaire. EBV infection was screened by the semiquantitative detection of anti-viral capsid antigen (VCA) EBV IgM and IgG, followed by molecular detection of the EBNA-3C gene. The plasma viral loads of HIV-1 and EBV were quantified using a commercial kit. The prevalence of primary coinfection was 7.12%. The associated risk factors were education level, family income, history of illicit drug use and sexually transmitted infections, homosexual contact and condom nonuse. Approximately 58.5% had late initiation of highly active antiretroviral therapy, which influenced the risk of HIV-EBV 1/2 multiple infection (odds ratio (OR): 4.76; 95% CI 1.51–15.04) and symptom development (p = 0.004). HIV viral load was associated with patient age (OR: 2.04; 95% CI 2.01–2.07; p = 0.026) and duration of illicit drug use (OR: 1.57; 95% CI 1.12–2.22; p = 0.0548). EBV viral load was associated with younger age (OR: 0.82; 95% CI 0.79–1.03; p = 0.0579). The replication of both viruses was associated with symptom development (HIV = OR: 2.06; 95% CI 1.22–3.50; p = 0.0073; EBV = OR: 8.81; 95% CI 1–10; p = 0.0447). The prevalence of HIV/EBV coinfection was lower than that observed in other studies, and social vulnerability and promiscuous sexual behavior were associated risk factors. A long time of HIV-1 infection, without therapy, influenced the risk of coinfection and disease progression. The viral loads of both viruses may be associated with some epidemiological aspects of the population.
Similar content being viewed by others
Introduction
The Epstein–Barr virus (EBV), also known as human gammaherpesvirus 4 (HHV4), belongs to the family Herpesviridae, genus Lymphocryptovirus1,2. Two EBV genotypes are classically known, EBV-1 and EBV-2, whose genetic differences lie in the sequence encoding the viral nuclear antigens (EBNA2, -3A, -3B and -3C)3,4. Both genotypes have a cosmopolitan distribution, although EBV-1 is more prevalent, especially in Western countries and Asia5, while EBV-2 is more prevalent in Central African countries6.
In developed countries, EBV infection is common in adolescence, ranging from 0 to 70% in childhood and reaching more than 90% in adulthood; in the latter age group, primary infection is clinically symptomatic7,8,9. In developing countries, primary EBV infection occurs in early childhood and is usually asymptomatic, reaching 97% seroprevalence in adulthood in heterogeneous populations, a value that is similar at the global scale10,11. In these countries, there is an alert for seroprevalence in children and adolescents with low socioeconomic status12 and for the predominance of infection in individuals with suspected coinfection with human immunodeficiency virus (HIV)-113.
Because it is a precursor of lymphatic neoplasms, EBV is identified as one of the factors that contributes to the morbidity of patients infected with HIV-114,15,16. Coinfection is related to progressive dysfunction events and impaired immune surveillance caused by HIV that favor persistent pathogens17. This factor becomes critical when the infection progresses to AIDS, in which severe immunosuppression results in clinical signs and symptoms related to malignant neoplasms18,19.
Although most studies focus on the pathological basis of viral carcinogenesis, it is still necessary to understand aspects of primary EBV infection in the context of coinfection. In this sense, EBV coinfection has been studied in HIV-1-seropositive children, in whom EBV DNA was isolated from the oral mucosa20 and blood tissue21,22. It is assumed that due to the high incidence of EBV infection during the first 5 years of life, maternal transmission is an important route in maintaining coinfection21,23. Additionally, microbial translocation and persistent immune activation induced by HIV are factors that may influence EBV replication and the expansion of infected B cells22.
In adults, exposure through unprotected sex can favor HIV/EBV coinfection24. However, there is controversy regarding the detection of EBV in different HIV-1 infection profiles25. A recent study reports higher than 68% primary EBV infection in patients with HIV not on highly active antiretroviral therapy (HAART)26, similar to the rate observed in patients on the HAART regimen27. These findings indicate the complexity of the relationship between viruses that can be attributed to intrinsic factors of coinfection, regardless of the therapeutic status of the patient28.
There are few scientific studies on the epidemiology of HIV/EBV coinfection in the Brazilian Amazon and northern region of the country. Most research is focused on the carcinogenic effects of viral interactions29,30. To date, we have only found 1 study, by Jacome-Santos et al., that reported the overall prevalence of coinfection by strictly analyzing the presence of EBV in the oral mucosa of people living with HIV (PLHIV)31. Thus, the aim of the present study was to describe the prevalence of primary EBV infection in HIV-positive adult therapy-naïve and to identify the epidemiological characteristics associated with this profile.
Materials and methods
Sample characterization and ethical aspects
This was an observational, cross-sectional and analytical study conducted in mutual collaboration between the Laboratory of Virology of the Federal University of Pará (LABVIR-UFPA), the Evandro Chagas Institute (IEC) and the Hemotherapy and Hematology Foundation of Pará (HEMOPA), with the selection of participants who regularly resided in municipalities of the state of Pará, Brazil, from January 2018 to December 2019, originating from the Center for Health Care in Acquired Infectious Diseases (CASA DIA), HEMOPA and IEC.
All participants were clinically evaluated at the respective referral centers and subjected to complementary investigation via serological and molecular biology tests, based on which the participants were categorized as follows: 249 HIV-1 monoinfected patients (p24+; anti-HIV-1 IgG+); 19 HIV-1-positive patients with primary EBV infection (p24+; anti-HIV-1 IgG+; anti-viral capsid antigen (VCA)-EBV IgM+; anti-VCA-EBV IgG−; confirmation by molecular biology); and 65 uninfected individuals (volunteer blood donors, based on a screening panel of blood banks defined by ministerial ordinance32).
Epidemiological data, such as sex, age, place of residence, and clinical anamnesis of patients, were obtained from clinical records and an epidemiological questionnaire administered at the time of care. In compliance with resolutions 466/2012 and 347/05 of the Brazilian National Health Council, which establishes guidelines and regulatory standards for research involving human beings, the project proposal was submitted for ethical review and approved by the Human Research Ethics Committee of IEC (Protocol n. 3.121.265; CAAE n. 73927717.3.0000.0019). All participants were informed about the study objectives and signed an informed consent form. The collected biological samples were stored in a biorepository until use.
Participants included in the study were 18 years of age or older, of both sexes, carriers of HIV-1 and/or EBV, antiretroviral therapy-naïve and signed the informed consent form. Participants who did not meet the inclusion criteria or who were on antiviral therapy before sample collection were excluded.
Collection, extraction and confirmatory methods
Five milliliters of peripheral blood was collected into vacuum tubes containing EDTA as an anticoagulant. DNA was extracted from whole blood following the protocol provided with the QiaAmp DNA Mini Kit (Qiagen, Germany).
HIV-1 infection was screened by qualitative and simultaneous detection of p24 antigen and anti-HIV-1 and anti-HIV-2 IgG antibodies by enzyme immunoassay (Murex HIV Ag/Ab Combination, DiaSorin, UK); serological confirmation was performed using an Immunoblot rapid DPP HIV-1/2 kit (Bio-Manguinhos, FIOCRUZ) following the manufacturer's recommendations. The samples from CASA DIA did not require complementary tests for the diagnosis of HIV-1 because the institution has its own screening panel, to which patients enrolled in the institution are subjected.
Infection by EBV was screened by semiquantitative detection of anti-EBV IgM and IgG antibodies by enzyme immunoassay (Ridascreen EBV VCA R-Biopharm, Germany) following the manufacturer's recommendations. The identification of EBV genotypes was performed by nested PCR, targeting the EBNA-3C gene, using the primers described by Lorenzetti et al.33 ((1st round) (F: 5′-AGATGGTGAGCCTGACGTG-3′/R: 5′-GCATCCTTCAAAACCTCAGC-3′)) and by Sample et al.3 ((2nd round) (F: 5′-AGAAGGGGAGCGGTGTGTTGT-3′/R: 5′-GGCTGTTTTTGACGTCGGC-3′)) and following the recommended conditions: primers (10 pmol/µL); MgCl2 (50 mM); dNTP (10 mM); and Taq (5U/µL); 1st round cycles—1 cycle of 95° C/3′, 20 cycles of (94 °C/45″; 56 °C/45″; 72 °C/45″), 1 cycle of 72 °C/7′; and 2nd round cycles—1 cycle of 95° C/3′; 35 cycles of (94 °C/45″; 56 °C/45″; 72 °C/45″); 1 cycle of 72 °C/7′. The presence of a 153-bp fragment was considered positive for EBV-1, and the presence of a 246-bp fragment was considered positive for EBV-2. The positive EBV-1 and EBV-2 controls were isolated from the B95 and P3HP1 lymphoid cell lines, respectively.
Quantification of HIV-1 and EBV viral load
The HIV-1 plasma viral load was quantified by real-time PCR using an Abbott mSample Preparation System RNA extraction kit and an Abbott Real-Time HIV-1 amplification reagent kit (Abbott, Chicago, Illinois, USA) following the manufacturer’s recommendations.
The EBV viral load was quantified by real-time PCR following the protocol provided with the XGEN Master EBV kit (Mobius Life Science, Pinhais, Paraná, Brazil). Plasma samples were used to quantify the viral load.
Statistical analysis
The epidemiological data were compared among the study groups using the G test. Fisher’s exact test was applied exclusively in comparisons whose data were arranged in 2 × 2 contingency tables.
For significant comparisons, the degree of dependence between the epidemiological data and the study groups was calculated using simple logistic regression for 1 variable and multiple logistic regression for all variables, in which epidemiological data were included as independent variables and the presence or absence of infections as dependent variables. Due to the statistical similarity between the epidemiological profile of HIV-1 monoinfected and HIV-1/EBV coinfected patients, we assumed the presence of monoinfection or coinfection as success (1) and the absence of both as nonsuccess (0). The epidemiological factors that maintained statistical significance in the multiple regression were considered risk factors for monoinfection or coinfection.
We also calculated the dependence of the increase in viral load on the epidemiological and behavioral variables of patients. In this context, we calculated the mean viral load values and classified them, for HIV, into low (0), when between undetectable and up to 10,000 RNA copies and high (1), when greater than 10,000 copies, and, for EBV, into low (0), when between undetectable and up to 45 DNA copies, and high (1), when higher than 45 copies. The quantitative epidemiological variables were included in the function as nonbinary data, and exclusively for the length of illicit drug use, we discarded patients with noncontinuous sporadic use and grouped the remaining time of use into less than 1 year, 1 to 5 years, or greater than 5 years.
The G test was applied in other analyses of categorical data, such as in the comparison of the profile of sexually transmitted infections (STIs); behavioral changes according to the HIV-1 infection time (since diagnosis) and symptomatology. We used curve fitting to determine the frequency distribution model of cases of monoinfection and coinfection according to the collection time.
All statistical analyses were performed using the programs GraphPad Prism 3.03 (San Diego, CA, USA) and Bioestat 5.034.
Ethics approval and consent to participate
All methods and experimental protocols were carried out in accordance with regulations 466/2012 and 347/05 of the Brazilian National Health Council and were approved by the Human Research Ethics Committee of Instituto Evandro Chagas (Protocol n. 3.121.265; CAAE n. 73927717.3.0000.0019). All participants were informed about the study objectives and signed an informed consent form. The collected biological samples were stored in a biorepository until use.
Results
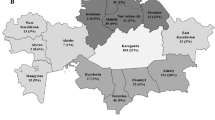
We detected primary EBV infection in only 19 of the 268 HIV+ patients evaluated (7.12%) (Table 1) and did not detect the presence of primary EBV infection in blood donor samples. The frequency of coinfection cases increased linearly throughout the study, approximately sixfold per month of collection (p = 0.0581; R2: 0.476), although the increase in HIV-1 monoinfection was slight (p = 0.1394; R2: 0.326) (Fig. 1A). The prevalence of coinfection cases was higher in the group of patients 18 to 28 years of age, specifically in men; the number of infected women stood out only in the group of patients 29 to 39 years of age (Fig. 1B). The EBV-1 genotype was the most prevalent among the coinfected patients (47.37%); EBV-2 and multiple infection by the 2 genotypes (EBV-1/2) occurred at the same frequency (26.32%) (Fig. 1C).
Frequency of cases and degree of exposure: (A) Frequency of new cases of HAART-free HIV-1 patients coinfected with HIV/EBV in the period between January 2018 and December 2019. (B) Prevalence of cases of HIV/EBV coinfection stratified based on age and sex. (C) Prevalence of EBV genotypes among HIV/EBV coinfected patients. (D) Potential risk/exposure to HIV-1 monoinfection or co-infection. The color gradient was proposed based on data on the frequency of individuals screened according to the intersection of social and sexual factors.
The epidemiological profiles of patients monoinfected with HIV-1 and coinfected with HIV/EBV were statistically similar; therefore, we grouped them for comparison with the noninfected group. Evaluating the variables individually, we observed that age was a risk factor for monoinfection or coinfection, being prevalent in the groups of young patients between 18 and 39 years of age (p = 0.0078; odds ratio (OR): 1.57; 95% CI 1.20–2.05). Only a history of cigarette use (p = 0.0013; OR: 2.95; 95% CI 1.64–5.30) and illicit drug use (p < 0.0001; OR: 24.53; 95% CI 3.34–180.14) were considered risk factors; present use was not relevant because most individuals claimed to have ceased these practices. Most aspects related to sexual practice and sexuality were associated with monoinfection or coinfection: history of STIs (p < 0.0001; OR: 14.05; 95% CI 4.29–45.95) and homosexual contact (p < 0.0001; OR: 10.87; 95% CI 4.32–27.34) had the highest probability ratios, followed by lack of a steady partner (p < 0.0001; OR: 4.81; 95% CI 2.25–10.25) and occasional condom use (p < 0.0001; OR: 1.46; 95% CI 1.06–2.01). An active sex life was associated with the noninfected group (p = 0.0110; OR: 0.36; 95% CI 0.18–0.72), whereas biological sex, history of sexual contact with sex workers and first sexual contact were not associated with infected patients (Table 1).
In contrast, higher education levels and higher family income were considered protective factors against monoinfection and coinfection, with the majority of noninfected individuals having completed secondary education or higher (p < 0.0001; OR: 0.21; 95% CI 0.13–0.32) and showing a greater representation of incomes ranging from 4 to greater than 10 times the minimum wage (p = 0.0418; OR: 0.48; 95% CI 0.33–0.70). Notably, current alcohol consumption was more strongly associated with noninfected patients (p = 0.0006; OR: 0.29; 95% CI 0.15–0.58). A family history of cancer and route of illicit drug administration were not associated with infected patients; most individuals reported no cases of cancer in the family, and for patients with a history of illicit drug use, most ingested them (Table 1).
We defined the risk factors for monoinfection or coinfection based on the multiple logistic regression results. In this context, education level, family income, history of illicit drug use, history of STIs, sexual orientation, and presence of a steady partner and condom nonuse remained significant. History of illicit drug use was the strongest risk factor for monoinfection or coinfection (p = 0.0367; OR: 13.74; 95% CI 1.18–160.46), and high family income was the strongest protective factor (p = 0.0002; OR: 0.45; 95% CI 0.27–0.67) (Table 1).
We generated a risk/potential matrix of exposure to monoinfection or co-infection that compiled risk factors categorized as sexual or social; the color gradient was based on the frequency of cases screened by the intersection of social and sexual factors (Fig. 1D). The level of risk/exposure was higher in individuals who did not use condoms, used illicit drugs, had low education and low income. High-income individuals had a low level of risk/exposure only when related to non-promiscuous sexual behaviors. Heterosexual orientation was considered a low risk/exposure factor only when associated with not using illicit drugs or high education and high income.
We also investigated the time of HIV-1 infection since diagnosis in the monoinfected and coinfected groups. A considerable portion of the patients (48.42%) lived for less than 1 month with HIV-1 and sought specific care immediately after diagnosis; however, approximately 51.58% of the patients lived between 1 and more than 12 months with the virus and without the use of HAART. When asked, patients with a longer time since diagnosis reported initial skepticism of the infection. Their point of view changed with the onset of symptoms, including through advice from third parties (data not shown).
In an intergroup analysis, we observed that a time of HIV-1 infection greater than 12 months was more frequent in patients coinfected with HIV/EBV-1/2 (40%; p = 0.0017) and that the chance of multiple infections was approximately 5 times higher (OR: 4.76; 95% CI 1.51–15.04). Most patients in the HIV and HIV/EBV-2 groups had a recent infection diagnosis (< 1 month) (49% and 80%, respectively). A time of infection between 1 and 6 months (78%) prevailed among HIV/EBV-1 coinfected patients, but without statistical significance (Fig. 2A).
Late initiation of treatment for HIV-1 infection: (A) Frequency for initiation of HIV-1 infection treatment and monitoring, in months, after the primary diagnosis. Patients with multiple infection (HIV/EBV-1/2) initiated treatment later. *p˂0.05. (B) Evaluation of behavioral profiles at different initiation times for HIV-1 infection treatment and monitoring. Condom use after diagnosis was the most frequent behavior at all treatment initiation times.
We evaluated the behavior of patients based on the time of HIV-1 infection since diagnosis (Fig. 2B). We did not observe significant differences in behavioral profiles; however, regarding sexual practice, we noted an increase in contact with sex workers for patients living longer with the infection; in contrast, the frequency of condom use after diagnosis, an active sex life, and the presence of a steady partner decreased. Regarding nonsexual behavior, alcohol consumption was a constant practice among patients, but we observed a gradual decline in the use of illicit drugs. Additionally, we observed an increase in the frequency of smokers only among patients diagnosed between 7 and 12 months prior.
We asked the patients about the symptoms presented at the time of collection and grouped them as asymptomatic (without symptoms), oligosymptomatic (from 2 to 3 symptoms) and polysymptomatic (from 4 to 5 symptoms). Asymptomatic patients prevailed in our analyses (51.91%); only in the group of patients coinfected with HIV/EBV-1/2 were oligosymptomatic patients predominant (60.0%), but the difference was not statistically significant (p = 0.96) (Fig. 3A). Sore throat and fever were the most frequent symptoms in all groups (77.78% and 71.72%, respectively), muscle and joint pain were not reported in the HIV/EBV-1/2 group (Fig. 3C), and no significant prevalence of symptoms was observed in the intergroup analysis (p = 0.96).
Symptoms: (A) Frequency of symptomatological categories among the studied groups. (B) STI diversity among individuals with a history of sexually transmitted infections in the studied groups. Blood donors had a poorly diversified history. *** ***: p˂0.001. (C) Diversity of symptoms between the studied groups. Fever and sore throat were the most frequent. (D) Evaluation of the frequency of the symptomatological categories at different initiation times for HIV-1 infection treatment. There was an extreme reduction in the frequency of asymptomatic patients among patients for whom treatment was initiated greater than 12 months after diagnosis.
We evaluated the profile of STIs among individuals with a history of these infections. The occurrence of syphilis and gonorrhea were the most frequent reports in the monoinfected and coinfected groups. The profile of STIs of noninfected individuals was significantly different from that of the other groups (p < 0.0001), with other STIs (66.67%), such as herpes (33.35%) and HPV infection (33.33%), prevailing; candidiasis was an alleged condition in 33.5% of the control group (Fig. 3B).
We evaluated the frequency of the symptomatological groups based on the time since HIV-1 diagnosis (Fig. 3D). We observed a significant decrease in the frequency of asymptomatic patients with an increase in the time since diagnosis (p = 0.04). Conversely, the rate of oligosymptomatic and polysymptomatic patients increased among patients living with HIV-1 for longer.
Increased HIV viral load was associated with patient age at the time of collection (OR: 2.04; 95% CI 2.01–2.07; p = 0.026) and with the length of illicit drug use in individuals with an illicit drug use history (OR: 1.57; 95% CI 1.12–2.22; p = 0.0548) (Fig. 4A). For EBV infection, no significant association was observed between viral load and patient’s age, although there seems to be a tendency for older age to be associated with lower viral load values (OR: 0.82; 95% CI 0.79–1.03; p = 0.0579) (Fig. 4B). The replication of both viruses was associated with patient symptoms, especially the EBV viral load, which was approximately 9 times higher in polysymptomatic patients (HIV = OR: 2.06; 95% CI 1.22–3.50; p = 0.0073; EBV = OR: 8.81; 95% CI 1–10; p = 0.0447).
Epidemiological factors associated with increased viral loads: (A) epidemiological factors associated with increased HIV viral load. (B) Epidemiological factors associated with increased EBV viral load. The x-axis is the odds ratio (OR) values. Horizontal lines represent the 95% confidence interval (CI) for each OR value. Vertical dashed lines delimit an OR equal to 1; therefore, OR values greater than 1 were considered risk factors for increased viral loads. For EBV, when the age of the patients obtained an OR value less than 1, the increase in viral load was inversely proportional to the factor. Symptomatology was a factor dependent on the viral load.
Discussion
In this study, we identified a prevalence rate of approximately 7% of primary EBV infection in adult HAART-free PLHIV in a state of the Brazilian Amazon region. Our findings were similar to the epidemiological data on the general prevalence of primary infection among immunocompetent adults in emerging countries35,36,37. However, there were discrepancies when compared to data from other studies with patients immunocompromised by HIV-1; our findings were lower than the values observed even in countries with similar socioeconomic profiles26,38,39.
Most of the differences between studies are due to the method used for sample screening, which is based on the detection of anti-VCA or anti-Epstein–Barr virus nuclear antigen (EBNA) as markers of EBV infection. The serological panel adopted in the present study was based on the detection of anti-VCA IgM and IgG antibodies, for which we assumed the profile IgM (+) IgG (−) as suspected primary infection, which was confirmed by molecular biology. Although the detection of anti-EBNA IgG antibodies is also suggested as a parameter to distinguish the infectious phases of the disease40, methods similar to those used in the present study were indicated for the recognition of primary infection by EBV, with an estimated sensitivity above 98%, and applied in the discrimination of false positive cases41. We emphasize that serum anti-VCA IgM levels emerge early and reach their serological peak within the first 5 days of disease onset, a period in which there is an increase in the clinical severity and viral load of EBV in the oral mucosa and peripheral blood42. Therefore, we suggest that the panel used in the present study may be a clinically relevant method in the identification of primary and recent EBV infection.
In Brazil, most studies have focused on the detection of EBV in specific organ sites of PLHIV with particular clinical profiles31,43,44. The methodologically closest study did not detect the same clinical profile in patients on HAART in southeastern Brazil45. We emphasize, however, that in the relatively time period included in the present study, the coinfection rate increased approximately sevenfold per month of collection, while the rate of new cases of PLHIV, understood as therapy-naïve based on the continuous care cascade recommended by the Brazilian Ministry of Health46, tended to be stable, with a nonsignificant increase of twofold per month of collection.
Although the number of new HIV cases identified is stable nationally, especially in the northern region of the country47,48, our data warn of an increase in coinfections in PLHIV, a predisposition observed in Brazil49 and internationally50, especially among young men.
We observed a prevalence of EBV-1 among HIV/EBV coinfected patients, confirming the epidemiology of the herpesvirus analyzed in different organ tissues of PLHIV with different clinical profiles51,52. Reports of EBV-2 prevalence in coinfected patients occur in specific clinical conditions after transient reactivation events of cell sites infected by different types of EBV53.
There is controversy regarding the predominant epidemiological profile among HIV/EBV-1 coinfected patients. Reports indicate that male sex, Caucasian ethnicity and heterosexual preference are factors that influence the distribution of cases54,55,56. In the present study, male individuals predominated, as well as a singular representation of homosexual and bisexual orientation, and we did not evaluate ethnic markers, such as self-reported race. Therefore, we assume a multiethnic representation in our sample with a probable predominance of European ancestry, as already described for populations in the Brazilian Amazon57,58. HIV/EBV-2 coinfection combined with multiple infection by both genotypes accounted for more than half the number of coinfections, a finding that emphasizes the susceptibility to the less frequent EBV genotype or to superinfection in HIV-immunocompromised patients59,60. It is argued that this is due both to changes in the immunological profile61 and in the exposure behavior of the host62.
The epidemiological analysis revealed similarities between the monoinfected and coinfected profiles. Apparently, in the context of HIV coinfection, the presence of primary EBV infection is not associated with specific risk factors. In fact, both infections have points of convergence related mainly to unprotected sexual exposure and possible contact with contaminated blood products63,64,65,66. In the present study, we found a prevalence of factors that are directly and indirectly related to sexual exposure, which makes this the main route of coinfection in the studied context.
The predominant socioeconomic profile was young adult men with a low education level, low family income, and history of illicit drug use. Unsatisfactory education is a risk factor congruent with HIV infection due to the lack of aggregate knowledge about prevention mechanisms of STIs and unprotected sexual exposure67,68. When functional, education empowers individuals to both reflect on the extent of contact between people and improves their ability to understand and act in favor of preventive health69,70.
It is argued that the lifestyle associated with a high education level can lead to both an increase and a decrease in the risk of HIV infection, depending on the balance of the different influences on behavior71. In the present study, more vulnerable socioeconomic profiles were associated with the risk of infection; in contrast, favorable profiles were prevalent among blood donors, a feature relevant to this altruistic activity72. This finding illustrates the sociodemographic complexity between the factors 'education' and 'income' that result in better conditions of access and social participation. That in Brazil they may be closely related to the way in which the individual is inserted in the community social organization and is motivated to act and support their relationships73. In contrast, low socioeconomic status contributes to less health knowledge and awareness of blood donation needs74.
It is suggested that most of the factors that predispose an individual to HIV are associated with family or individual income because this creates environmental risks inherent to precarious access to health, the practice of “sex for survival” (sex as a means of earning income), and even the ability to deal with the consequences of the condition75,76. In a context of progressive poverty, HIV mortality and morbidity rates were predicted by lower incomes77. Analogously, there is an aggravation of social vulnerability in PLHIV of specific ethnic groups, women, and those with physical health limitations78. In contrast, even in already-infected key populations, a relative increase in income results in improvements in access to basic health care79.
A history of illicit drug use was the greatest risk factor for monoinfection or coinfection in the present study, among which drugs taken orally prevailed. Recent studies have shown that an increase in the number of noninjectable drug users (NIDUs) infected by HIV trigger epidemiological surveillance in the Amazon region because although this group does not expose itself, a priori, to parenteral transmission, they may be at the mercy of other risk variables, especially sexual factors80,81, as observed in the decrease in condom use among NIDU sex workers, even between serodiscordant partners82. A worrying problem is the use of polydrug use combined with unprotected sex with multiple partners83, especially in Brazil, where although HIV prevalence rates among drug users have been declining in recent years48, they can still vary between 10 and 25-fold higher than that estimated in the general population84, and most users do not have basic knowledge about the severity of the infection85.
From the point of view of sexual practice, homosexual or bisexual orientation without the presence of a steady partner and occasional condom use were predominant characteristics among monoinfected and coinfected individuals. In fact, men who have sex with men (MSM) correspond to one of the main groups particularly vulnerable to HIV (key populations) and who historically do not have adequate access to health services. Data suggest that approximately 47% of new infections worldwide were associated with key populations and their sexual partners in 2017; in that evaluation, the MSM group represented 57% of new cases in Western Europe and Central and North America86,87. However, in developed countries, MSM exposed to high risks report willingness to participate in prophylaxis measures once informed of these prevention efforts88.
In developing countries, the high proportion of seropositive MSM who are unaware of their serological status is highly worrying and represents a large gap between basic health care practices and national guidelines89. In Brazil, recent reports highlight the high prevalence of HIV among MSM, whose main risk factors include environments of vulnerability; stigma and discrimination; behavioral profiles; sexual practices; and lackluster policies and programs90,91. Notably, there is a low rate of satisfactory knowledge on HIV infection observed among Brazilian MSM92.
As already mentioned, multiple sex partners is one of the characteristics composing the behavioral profiles of MSM83,93, and a history of STIs is one of the consequences of this practice94, as suggested in the present study. Recent warnings highlight the low rates of disclosure of serological status among sex partners, which is worrisome given the persistence of viral transmissibility, especially when the majority continues to practice unprotected sex95. It is argued that specific social networks may be endorsing this exposure behavior96. In the present study, we showed that unprotected sex with multiple partners, particularly for young men, are relevant attributes in the prevalence of monoinfection or coinfection. Notably, most infected individuals had their first sexual contact in preadolescence (10 to 15 years), a trend that has been observed in previous studies97.
Regarding protected sex, a recent report points to the existence of accessibility barriers for purchasing condoms, even in developed countries, and the need for a multifaceted approach to overcome these barriers98. In Brazil, despite an ongoing policy of free provision of condoms in referral units99, there is still a deficit in adherence to condom use, as suggested in the present study. We do not know whether this scenario is due to poor adherence itself, as a behavioral attitude100, to stigmatization in accessibility101, or to population ignorance regarding the national condom distribution policy. Future studies addressing this issue are essential as a strategic basis for preventive measures in the Brazilian Amazon region.
A history of STIs was the second factor most strongly associated with monoinfection or coinfection, with a prevalence of reported cases of syphilis and gonorrhea, respectively. Recent studies have shown that the highest risk for STIs is observed in young MSM from the most vulnerable socioeconomic groups50,62; among these, those diagnosed with syphilis and gonorrhea have the highest rate of HIV coinfection102, similar to our findings. These data are particularly worrying for Brazil because the incidence rates of acquired syphilis have been increasing since 2010103, and there is an estimated global increase in cases of gonorrhea, especially in MSM in developing countries104. The specific detection of these STIs can guide resource-intensive interventions, such as pre-exposure prophylaxis (PrEP), among PLHIV102.
In summary, we propose a risk/exposure potential matrix for monoinfection or coinfection based on sexual and social risk factors. We showed that the highest risk/exposure occurs among individuals with social vulnerability and promiscuous sexual behavior, among whom condom nonusers were more susceptible to viral infections.
However, condom use had a risk more associated with low education and low family income, similar to that presented among immigrants with similar socioeconomic profiles. In this scenario of preventive failure, we assume that social factors can directly influence the quality of aggregated knowledge about proper condom handling and use and awareness of STI/AIDS105. Alternatively, high education is associated with affordable family income and consequent adoption of safer sexual practices and access to preventive health services106, as suggested for individuals with high purchasing power, whose risk/exposure remained modest regardless of sexual factors.
We noted that on a monthly scale, the care and monitoring of HIV infection in a portion of patients was initiated late, with an interval between 1 and 24 months after becoming aware of the infection. However, the majority started the process quickly, approximately 1 month after the primary diagnosis.
These results are promising given persistent reports of late diagnosis among PLHIV. The greatest concern lies with the increase in the degree of morbidity and mortality inherent to the late initiation of treatment, although a trend toward a decrease in overall lethality is observed in these patients, despite maintaining a high frequency of late or advanced presentation of HIV-infected patients and the evolution of associated factors107. It is argued that in these cases, the immediate initiation of HAART may have favored the gradual immune recovery of patients and impacted mortality108. In Brazil, management guidelines advocate the immediate start of therapeutic regimens regardless of the patient’s clinical or immunological status46.
One strategy to encourage the early diagnosis of STIs is to promote testing outside the health setting109,110. Brazil again stands out for encouraging this practice in official and independent campaigns that aim not only to diagnosis but also to provide information regarding and encourage condom use111,112.
It is understood that older age and heterosexual orientation are the main factors influencing late start of monitoring in PLHIV113,114,115,116; however, little is discussed about the risk behavior profile of this group. In the present study, we observed that the exposure behavior was similar between the different times since diagnosis, indicating that prolonged experience with HIV did not significantly alter the behavioral profile of patients who maintained promiscuous practices related to infection and inherent comorbidities. Although there was a decrease in the percentage of sexual practice in patients for whom treatments was initiated late, most patients had no steady partner and maintained sexual contact with sex workers, some of whom did not use condoms.
We also show that the risk of HIV/EBV-1/2 multiple infection was higher in patients with longer HIV-1 infection, which emphasizes our warning because coinfections are common in patients who start treatment late, have the highest associated risk rate, and are also correlated with advanced clinical disease stages117,118. For EBV, this becomes more specific because the prevalence of EBV-1 and of multiple infection predominate in the most immunosuppressed patients; however, it is not known which factors may be involved in this scenario44,119.
Regarding the symptomatological aspects, asymptomatic individuals prevailed in all groups. Symptoms, when present, although still noncomplex and clinically nonspecific, were similar between both the initial phase of HIV infection and primary symptomatic EBV infection120,121,122,123. Particularly with EBV, these symptoms are associated with age groups above 18 years old and the host's aggravated immune status64, attributes that were prevalent among coinfected patients in the present study.
With the late initiation of HAART, there was a clear progression in coinfection symptoms, evidenced by the increase in cases of oligosymptomatic and polysymptomatic patients with HAART initiation greater than 12 months after the primary diagnosis. Although our results indicate that most patients sought specific medical care at the onset of the disease, we show how neglect of the condition can affect the pathological status of HIV infection, given that antiretroviral therapy initiated as soon as possible delays the progression of the disease and viral transmission124 and has secondary benefits125,126.
We observed that some of the epidemiological and behavioral factors were also associated with biological aspects of coinfection. Exclusively for HIV, patients with longer use of illicit drugs were more likely to have a high viral load. Specifically, we noted that most users used cocaine or marijuana, which are associated with effects on the immune system indicative of compromised resistance to HIV127, which favors not only the risk of infection but also intensive therapeutic control128. We suggest that the continued use of these illicit drugs may be conducive to both the acquisition and progression of infection because the time of initiation of HAART was not associated with viral load, which points to a complex interaction between illicit drug use and virological activity, which may be as significant as exposure to therapy129.
The advancement of age as a risk factor for increased HIV viral load was a finding different from those reported in previous studies130,131,132. However, in the present study, all patients were treatment-naïve, unlike those participating in the aforementioned studies, revealing the possible effects of immunosenescence in the face of HIV infection133, even in a predominantly young population.
In contrast, the chances of the EBV viral load increasing were higher in younger patients, in contrast that what was observed in HIV patients. Our findings reflect those observed in other epidemiological studies, in which it is argued that the early acquisition of primary infection results in higher and more sustained levels of EBV viremia. The presence of coinfections may also impair specific immune surveillance for one or both pathogens, leading to worsening of the disease134,135.
For both viruses, symptomatology was a factor dependent on viral load; the higher the viral load is, the greater the chances of patients presenting with a polysymptomatic condition, especially in EBV infection. For HIV, in cohorts with a high prevalence of infection, it was shown that viral load is a determining factor of disease progression136, an observation more characteristic of the acute phase of infection123. However, studies indicate that viral subtype and chronic immune activation are also aspects that predict the course of progression in populations of different ethnic origins137,138. For EBV, the plasma viral load is directly proportional to the patient's symptoms, especially within the first 2 weeks of symptom onset; in the acute phase, viral replication is intense in peripheral blood, with the virus contained in infected B cells and a portion being released into the plasma64,139. In specific clinical cases, the plasma viral load, even when low, is already an effective biomarker140,141.
Conclusions
Based on the results of the present study, we conclude that the prevalence of primary coinfection with EBV in HIV therapy-naïve patients in a state of the Brazilian Amazon region was lower than that observed in other similar regions. This finding may be associated with the methods used in the different studies, with social vulnerability and promiscuous sexual behavior being the risk factors associated with this population. We warn of the effect of the late initiation of HIV infection monitoring and the consequences of this decision on the risk of coinfections by EBV and disease progression, and we suggest that some epidemiological factors may influence the increase in viral load of both viruses.
Data availability
Data supporting the findings of this study are available from Igor Brasil Costa, but restrictions apply to the availability of these data, which were used under license for the present study and therefore are not publicly available. However, data are available from the authors upon reasonable request and with permission from Igor Brasil Costa.
Change history
17 May 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41598-022-12511-y
References
Odumade, O. A., Hogquist, K. A. & Balfour, H. H. Jr. Progress and problems in understanding and managing primary Epstein–Barr virus infections. Clin. Microbiol. Rev. 24(1), 193–209. https://doi.org/10.1128/CMR.00044-10 (2011).
International Committee on Taxonomy of Viruses (ICTV). https://talk.ictvonline.org/taxonomy/ (Acessado em 04 Sept 2020).
Sample, J. et al. Epstein–Barr virus types 1 and 2 differ in their EBNA-3A, EBNA-3B, and EBNA-3C genes. J. Virol. 64(9), 4084–4092 (1990).
Cohen, J. I. Epstein–Barr virus infection. N. Engl. J. Med. 343(7), 481–492. https://doi.org/10.1056/NEJM200008173430707 (2000).
Tzellos, S. & Farrell, P. J. Epstein–Barr virus sequence variation biology and disease. Pathogens. 1(2), 156–174. https://doi.org/10.3390/pathogens1020156 (2012).
Ibrahim Hazem, A. H. et al. Epstein–Barr virus (EBV) genotypes among human immunodeficiency virus (HIV)-related B-cell lymphomas and B-cell post-transplant lymphoproliferative disorders (B-PTLD)–late-onset lymphomas, especially in the HIV setting, are associated with type-B-EBV. Eur. J. Haematol. 85(3), 227–230. https://doi.org/10.1111/j.1600-0609.2010.01460.x (2010).
Hjalgrim, H., Friborg, J. & Melbye, M. The epidemiology of EBV and its association with malignant disease. In Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis (Cambridge University Press, 2007).
Trottier, H. et al. Transfusion-related Epstein–Barr virus infection among stem cell transplant recipients: A retrospective cohort study in children. Transfusion 52(12), 2653–2663 (2012).
Fourcade, G. et al. Evolution of EBV seroprevalence and primary infection age in a French hospital and a city laboratory network, 2000–2016. PLoS ONE 12(4), e0175574. https://doi.org/10.1371/journal.pone.0175574 (2017) (eCollection 2017).
Smatti, M. K. et al. Prevalence and molecular profiling of Epstein Barr virus (EBV) among healthy blood donors from different nationalities in Qatar. PLoS ONE 12(12), e0189033. https://doi.org/10.1371/journal.pone.0189033 (2017).
Gerpe, N. M. F. et al. Distinctive EBV infection characteristics in children from a developing country. Int. J. Infect. Dis. 93, 139–145. https://doi.org/10.1016/j.ijid.2020.01.044 (2020).
Figueira-Silva, C. M. & Pereira, F. E. L. Prevalence of Epstein–Barr virus antibodies in healthy children and adolescents in Vitória, State of Espírito Santo, Brazil. Rev. Soc. Bras. Med. Trop. 37(5), 409–412 (2004).
Bastos, M. S. et al. Detection of Herpesvirus, Enterovirus, and Arbovirus infection in patients with suspected central nervous system viral infection in the Western Brazilian Amazon. J. Med. Virol. 86(9), 1522–1527 (2014).
Lacoste, V. et al. Virological and molecular characterisation of a new B lymphoid cell line, established from an AIDS patient with primary effusion lymphoma, harbouring both KSHV/HHV8 and EBV viruses. Leuk. Lymphoma. 38(3–4), 401–409. https://doi.org/10.3109/10428190009087032 (2000).
Chao, C. et al. Epstein–Barr virus infection and expression of B-cell oncogenic markers in HIV-related diffuse large B-cell Lymphoma. Clin. Cancer Res. 18(17), 4702–4712 (2012).
Lang, F., Pei, Y., Lamplugh, Z. L. & Robertson, E. S. Molecular biology of EBV in relationship to HIV/AIDS-associated oncogenesis. Cancer Treat. Res. 177, 81–103. https://doi.org/10.1007/978-3-030-03502-0_4 (2019).
Hernández, D. M. et al. Loss of T-cell multifunctionality and TCR-Vβ repertoire against Epstein–Barr virus is associated with worse prognosis and clinical parameters in HIV+ patients. Front. Immunol. 9, 2291. https://doi.org/10.3389/fimmu.2018.02291 (2018).
Friis, A. M., Åkerlund, B., Gyllensten, K., Aleman, A. & Ernberg, I. Host-Epstein–Barr virus relationship affected by immunostimulation in HIV-infected patients representing distinct progressor profile groups. Scand. J. Infect. Dis. 44(5), 388–392 (2012).
Riedel, D. J., Tang, L. S. & Rositch, A. F. The role of viral co-infection in HIV-associated non-AIDS related cancers. Curr. HIV/AIDS Rep. 12(3), 362–372. https://doi.org/10.1007/s11904-015-0276-6 (2015).
Grando, L. J. et al. Viral coinfection in the oral cavity of HIV-infected children: Relation among HIV viral load, CD4+ T lymphocyte count and detection of EBV, CMV and HSV. Braz. Oral Res. 19(3), 228–234 (2005).
Slyker, J. A. et al. Clinical and virologic manifestations of primary Epstein–Barr virus (EBV) infection in Kenyan infants born to HIV-infected women. J. Infect. Dis. 207(12), 1798–1806 (2013).
Petrara, M. R. et al. Epstein–Barr virus load in children infected with human immunodeficiency virus type 1 in Uganda. J. Infect. Dis. 210(3), 392–399 (2014).
Gumbo, H. et al. Congenital and postnatal CMV and EBV acquisition in HIV-infected Zimbabwean infants. PLoS ONE 9(12), e114870. https://doi.org/10.1371/journal.pone.0114870 (2014).
Moriuchi, M. & Moriuchi, H. Increased susceptibility to HIV-1 of peripheral blood lymphocytes in acute infection with Epstein–Barr vírus. J. Med. Virol. 71, 343–346. https://doi.org/10.1002/jmv.10494 (2003).
Fellner, M. D. et al. Circulating Epstein–Barr virus (EBV) in HIV-infected patients and its relation with primary brain lymphoma. Int. J. Infect. Dis. 11(2), 172–178. https://doi.org/10.1016/j.ijid.2006.04.001 (2007).
Yan, Y. et al. Evaluation of Epstein–Barr virus salivary shedding in HIV/AIDS patients and HAART use: A retrospective cohort study. Virol. Sin. 33(3), 227–233. https://doi.org/10.1007/s12250-018-0028-z (2018).
Ling, P. D. et al. Epstein–Barr virus DNA loads in adult human immunodeficiency virus type 1-infected patients receiving highly active antiretroviral therapy. Clin. Infect. Dis. 37(9), 1244–1249. https://doi.org/10.1086/378808 (2003).
O’Sullivan, C. E. et al. Epstein–Barr virus and human immunodeficiency virus serological responses and viral burdens in HIV-infected patients treated with HAART. J. Med. Virol. 67(3), 320–326. https://doi.org/10.1002/jmv.10080 (2002).
Guimarães, A. G. D. P. et al. Coinfection of Epstein–Barr virus, cytomegalovirus, herpes simplex virus, human papillomavirus and anal intraepithelial neoplasia in HIV patients in Amazon, Brazil. J. Coloproctol. 32(1), 18–25. https://doi.org/10.1590/S2237-93632012000100003 (2012).
Rebelo-Pontes, H. A. et al. Burkitt’s lymphoma of the jaws in the Amazon region of Brazil. Med. Oral Patol. Oral Cir. Bucal. 19(1), e32–e38. https://doi.org/10.4317/medoral.18936 (2014) (Published 2014 Jan 1).
Jácome-Santos, H. et al. Epstein–Barr virus (EBV) in periodontal sites of human immunodeficiency virus (HIV)-positive individuals in North Brazil: A cross-sectional study. Quintessence Int. 51(1), 18–26. https://doi.org/10.3290/j.qi.a43616 (2020).
BRASIL. Ministério da Saúde. Anexo 1 Do Anexo IV-Portaria De Consolidação Nº 5, DE 28 DE Setembro De 2017. (Gabinete do ministro, 2017).
Lorenzetti, M. A. et al. EBNA1 sequences in Argentinean pediatric acute and latent Epstein–Barr virus infection reflect circulation of novel South American variants. J. Med. Virol. 82(10), 1730–1738 (2010).
Ayres, M., Ayres Júnior, M., Ayres, D. L. & Santos, A. S. BioEstat 5.0: aplicações estatísticas nas áreas de ciências biológicas e médicas, CNPq, 272 (Sociedade Civil Mamirauá, 2008).
Moeini, M., Ziyaeyan, M., Asaei, S. & Behzadi, M. A. The incidence of Epstein–Barr virus primary infection among suspected patients referred to Namazi Hospital of Shiraz, Iran. Jundishapur J. Microbiol. 8(4), e16109. https://doi.org/10.5812/jjm.8(4)2015.16109 (2015).
Cui, J. et al. Anti-Epstein–Barr virus antibodies in Beijing during 2013–2017: What we have found in the different patients. PLoS ONE 13(3), e0193171. https://doi.org/10.1371/journal.pone.0193171 (2018).
Beader, N., Kolarić, B., Slačanac, D., Tabain, I. & Vilibić-Čavlek, T. Seroepidemiological study of Epstein–Barr virus in different population groups in Croatia. Isr. Med. Assoc. J. 20(2), 86–90 (2018).
Abdollahi, A., Shoar, S., Rasoulinejad, M. & Sheikhbahaei, S. Seroprevalence of Epstein–Barr virus among HIV positive patients moreover and its association with CD4 positive lymphocyte count. Acta Med. Iran. 52(12), 916–921 (2014).
Okonko, I. O., Makinde, T. S., Okonko, B. J. & Ogbu, O. Immunological and epidemiological evaluation of EBV infections among HIV-1 infected individuals in Abakaliki, Nigeria supports the potential use of neutrophils as a marker of EBV in HIV disease progression and as useful markers of immune activation. J. Immunoassay Immunochem. 41(2), 158–170. https://doi.org/10.1080/15321819.2019.1705483 (2020).
De Paschale, M. & Clerici, P. Serological diagnosis of Epstein–Barr virus infection: Problems and solutions. World J. Virol. 1(1), 31–43. https://doi.org/10.5501/wjv.v1.i1.31 (2012).
Guerrero-Ramos, A., Patel, M., Kadakia, K. & Haque, T. Performance of the architect EBV antibody panel for determination of Epstein–Barr virus infection stage in immunocompetent adolescents and young adults with clinical suspicion of infectious mononucleosis. Clin. Vaccine Immunol. 21(6), 817–823. https://doi.org/10.1128/CVI.00754-13 (2014).
Grimm, J. M. et al. Prospective studies of infectious mononucleosis in university students. Clin. Transl. Immunol. 5(8), e94. https://doi.org/10.1038/cti.2016.48 (2016).
Carvalho, K. S. S. et al. PCR detection of multiple human herpesvirus DNA in saliva from HIV-infected individuals in Teresina, State of Piauí, Brazil. Rev. Soc. Bras. Med. Trop. 43(6), 620–623. https://doi.org/10.1590/S0037-86822010000600003 (2010).
Santos, L., Azevedo, K., Silva, L. & Oliveira, L. Epstein–Barr virus in oral mucosa from human immunodeficiency virus positive patients. Rev. Assoc. Med. Bras. 60(3), 262–269. https://doi.org/10.1590/1806-9282.60.03.016 (2014).
Kuschnaroff, T. M. et al. Prevalência da infecção pelo vírus Epstein–Barr em voluntários doadores de sangue e indivíduos com AIDS na cidade de São Paulo. Arq Med Hosp Fac Cienc Med Santa Casa São Paulo 52(1), 8–13 (2007).
Brasil. Ministério da Saúde. Protocolo Clínico e Diretrizes Terapêuticas para Manejo da Infecção pelo HIV em adultos (Departamento de Vigilância, Prevenção e Controle das Infecções Sexualmente Transmissíveis, do HIV/Aids e das Hepatites Virais, 2018).
UNAIDS Data 2017. http://www.unaids.org/sites/default/files/media_asset/20170720_Data_book_2017_en.pdf (Acessado em 28 Oct 2020).
Brasil. Ministério da Saúde. Boletim Epidemiológico HIV/AIDS 2019. (Departamento de Vigilância, Prevenção e Controle das Infecções Sexualmente Transmissíveis, do HIV/Aids e das Hepatites Virais, 2019).
Luppi, C. G. et al. Factors associated with HIV co-infection in cases of acquired syphilis reported in a Reference Center for Sexually Transmitted Diseases and AIDS in the municipality of São Paulo, Brazil, 2014. Epidemiol. Serv. Saude. 27(1), e20171678. https://doi.org/10.5123/s1679-49742018000100008 (2018) (English, Portuguese).
Secco, A. A. et al. Sexually transmitted infections in persons living with HIV infection and estimated HIV transmission risk: Trends over time from the DC Cohort. Sex Transm. Infect. 96(2), 89–95. https://doi.org/10.1136/sextrans-2019-054216 (2020).
Willoughby, C. E. et al. Epstein–Barr virus (types 1 and 2) in the tear film in Sjogren’s syndrome and HIV infection. J. Med. Virol. 68(3), 378–383. https://doi.org/10.1002/jmv.10214 (2002).
Grande, S. R. et al. Relationship between herpesviruses and periodontopathogens in patients with HIV and periodontitis. J. Periodontol. 82(10), 1442–1452. https://doi.org/10.1902/jop.2011.100723 (2011).
Buisson, M. et al. Development of an Epstein–Barr virus type 2 (EBV-2)-associated hepatic B cell non-Hodgkin’s lymphoma in an HIV-1-infected patient following a change in the EBV dominant type. Leukemia 13(2), 298–301. https://doi.org/10.1038/sj.leu.2401268 (1999).
Van Baarle, D. et al. High prevalence of Epstein–Barr virus type 2 among homosexual men is caused by sexual transmission. J. Infect. Dis. 181(6), 2045–2049 (2000).
Robaina, T. F. et al. Polymerase chain reaction genotyping of Epstein–Barr virus in scraping samples of the tongue lateral border in HIV-1 seropositive patients. Mem. Inst. Oswaldo Cruz. 103(4), 326–331. https://doi.org/10.1590/s0074-02762008000400002 (2008).
Giron, L. B. et al. Impact of Epstein–Barr virus load, virus genotype, and frequency of the 30 bp deletion in the viral BNLF-1 gene in patients harboring the human immunodeficiency virus. J. Med. Virol. 85(12), 2110–2118. https://doi.org/10.1002/jmv.23722 (2013).
Salzano, F. M. & Sans, M. Interethnic admixture and the evolution of Latin American populations. Genet. Mol. Biol. 37, 151–170 (2014).
da Silva, E. M. et al. Effect of genetic ancestry to the risk of susceptibility to gastric cancer in a mixed population of the Brazilian Amazon. BMC Res. Notes. https://doi.org/10.1186/s13104-017-2963-4 (2017).
Buisson, M. et al. Changes in the dominant Epstein–Barr virus type during human immunodeficiency virus infection. J. Gen. Virol. 75(Pt 2), 431–437. https://doi.org/10.1099/0022-1317-75-2-431 (1994).
Correa, R. M. et al. Epstein Barr virus genotypes and LMP-1 variants in HIV-infected patients. J. Med. Virol. 79(4), 401–407. https://doi.org/10.1002/jmv.20782 (2007).
Gianella, S., Ginocchio, C. C., Daar, E. S., Dube, M. P. & Morris, S. R. Genital Epstein Barr Virus is associated with higher prevalence and persistence of anal human papillomavirus in HIV-infected men on antiretroviral therapy. BMC Infect. Dis. 16, 24 (2016).
Chen, M. J., Scheer, S., Nguyen, T. Q., Kohn, R. P. & Schwarcz, S. K. HIV coinfection among persons diagnosed as having sexually transmitted diseases, San Francisco, 2007 to 2014. Sex. Transm. Dis. 45(8), 563–572. https://doi.org/10.1097/OLQ.0000000000000789 (2018).
Crawford, D. H. et al. A cohort study among university students: Identification of risk factors for Epstein–Barr virus seroconversion and infectious mononucleosis. Clin. Infect. Dis. 43(3), 276–282. https://doi.org/10.1086/505400 (2006).
Dunmire, S. K., Verghese, P. S. & Balfour, H. H. Jr. Primary Epstein–Barr virus infection. J. Clin. Virol. 102, 84–92. https://doi.org/10.1016/j.jcv.2018.03.001 (2018).
Atuhaire, L., Adetokunboh, O., Shumba, C. & Nyasulu, P. S. Effect of female sex work-targeted community-based interventions along the HIV treatment cascade in sub-Saharan Africa: A systematic review protocol. BMJ Open 10(10), e039495. https://doi.org/10.1136/bmjopen-2020-039495 (2020).
Kumari, S. Prevalence and trends of hepatitis B virus, hepatitis C virus, human immunodeficiency virus 1, 2 and syphilis infections among blood donors in a regional transfusion center in Punjab, India: A 3 years study. Indian J. Sex. Transm. Dis. AIDS. 41(1), 22–29. https://doi.org/10.4103/0253-7184.196887 (2020).
Szwarcwald, C. L. et al. Factors associated with HIV infection among female sex workers in Brazil. Medicine 97(1S Suppl 1), S54–S61. https://doi.org/10.1097/MD.0000000000009013 (2018).
da Fonseca, A. J. et al. Knowledge, perception and seroprevalence of HIV/STIS among young adults in Brazilian Amazon region: A population-based study. J. AIDS Clin. Res. 10, 784. https://doi.org/10.4172/2155-6113.1000784 (2019).
Kilian, A. H. et al. Reductions in risk behaviour provide the most consistent explanation for declining HIV-1 prevalence in Uganda. AIDS 13(3), 391–398. https://doi.org/10.1097/00002030-199902250-00012 (1999).
Fylkesnes, K. et al. The HIV epidemic in Zambia: Socio-demographic prevalence patterns and indications of trends among childbearing women. AIDS 11(3), 339–345. https://doi.org/10.1097/00002030-199703110-00012 (1997).
Hargreaves, J. R. & Glynn, J. R. Educational attainment and HIV-1 infection in developing countries: A systematic review. Trop. Med. Int. Health. 7(6), 489–498. https://doi.org/10.1046/j.1365-3156.2002.00889.x (2002).
Zucoloto, M. L., Gonçalez, T., Custer, B., McFarland, W. & Martinez, E. Z. Comparison of the demographic and social profile of blood donors and nondonors in Brazil. Health Soc. Care Community. 27(2), 330–336. https://doi.org/10.1111/hsc.12650 (2019).
Gonçalez, T. T. et al. Motivation and social capital among prospective blood donors in three large blood centers in Brazil. Transfusion 53(6), 1291–1301. https://doi.org/10.1111/j.1537-2995.2012.03887.x (2013).
Zago, A., da Silveira, M. F. & Dumith, S. C. Blood donation prevalence and associated factors in Pelotas, Southern Brazil. Rev. Saude Publica. 44(1), 112–120. https://doi.org/10.1590/s0034-89102010000100012 (2010) (English, Portuguese).
Farmer, P. Women, Poverty and AIDS: Sex, Drugs, and Structural Violence (University of California Press, 2002).
Mutenje, M. J., Nyakudya, I. W., Katsinde, C. & Chikuvire, T. J. Sustainable income-generating projects for HIV-affected households in Zimbabwe: Evidence from two high-density suburbs. Afr. J. AIDS Res. 6(1), 9–15. https://doi.org/10.2989/16085900709490394 (2007).
Bachmann, M. O. & Booysen, F. L. Relationships between HIV/AIDS, income and expenditure over time in deprived South African households. AIDS Care 16(7), 817–826. https://doi.org/10.1080/09540120412331290220 (2004).
Conover, C. J., Arno, P., Weaver, M., Ang, A. & Ettner, S. L. Income and employment of people living with combined HIV/AIDS, chronic mental illness, and substance abuse disorders. J. Ment. Health Policy Econ. 9(2), 71–86 (2006).
da Costa, L. M. et al. Prevalence and risk factors for human immunodeficiency virus infection among female sex workers: Distinct offers of sexual services in a municipality of the Brazilian Amazon. AIDS Res. Hum. Retroviruses. 35(9), 826–832. https://doi.org/10.1089/AID.2019.0032 (2019).
Gomes, S. T. M. et al. Immunological and virological characterization of HIV-1 viremia controllers in the North Region of Brazil. BMC Infect. Dis. 17(1), 381. https://doi.org/10.1186/s12879-017-2491-9 (2017).
Machado, L. F. et al. Lower genetic variability of HIV-1 and antiretroviral drug resistance in pregnant women from the state of Pará, Brazil. BMC Infect. Dis. 17(1), 270. https://doi.org/10.1186/s12879-017-2392-y (2017).
Avila, M. M. et al. High frequency of illegal drug use influences condom use among female transgender sex workers in Argentina: Impact on HIV and syphilis infections. AIDS Behav. 21(7), 2059–2068. https://doi.org/10.1007/s10461-017-1766-x (2017).
Grabovac, I., Meilinger, M., Schalk, H., Leichsenring, B. & Dorner, T. E. Prevalence and associations of illicit drug and polydrug use in people living with HIV in Vienna. Sci. Rep. 8(1), 8046. https://doi.org/10.1038/s41598-018-26413-5 (2018).
Guimarães, M. L. et al. Assessing the HIV-1 epidemic in Brazilian drug users: A molecular epidemiology approach. PLoS ONE 10(11), e0141372. https://doi.org/10.1371/journal.pone.0141372 (2015).
Bertoni, N. et al. Knowledge of AIDS and HIV transmission among drug users in Rio de Janeiro. Brazil. Harm Reduct J. 8, 5. https://doi.org/10.1186/1477-7517-8-5 (2011).
UNAIDS. Key Population. http://www.unaids.org/en/topic/key-populations (Acessado em 29 Oct 2020).
WHO. HIV Key Populations. http://www.euro.who.int/en/health-topics/communicable-diseases/hivaids/policy/policy-guidance-for-key-populations-most-at-risk2 (Acessado em 04 Nov 2020).
Connochie, D., Tingler, R. C. & Bauermeister, J. A. Young men who have sex with men’s awareness, acceptability, and willingness to participate in HIV vaccine trials: Results from a nationwide online pilot study. Vaccine. 37(43), 6494–6499. https://doi.org/10.1016/j.vaccine.2019.08.076 (2019).
Lahuerta, M. et al. HIV Prevalence and related risk factors in men who have sex with men in Bamako, Mali: Findings from a bio-behavioral survey using respondent-driven sampling. AIDS Behav. 22(7), 2079–2088. https://doi.org/10.1007/s10461-017-1793-7 (2018).
Kerr, L. et al. HIV prevalence among men who have sex with men in Brazil: Results of the 2nd national survey using respondent-driven sampling. Medicine 97(1S Suppl 1), S9–S15. https://doi.org/10.1097/MD.0000000000010573 (2018).
Rios, L. F., Paiva, V. & Brignol, S. Passivos, ativos and versáteis: Men who have sex with men, sexual positions and vulnerability to HIV infection in the northeast of Brazil. Cult. Health Sex. 21(5), 510–525. https://doi.org/10.1080/13691058.2018.1491063 (2019).
Guimarães, M. D. C. et al. HIV/AIDS knowledge among MSM in Brazil: A challenge for public policies. Rev Bras Epidemiol. 22(Suppl 1), e190005. https://doi.org/10.1590/1980-549720190005.supl.1 (2019) (English, Portuguese).
Guimarães, M. D. C. et al. Comparing HIV risk-related behaviors between 2 RDS national samples of MSM in Brazil, 2009 and 2016. Medicine 97(1S Suppl 1), S62–S68. https://doi.org/10.1097/MD.0000000000009079 (2018).
Wang, Z. et al. Co-occurring psychosocial problems and multiple sexual partners among men who have sex with men in Shanghai, China: A syndemic approach. J. Sex. Res. 55(7), 892–901. https://doi.org/10.1080/00224499.2017.1399333 (2018).
Mengwai, K., Madiba, S. & Modjadji, P. Low disclosure rates to sexual partners and unsafe sexual practices of youth recently diagnosed with HIV; Implications for HIV prevention interventions in South Africa. Healthcare 8(3), 253. https://doi.org/10.3390/healthcare8030253 (2020).
Chittamuru, D., Icard, L. D., Jemmott, J. B. 3rd. & O’Leary, A. Prospective predictors of multiple sexual partners among African American men who have sex with men. Arch Sex Behav. 47(7), 2081–2090. https://doi.org/10.1007/s10508-018-1207-6 (2018).
Heeren, G. A. et al. Multiple partners and condom use among students at a South African University. J. Evid. Based Soc. Work. 11(5), 437–444. https://doi.org/10.1080/15433714.2012.759468 (2014).
McCool-Myers, M., Myo, A. & Carter, J. A. Barriers to purchasing condoms in a high HIV/STI-risk urban area. J. Community Health. 44(4), 836–843. https://doi.org/10.1007/s10900-019-00670-5 (2019).
Brasil. Ministério da Saúde. ANEXO 05 da Norma Técnica–Incentivo HIV/Aids e outras DST – Nº 01/2002 (Portaria Nº 2314, de 20 de dezembro de 2002) (Gabinete do ministro, 2002).
Rutakumwa, R., Mbonye, M., Kiwanuka, T., Bagiire, D. & Seeley, J. Why do men often not use condoms in their relationships with casual sexual partners in Uganda?. Cult. Health Sex. 17(10), 1237–1250. https://doi.org/10.1080/13691058.2015.1053413 (2015).
Wilson, A. M. & Ickes, M. J. Purchasing condoms near a college campus: Environmental barriers. Sex. Health. 12(1), 67–70. https://doi.org/10.1071/SH14155 (2015).
Katz, D. A., Dombrowski, J. C., Bell, T. R., Kerani, R. P. & Golden, M. R. HIV incidence among men who have sex with men after diagnosis with sexually transmitted infections. Sex. Transm. Dis. 43(4), 249–254. https://doi.org/10.1097/OLQ.0000000000000423 (2016).
Brasil. Ministério da Saúde. Boletim Epidemiológico Sífilis 2020 (Departamento de Vigilância, Prevenção e Controle das Infecções Sexualmente Transmissíveis, do HIV/Aids e das Hepatites Virais, 2020).
Kirkcaldy, R. D., Weston, E., Segurado, A. C. & Hughes, G. Epidemiology of gonorrhoea: A global perspective. Sex. Health. 16(5), 401–411. https://doi.org/10.1071/SH19061 (2019).
Zoboli, F. et al. Correlation between knowledge on transmission and prevention of HIV/STI and proficiency in condom use among male migrants from Africa and Middle East evaluated by a Condom Use Skills score using a wooden penile model. BMC Res. Notes. 10(1), 216. https://doi.org/10.1186/s13104-017-2520-1 (2017).
Hargreaves, J. R. et al. Systematic review exploring time trends in the association between educational attainment and risk of HIV infection in sub-Saharan Africa. AIDS 22(3), 403–414. https://doi.org/10.1097/QAD.0b013e3282f2aac3 (2008).
Raffetti, E. et al. The risk of late or advanced presentation of HIV infected patients is still high, associated factors evolve but impact on overall mortality is vanishing over calendar years: Results from the Italian MASTER Cohort. BMC Public Health 16(1), 878. https://doi.org/10.1186/s12889-016-3477-z (2016).
Cid-Silva, P. et al. Late HIV diagnosis but earlier antiretroviral treatment initiation in northwest Spain: Impact of current treatment guidelines. J. Int. Assoc. Provid. AIDS Care. 18, 2325958218821940. https://doi.org/10.1177/2325958218821940 (2019).
Goodall, L. & Leen, C. Late diagnosis of HIV: Could this be avoided?. Scott. Med. J. 56(2), 84–86. https://doi.org/10.1258/smj.2011.011032 (2011).
Meléndez, J. et al. Late Presentation and missed opportunities for HIV diagnosis in Guatemala. AIDS Behav. 23(4), 920–928. https://doi.org/10.1007/s10461-018-2331-y (2019).
UNODC. HIV e AIDS: Campanhas. Extraído de: https://www.unodc.org/lpo-brazil/pt/hiv-aids/campanhas.html (Acessado em 15 Jan 2021).
Brasil. Ministério da Saúde. Nova campanha contra HIV/aids estimula público jovem a realizar a testagem. Brasília: Departamento de Doenças de Condições Crônicas e Infecções Sexualmente Transmissíveis. Extraído de: http://www.aids.gov.br/pt-br/noticias/nova-campanha-contra-hivaids-estimula-publico-jovem-realizar-testagem (Acessado em 15 Jan 2021).
Kundro, M. A., Terwel, S. R., Toibaro, J. J., Viloria, G. A. & Losso, M. H. Late diagnosis of HIV infection in asymptomatic patients. Medicina 76(5), 273–278 (2016).
Gardner, A. T., Napier, R. & Brown, B. Risk factors for “late-to-test” HIV diagnosis in Riverside County, California. Medicine 95(39), e5021. https://doi.org/10.1097/MD.0000000000005021 (2016).
Bath, R. E., Emmett, L., Verlander, N. Q. & Reacher, M. Risk factors for late HIV diagnosis in the East of England: Evidence from national surveillance data and policy implications. Int. J. STD AIDS. 30(1), 37–44. https://doi.org/10.1177/0956462418793327 (2019).
Palacios-Baena, Z. R., Martín-Ortega, M. & Ríos-Villegas, M. J. Profile of new HIV diagnoses and risk factors associated with late diagnosis in a specialized outpatient clinic during the 2014–2018 period. Med. Clin. 155(11), 482–487. https://doi.org/10.1016/j.medcli.2020.01.035 (2020).
Scognamiglio, P. et al. Unawareness of HCV serostatus among persons newly diagnosed with HIV. J. Infect. Public Health. 12(5), 733–737. https://doi.org/10.1016/j.jiph.2019.01.055 (2019).
Ríos-Hincapié, C. Y. et al. Delays in HIV and TB diagnosis and treatment initiation in co-infected patients in Colombia. Int. J. STD AIDS. 31(5), 410–419. https://doi.org/10.1177/0956462419881074 (2020).
Traore, L. et al. EBV and HHV-6 circulating subtypes in people living with HIV in Burkina Faso, impact on CD4 T cell count and HIV viral load. Mediterr. J. Hematol. Infect. Dis. 9(1), e2017049. https://doi.org/10.4084/MJHID.2017.049 (2017).
Son, K. H. & Shin, M. Y. Clinical features of Epstein–Barr virus-associated infectious mononucleosis in hospitalized Korean children. Korean J. Pediatr. 54(10), 409–413 (2011).
Oliveira, R. S. M., Benzaken, A. S., Saraceni, V. & Sabidó, M. HIV/AIDS epidemic in the State of Amazonas: Characteristics and trends from 2001 to 2012. Rev. Soc. Bras. Med. Trop. 48(1), 70–78 (2015).
Braun, D. L. et al. Frequency and spectrum of unexpected clinical manifestations of primary HIV-1 infection. Clin. Infect. Dis. 61(6), 1013–1021 (2015).
Melhuish, A. & Lewthwaite, P. Natural history of HIV and AIDS. Medicine 46(6), 356–361 (2018).
Maartens, G., Celum, C. & Lewin, S. R. HIV infection: Epidemiology, pathogenesis, treatment, and prevention. Lancet 384(9939), 258–271. https://doi.org/10.1016/S0140-6736(14)60164-1 (2014) (Erratum in: Lancet. 2014 20;384(9948): 1098).
Almeida, F. J., Kochi, C. & Sáfadi, M. A. P. Influence of the antiretroviral therapy on the growth pattern of children and adolescents living with HIV/AIDS. J. Pediatr. 95(Suppl 1), 95–101. https://doi.org/10.1016/j.jped.2018.12.007 (2019).
Dharan, N. J. et al. Benefit of early versus deferred antiretroviral therapy on progression of liver fibrosis among people with HIV in the START randomized trial. Hepatology 69(3), 1135–1150. https://doi.org/10.1002/hep.30296 (2019).
Cabral, G. A. Drugs of abuse, immune modulation, and AIDS. J. Neuroimmune Pharmacol. 1(3), 280–295. https://doi.org/10.1007/s11481-006-9023-5 (2006).
Ladak, F. et al. Substance use patterns and HIV-1 RNA viral load rebound among HIV-positive illicit drug users in a Canadian setting. Antivir. Ther. 24(1), 19–25. https://doi.org/10.3851/IMP3265 (2019).
Liang, J. et al. Longitudinal patterns of illicit drug use, antiretroviral therapy exposure and plasma HIV-1 RNA viral load among HIV-positive people who use illicit drugs. AIDS 34(9), 1389–1396. https://doi.org/10.1097/QAD.0000000000002551 (2020).
European Collaborative Study. Level and pattern of HIV-1-RNA viral load over age: Differences between girls and boys?. AIDS 16(1), 97–104. https://doi.org/10.1097/00002030-200201040-00012 (2002).
Goodkin, K. et al. Older age and plasma viral load in HIV-1 infection. AIDS 18(Suppl 1), S87-98 (2004).
George, S., McGrath, N. & Oni, T. The association between a detectable HIV viral load and non-communicable diseases comorbidity in HIV positive adults on antiretroviral therapy in Western Cape, South Africa. BMC Infect. Dis. 19(1), 348. https://doi.org/10.1186/s12879-019-3956-9 (2019).
Deeks, S. G. HIV infection, inflammation, immunosenescence, and aging. Annu. Rev. Med. 62, 141–155. https://doi.org/10.1146/annurev-med-042909-093756 (2011).
Piriou, E. et al. Early age at time of primary Epstein–Barr virus infection results in poorly controlled viral infection in infants from Western Kenya: Clues to the etiology of endemic Burkitt lymphoma. J. Infect. Dis. 205(6), 906–913. https://doi.org/10.1093/infdis/jir872 (2012).
Balfour, H. H. Jr. & Verghese, P. Primary Epstein–Barr virus infection: Impact of age at acquisition, coinfection, and viral load. J. Infect. Dis. 207(12), 1787–1789. https://doi.org/10.1093/infdis/jit096 (2013).
Eller, M. A. et al. HIV type 1 disease progression to AIDS and death in a rural Ugandan cohort is primarily dependent on viral load despite variable subtype and T-cell immune activation levels. J. Infect. Dis. 211(10), 1574–1584. https://doi.org/10.1093/infdis/jiu646 (2015).
Touloumi, G. et al. Impact of HIV-1 subtype on CD4 count at HIV seroconversion, rate of decline, and viral load set point in European seroconverter cohorts. Clin. Infect. Dis. 56(6), 888–897. https://doi.org/10.1093/cid/cis1000 (2013).
McPhee, E. et al. Short Communication: The interaction of HIV set point viral load and subtype on disease progression. AIDS Res. Hum. Retroviruses. 35(1), 49–51. https://doi.org/10.1089/AID.2018.0165 (2019).
Balfour, H. H. Jr. et al. A prospective clinical study of Epstein–Barr virus and host interactions during acute infectious mononucleosis. J. Infect. Dis. 192(9), 1505–1512. https://doi.org/10.1086/491740 (2005).
Chan, K. C. A. et al. Analysis of plasma Epstein–Barr virus DNA to screen for nasopharyngeal cancer. N. Engl. J. Med. 377(6), 513–522. https://doi.org/10.1056/NEJMoa1701717 (2017) (Erratum in: N Engl J Med. 2018 Mar 8;378(10 ):973).
Sorgato, C. C. et al. EBV and CMV viral load in rheumatoid arthritis and their role in associated Sjögren’s syndrome. J. Oral Pathol. Med. 49(7), 693–700. https://doi.org/10.1111/jop.13036 (2020).
Acknowledgements
We thank the entire technical and administrative staff of the participating institutions for helping to recruit the study patients, as well as all patients and blood donors for their trust and willingness to participate in the study.
Funding
This study was funded by specific incentives from the Health Surveillance Department of the Brazilian Ministry of Health and Conselho Nacional de Desenvolvimento Científico e Tecnológico–CNPQ (#301869/2017-0). We thank the Coordination for the Improvement of Higher Education Personnel (CAPES) for granting a scholarship (process number: 88882.183970/2018-01).
Author information
Authors and Affiliations
Contributions
T.A.F.M., O.M., R.C.M.S., F.B.F., I.B.C. and A.C.R.V. were the creators of the project. L.M.S.P., E.S.F., A.B.C.F. and F.L.P.R. were responsible for collecting the samples in the reference centers. L.M.S.P., E.S.F., I.B.C. and I.T.L. developed the project's methodological activities. L.M.S.P. applied the statistical analysis and wrote the article. I.B.C. and A.C.R.V. reviewed the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: The original version of this Article contained an error in Figure 1, where Panel B was incorrect and Panel C was published as Panel B.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pereira, L.M.S., dos Santos França, E., Costa, I.B. et al. Epidemiological risk factors associated with primary infection by Epstein–Barr virus in HIV-1-positive subjects in the Brazilian Amazon region. Sci Rep 11, 18476 (2021). https://doi.org/10.1038/s41598-021-97707-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-97707-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.