Abstract
Sn-doped BaTi4O9 (BT4) dielectric ceramics were prepared by a mixed oxide route. Preliminary X-ray diffraction (XRD) structural study shows that the ceramic samples have orthorhombic symmetry with space group (Pnmm). Scanning electron microscopy (SEM) shows that the grain size of the samples decreases with an increase in Sn4+ content. The presence of the metal oxide efficient group was revealed by Fourier transform infrared (FTIR) spectroscopy. The photoluminescence spectra of the ceramic samples reported red color ~ 603, 604, 606.5 and 605 nm with excitation energy ~ 2.06, 2.05, 2.04 and 2.05 eV for Sn4+ content with x = 0.0, 0.3, 0.5, and 0.7, respectively. The microwave dielectric properties of these ceramic samples were investigated by an impedance analyzer. The excellent microwave dielectric properties i.e. high dielectric constant (εr = 57.29), high-quality factor (Qf = 11,852), or low-dielectric loss (3.007) has been observed.
Similar content being viewed by others
Introduction
Dielectric ceramic materials are frequently used in modern telecommunication systems like satellite modules and cellular mobile phones. Layered-structured dielectric materials have been widely investigated for their potential use in ferroelectric random access memories (FeRAM) and piezoelectric devices. Ferroelectric and piezoelectric ceramic materials are broadly used in a range of applications such as sensors, actuators, transducers, pulse signal circuits, spacecraft, X-ray equipment, weapons, medical devices, and transportation. The dielectric component used in these devices is called dielectric resonators (DR). The optimum commercial dielectric properties of these ceramics included excellent dielectric constant (εr), good quality factor (Qf) or low tangent loss (tan σ), and near to zero temperature coefficient at resonant frequency (τf)1,2,3,4,5,6. For example, a dielectrically loaded antenna requires; εr from 20 to 85, (Q) ≥ 10,000, and τf close to zero7,8. Sometimes quality factor is expressed as the product of Q and fo (resonant frequency) i.e. (Q × fo). The dielectric constant (εr) and dielectric loss (tan σ) decrease with resonant frequency (fo), which suggests that electric dipoles and interfaces play an important role at the lower frequency range. The decrease in dielectric constant with resonant frequency can be explained based on Koops theory, reported by Praveena and Varma9. A relatively good dielectric constant is required for the miniaturization of devices and low tangent loss is required for noise reduction with zero \({\uptau }_{{\text{f }}}\), which is important for the temperature stability of the DR2. From the manufacturing point of view, it is very difficult to obtain compounds with all three optimum properties along with a low cost. Among oxides compounds, BaTi4O9 (BT4) is one of the dielectric materials that may be used in the microwave domain as first reported by Rase and Roy10. It has been recorded that BaTi4O9 ceramics have εr = 37.3, Q = 27,200 GHz and τf = + 15 ppm/°C11. On the other side, wet chemical methods (i.e. sol–gel, co-precipitation, or hydrothermal) can be used for the preparation of titanates ceramics12. The optical, structural, microstructural, and microwave properties of BaTi4O9 ceramic samples with various dopant elements have been extensively studied at microwave frequency. Many researchers studied the effects of B site cation substitutions and dielectric properties of those samples affected by the substitution of larger ionic radius (Sn4+) for smaller ionic radius (Ti4+) ions13,14,15. In this way, Veenhuis et al. processed compounds with good dielectric properties which have been used in the field of advanced laser technology and optical storage devices16.
In the present time, BT4 has been investigated broadly because of its good Qf, high εr, and small τf. Because used widely in microwave dielectric resonator applications, patch antenna, microwave telecommunication devices, etc. During the densification of these titanates at the very high sintering temperature, enhanced dielectric properties are observed due to the phase and compositional defects (fluctuations) (i.e. because of the partial reduction of Ti4+ to Ti3+ ion)17. The aim of the present work, to achieve a material with enhanced structural and dielectric properties for device application. In this report, we describe the synthesis (i.e.via the mixed oxide method) and the structural, optical, and microwave dielectric properties of Sn-doped BaTi4O9 ceramics. The calcined powders and sintered pellets obtained were characterized by XRD (X-ray diffraction), SEM (scanning electron microscopy), impedance analyzer, and FTIR (Fourier transform infra-red). The microwave dielectric properties of ceramic samples are discussed in terms of their physical and chemical characteristics.
Materials and methods
The starting raw materials along with purity grades were: BaCO3 (Merck, Germany, 99.9%), TiO2 (Aldrich Chemical Company, Inc., U.S.A, 99.9%), and SnO2 (Strem, Chemicals, U.S.A, 99.9%) used to make the solid solutions of Ba(Ti1−xSnx)4O9, 0 ≤ x ≤ 0.7 by using mixed oxides route. These raw materials were weighted according to stoichiometric ratio and mixed for 12 h in distilled water by using horizontal ball milling. Then the slurry was dried in a microwave oven at 100 °C for one day and calcined at 1100 °C for 3 h in a nickel crucible in the air atmosphere with a heating–cooling rate of 10 °C/min. The calcined powders were grinded for 60 min with a mortar and pistol manually to avoid agglomerations. Then pressed 0.6–0.8 gm of powder in cylindrical pellets of thickness 2 mm and diameter 10 mm by using a hydraulic press (CARVER, USA) with a pressure of 80 MPa. Thereafter, these green pellets were sintered at 1320 °C temperature in the open air for 4 h with a heating–cooling rate of 10 °C/min. The XRD patterns of the compounds were recorded at room temperature using an X-ray powder diffractometer (JDX-3532, JEOL, Japan) with Cu Ka radiation (k = 1.5405 Å) in a wide range of Bragg angles (20° ≤ 2θ ≤ 60°) at a scanning rate of 2 deg min-1. The experimental density of the samples was measured by Archimedes’ principle using a density meter (MD-3005, Germany). Scanning electron microscopy (SEM, JEOL 7600F) was used to study the microstructure of the dense pellets. The optical properties of these ceramics were done by using the Fourier transformation infrared spectroscopy (FTIR, Perkin-Elmer) and PL spectroscopy. Dielectric properties were measured at microwave frequencies by LCR meter (Agilent 4287 A).
Results and discussions
XRD analysis
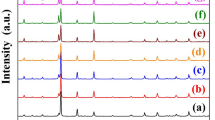
Figure 1 represents the XRD patterns of Ba(Ti1−xSnx)4O9, 0 ≤ x ≤ 0.7 ceramics at room temperature. The main peaks corresponding to (200), (121), (150), (211), (230), (320), (213), (503) planes are well-matched with PDF card number 34–70 of BaTi4O9 ceramics and have an orthorhombic structure with space group (Pnmm). Some of the secondary phases of Ba2Ti8O18 are detectable with PDF card number 0080–0916. It can be noted from Fig. 1 that the peaks shifted toward the lower 2θ values and representing the cell volume expansion with increasing the Sn4+ contents. This might be due to the inhomogeneity, micro-strain, or maybe due to the substitution of the relatively larger cation ions of Sn4+ (~ 0.69 Å) for the smaller cation of Ti4+ (~ 0.64 Å)18. The calculated lattice parameters (i.e. ‘a’, ‘b’, and ‘c’) increases with Sn4+ content. This increase in the lattice parameters may result in the phase transition from orthorhombic to a tetragonal structure. The average crystallite size of these samples was calculated by using Debye Scherer’s formula19. The observed average crystallite size and lattice parameters are given in Table 1.
where ‘θ’ is the brags angle, ‘λ’ is the wavelength of the incident radiation, and ‘β’ is the full-width of half-maximum (FWHM). The average crystallite size of these samples was lie in the range of 30–90 nm.
Microstructure analysis
SEM images were used to calculate the average grain size and density of Ba(Ti1−xSnx)4O9, 0 ≤ x ≤ 0.7 ceramics (as shown in Fig. 2). It is clear from the figure that two types of surface morphologies are present in all samples (i.e. rod-like and spherical-like particles). The relative densities are increased, as the Sn4+ doping content increased in the base sample. The existence of cavities in the denser pellets confirms the existence of porosity. Thus, an increase in the relative density and decrease in the porosity was observed as the Sn content increased20. The porosity of these samples was calculated using Eq. (2) shown in Table 2.
where ρth = theoretical density and ρex = experimental density (calculated using Archimedes’ principle). The observed average grain size varies from ~ 10.7 to ~ 2 µm as dopant content x varies from 0.0 to 0.7. At x = 0.5, the Sn4+ doped BaTi4O9 system has a smaller average grain size of 0.8 µm with a more uniform grain size distribution. Thus, high densification (~ 99.4%) and low porosity (~ 0.55%) were achieved with Sn4+ = 0.5 content which reduces the growth of grains.
Optical properties
Figure 3 represents the FTIR spectra of Ba(Ti1−xSnx)4O9, 0 ≤ x ≤ 0.7 ceramics at room temperature. The peaks that appear near ~ 2852, ~ 2922, and ~ 1433 cm−1 represents the symmetric, asymmetric, and bending vibrations of the H-C-H group, respectively21. Peaks appearing at ~ 854 cm−1 show the vibrational mode which relates to the stretching mode of the O-Ti–O system in the ceramic samples that confirmed the presence of BaTi4O9 structures22. The absorption mode appearing at ~ 690 cm−1 represents the Ti–O stretching mode of the octahedral group in complex perovskite structure23,24, as this mode appears only in BaTi4O9 ceramics. Furthermore, the structure of the BaTi4O9 ceramic sample has been confirmed by the XRD result.
Photoluminescence spectroscopy of Ba(Ti1−xSnx)4O9, 0 ≤ x ≤ 0.7 ceramics are shown in Fig. 4. The optical emission spectra are recognized as the recombination of electrons and holes in the state of transfer of carriers ions. By using Eq. (3), we have found the value of excitation energy of the samples.
where E = optical excitation energy, h = Plank’s constant (~ 6.63 × 10–34 Js) c = velocity of light (3 × 108 m/s) and λ is the emission wavelength. Emission at photoluminescence peak of the samples were recorded at ~ 603, 604, 606.5 and 605 nm with excitation energy ~ 2.06, 2.05, 2.04 and 2.05 eV for x = 0.0, 0.3, 0.5, and 0.7 content of Sn4+ dopant, respectively. Photoluminescence is a multi-photonic process that is an optical energy emission occurred in the optical range by many vibrational modes within the samples25. Within the energy band-gap, the photoluminescence process confirmed that due to localizing state the order/disorder structure may be affected directly. Hence, the structural order/disorder may be increased with increasing the energy band gap26. It was recorded that a broad-emission spectrum was located at ~ 604 nm and have an optical excitation energy (~ 2.06 eV) which was smaller than the energy band gap of extremely ordered BaTi4O9 ceramics located at ~ 558 nm (~ 2.23 eV) which may be due to the absence of oxygen vacancy27. In the photoluminescence spectrum, the red color may occur due to the oxygen vacancy.
Microwave dielectric properties
The variation of the relative permittivity (εr) and tangent loss (tanδ) values of Ba(Ti1−xSnx)4O9, 0 ≤ x ≤ 0.7 sintered ceramics versus frequency at room temperature is shown in Fig. 5. Due to increasing frequency, the values of εr and tanδ decrease exponentially in the samples. The high value of εr at resonant frequency (fo) can be described based on:
-
(1)
According to Maxwell–Wagner’s model, the dielectric materials are consist of fine conductive grains which are surrounded by grain boundaries. Large polarization is caused by the motion of charge carriers from grain to the grain surface.
-
(2)
The ionic polarization
-
(3)
The majority are due to crystal defects, vacancies, and grain defects etc28,29.
The increase in εr values with Sn4+ contents may be recognized by the substitution of a larger ionic radius of Sn4+ (~ 0.69 Å) cation for a smaller ionic radius of Ti4+ (~ 0.64 Å) cation30. To increasing the bond length of complex perovskite (i.e. AB4O9) the larger ionic radius cation may be substituted at B-site cation. The Sn4+ contents greatly affected the microwave dielectric properties due to high ionic polarization31,32. The maximum dielectric constant is obtained at x = 0.5 at maximum relative density (i.e. at low porosity). Because charge carriers need a medium to propagate and hence dielectric constant decreases with increasing material porosity33,34. Dielectric loss decreases with frequency due to the space charge polarization in all samples. At the lowest frequency, the maximum tangent loss occurs due to the presence of defects, impurities, and porosity in the ceramic samples35.
In general, the microwave dielectric properties of ceramics are dependent on intrinsic and extrinsic factors. The intrinsic properties are due to the interaction of materials phonons with the applied ac field. Thus the intrinsic properties also depend upon the crystal symmetry as observed in many single crystals36. The extrinsic properties are due to the imperfection in the crystal structure such as dopants or impurity atoms, grain boundaries, vacancies, order–disorder, secondary phases, etc36,37,38,39,40. Mostly, extrinsic factors are process-dependent and can be optimized. In this report, the sintering of these ceramic samples was done at a very high temperature (i.e. > 1300 °C for 4 h). The sintering at a high temperature may be causing the partial reduction of Ti4+ to Ti3+ ions. When Sn4+ is doped, it helps to maintain Ti4+ due to the following reaction:
and, thus control the reduction of Ti4+ to Ti3+ ions.
Therefore, at high-temperature sintering, a sintered layer acts as a shield that prevents the transport of oxygen to the core. Due to the lack of oxygen at the core, oxygen vacancies or titanium interstitials are produced. The presence of vacancies in the lattice is responsible for damping of phonon modes and hence maybe leads to enhancement of dielectric properties and Q-factors36,41,42.
The variation of quality factor (Qf) and relative density (%) of Ba(Ti1−xSnx)4O9 sintered ceramics as a function of composition (x) is shown in Fig. 6. Initially, the quality factor decreases from 9264.49 to 5681.16 with increasing Sn4+ content (from 0 to 0.3). The observed decrease in the value of Qf may be accepted due to the substitution of larger Sn4+ cation ion on the B-site cation, contributing to harmonic vibrational modes43,44,45 and another reason may be the phase transition. Additionally, an increase in Sn4+ content leads to a high Qf value, which may occur due to: (1) The phonon modes of B-site harmonic, and (2) Relative density of the ceramic samples.
The variation of ac conductivity of Ba(Ti1−xSnx)4O9, 0 ≤ x ≤ 0.7 sintered ceramics versus frequency is shown in Fig. 7. It is clear from the graph that ac conductivity depends upon the frequency and does not show any significant variation at the lowest frequency. The maximum value of ac conductivity at a lower frequency may be due to the rising state of localization in the hopping process. By the application of electric field, the hopping frequency of the charge carrier increased which result in the highest value of ac conductivity towards the high-frequency region46.
Conclusion
Solid solutions of Ba(Ti1−xSnx)4O9, (0 ≤ x ≤ 0.7) ceramics were fabricated through a mixed-oxide route. The average crystallite size of these ceramic samples lies in the range of 30–90 nm with phase change from orthorhombic (space group = Pnmm) to Tetragonal (P4mm) structure. Sintered ceramics attained 99.5% of the theoretical density at content (x = 0.5) and fine grain growth with uniformity was achieved. Photoluminescence confirmed that the present state of localization within the band-gap may affect the structural order/disorder. The dielectric properties of sintered ceramic samples showed εr = 57.29, and high Qf = 11,852. The increase in ac conductivity is due to the hopping mechanism. Based on the above-obtained results, these ceramic materials can be used for filter applications.
Data availability
The data of this study are available from the corresponding author upon reasonable request.
References
Richtmyer, R. Dielectric resonators. J. Appl. Phys. 10, 391–398 (1939).
Sebastian, M. T. Dielectric Materials for Wireless Communication (Elsevier, New York, 2010).
Vijay Kumar, H. & Muralidhar Nayak, M. A highly efficient iron doped BaTiO3 nanocatalyst for the catalytic reduction of nitrobenzene to azoxybenzene. RSC Adv. 4, 18881–18884 (2014).
Kumar, S. & Varma, K. Dielectric relaxation in bismuth layer-structured BaBi4Ti4O15 ferroelectric ceramics. Curr. Appl. Phys. 11, 203–210 (2011).
Patel, S. et al. Tuning of dielectric, pyroelectric and ferroelectric properties of 0.715 Bi0.5Na0.5TiO3–0065 BaTiO3–022 SrTiO3 ceramic by internal clamping. AIP Adv. 5, 087145 (2015).
Sebastian, M., Ubic, R. & Jantunen, H. Low-loss dielectric ceramic materials and their properties. Int. Mater. Rev. 60, 392–412 (2015).
Mirsaneh, M., Leisten, O. P., Zalinska, B. & Reaney, I. M. Circularly polarized dielectric-loaded antennas: Current technology and future challenges. Adv. Func. Mater. 18, 2293–2300 (2008).
Muhammad, R., Iqbal, Y. & Reaney, I. M. New low loss A9B9O31 (A= La; B= Ti, Mg, Sc, Fe, Al, Ga) ceramics for microwave applications. J. Alloys Compd. 646, 368–371 (2015).
Praveena, K. & Varma, K. Ferroelectric and optical properties of Ba5Li2Ti2Nb8O30 ceramics potential for memory applications. J. Mater. Sci. Mater. Electron. 25, 3103–3108 (2014).
Rase, D. & Roy, R. Phase equilibria in the system BaO–TiO2. J. Am. Ceram. Soc. 38, 102–113 (1955).
O’Bryan, H. Jr., Thomson, J. Jr. & Plourde, J. A new BaO–TiO2 compound with temperature-stable high permittivity and low microwave loss. J. Am. Ceram. Soc. 57, 450–453 (1974).
Cernea, M. Microwave dielectric properties of BaT14O9–Nd2O3, BaT14O9–Sm2O3 and BaT14O9–WO3 ceramics. J. Optoelectron. Adv. Mater. 9, 3790–3794 (2007).
Mueller, V., Beige, H. & Abicht, H.-P. Non-Debye dielectric dispersion of barium titanate stannate in the relaxor and diffuse phase-transition state. Appl. Phys. Lett. 84, 1341–1343 (2004).
Lu, S., Xu, Z. & Chen, H. Tunability and relaxor properties of ferroelectric barium stannate titanate ceramics. Appl. Phys. Lett. 85, 5319–5321 (2004).
Shvartsman, V., Kleemann, W., Dec, J., Xu, Z. & Lu, S. Diffuse phase transition in Ba Ti1–xSnxO3 ceramics: An intermediate state between ferroelectric and relaxor behavior. J. Appl. Phys. 99, 124111 (2006).
Veenhuis, H. et al. Light-induced charge-transport properties of photorefractive barium-calcium-titanate crystals doped with rhodium. Appl. Phys. B 70, 797–801 (2000).
Ma, H. et al. BaTi4O9 mesocrystal: Topochemical synthesis, fabrication of ceramics, and relaxor ferroelectric behavior. J. Alloys Compd. 777, 335–343 (2019).
Ali, A. et al. Dielectric and optical properties of tin-doped barium tetra titanate ceramics. Rev. Rom. Mater. 50, 448–452 (2020).
Lal, M. et al. Study of structural and magnetoelectric properties of 1–x (Ba096Ca004TiO3)–x (ZnFe2O4) ceramic composites. J. Mater. Sci. Mater. Electron. 29, 80–85 (2018).
Kumar, M. M., Srinivas, K. & Suryanarayana, S. Relaxor behavior in BaTiO3. Appl. Phys. Lett. 76, 1330–1332 (2000).
Gupta, S. & Subramanian, V. Encapsulating Bi2Ti2O7 (BTO) with reduced graphene oxide (RGO): An effective strategy to enhance photocatalytic and photoelectrocatalytic activity of BTO. ACS Appl. Mater. Interfaces. 6, 18597–18608 (2014).
Sun, D. et al. Investigation on FTIR spectrum of barium titanate ceramics doped with alkali ions. Ferroelectrics 355, 145–148 (2007).
Pavlović, N. & Srdić, V. V. Synthesis and structural characterization of Ce-doped bismuth titanate. Mater. Res. Bull. 44, 860–864 (2009).
Yadav, K. Optical and dielectric properties of Bi2Ti2O7/Bi4Ti3O12 nanocomposite. Mater. Today Proc. 28, 153–157 (2020).
Longo, V. et al. Structural conditions that leads to photoluminescence emission in SrTiO3: An experimental and theoretical approach. J. Appl. Phys. 104, 023515 (2008).
Leite, E. R. et al. The origin of photoluminescence in amorphous lead titanate. J. Mater. Sci. 38, 1175–1178 (2003).
El Marssi, M., Le Marrec, F., Lukyanchuk, I. & Karkut, M. Ferroelectric transition in an epitaxial barium titanate thin film: Raman spectroscopy and x-ray diffraction study. J. Appl. Phys. 94, 3307–3312 (2003).
Kaur, M. et al. Effect on the dielectric properties due to In–N co-doping in ZnO particles. J. Mater. Sci. Mater. Electron. 32, 8991–9004 (2021).
Liu, B., Hu, C. C., Huang, Y. H., Bafrooei, H. B. & Song, K. X. Crystal structure, infrared reflectivity spectra and microwave dielectric properties of CaAl2O4 ceramics with low permittivity. J. Alloys Compd. 791, 1033–1037 (2019).
Shannon, R. D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A Cryst. Phys. Diffract. Theor General Crystallogr. 32, 751–767 (1976).
Muhammad, R., Iqbal, Y. & Reaney, I. M. Structure and microwave dielectric properties of La5−xSrxTi4+xGa1−xO17 ceramics. J. Mater. Sci. 50, 3510–3516 (2015).
Iqbal, Y. & Muhammad, R. Microwave dielectric properties of Mg-doped SrLa4Ti5O17 layered perovskite. J. Mater. Sci. Mater. Electron. 27, 1314–1317 (2016).
Weng, M.-H., Liang, T.-J. & Huang, C.-L. Lowering of sintering temperature and microwave dielectric properties of BaTi4O9 ceramics prepared by the polymeric precursor method. J. Eur. Ceram. Soc. 22, 1693–1698 (2002).
Liu, B. et al. Enhancement of densification and microwave dielectric properties in LiF ceramics via a cold sintering and post-annealing process. J. Eur. Ceram. Soc. 41, 1726–1729 (2021).
Das, S., Das, S. & Sutradhar, S. Effect of Gd3+ and Al3+ on optical and dielectric properties of ZnO nanoparticle prepared by two-step hydrothermal method. Ceram. Int. 43, 6932–6941 (2017).
Freer, R. & Azough, F. Microstructural engineering of microwave dielectric ceramics. J. Eur. Ceram. Soc. 28, 1433–1441 (2008).
Reaney, I. M. & Iddles, D. Microwave dielectric ceramics for resonators and filters in mobile phone networks. J. Am. Ceram. Soc. 89, 2063–2072 (2006).
Muhammad, R., Iqbal, Y., Rambo, C. R. & Khan, H. Research trends in microwave dielectrics and factors affecting their properties: A review. Int. J. Mater. Res. 105, 431–439 (2014).
Kumar, S., Raju, V. & Kutty, T. Preparation of BaTi4O9 and Ba2Ti9O20 ceramics by the wet chemical gel-carbonate method and their dielectric properties. Mater. Sci. Eng., B 142, 78–85 (2007).
Liu, L., Flores, M. & Newman, N. Microwave loss in the high-performance dielectric Ba (Zn1/3Ta2/3) O3 at 4.2 K. Phys. Rev. Lett. 109, 257601 (2012).
Cheng, C.-M., Yang, C.-F., Lo, S.-H. & Tseng, T.-Y. Sintering BaTi4O9/Ba2Ti9O20-based ceramics by glass addition. J. Eur. Ceram. Soc. 20, 1061–1067 (2000).
Liu, B. et al. Novel low-εr MGa2O4 (M= Ca, Sr) microwave dielectric ceramics for 5 G antenna applications at the Sub-6 GHz band. J. Eur. Ceram. Soc. 41, 5170–5175 (2021).
Zhou, D., Randall, C. A., Wang, H., Pang, L. X. & Yao, X. Microwave dielectric properties trends in a solid solution (Bi1−xLnx) 2Mo2O9 (Ln= La, Nd, 0.0 ≤ x ≤ 0.2) system. J. Am. Ceram. Soc. 92, 2931–2936 (2009).
Liu, B. et al. High quality factor cold sintered LiF ceramics for microstrip patch antenna applications. J. Eur. Ceram. Soc. 41, 4835–4840 (2021).
Jin, D. H. et al. Boosting densification and microwave dielectric properties in cold sintered BaF2 ceramics for 5.8 GHz WLAN applications. J. Alloys Compd. 886, 161141 (2021).
Khan, R. et al. Influence of oxygen vacancies on the structural, dielectric, and magnetic properties of (Mn, Co) co-doped ZnO nanostructures. J. Mater. Sci. Mater. Electron. 29, 9785–9795 (2018).
Acknowledgements
Author Khaled Althubeiti is thankful to Researcher Supporting Project (TURSP-2020/241) at Taif University for the financial support.
Author information
Authors and Affiliations
Contributions
A.A. and A.Z. prepared samples and write the manuscript. S.U. helps in measurements. Z.I. supervises this research. M.L. and K.A. did the final writing-review, corrections, and editing. All the authors read and approve the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ali, A., Uddin, S., Lal, M. et al. Structural, optical and microwave dielectric properties of Ba(Ti1−xSnx)4O9, 0 ≤ x ≤ 0.7 ceramics. Sci Rep 11, 17889 (2021). https://doi.org/10.1038/s41598-021-97584-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-97584-x
This article is cited by
-
An investigation of microwave dielectric properties of BaZr0.25Ti0.75O3 and performance of DRA for 5G application
Journal of Materials Science: Materials in Electronics (2024)
-
Structural and microwave dielectric properties of high-permittivity Ca0.245Li0.325Nd0.395Ti1−xSnxO3 ceramics
Journal of Materials Science: Materials in Electronics (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.