Abstract
The objective of this study was to develop an accessible and accurate analysis method for microplastics that have been unintentionally added to cream cosmetic products. An experiment was performed on three cleansing creams in rich and viscous formulations. A spiked sample was prepared by adding polyethylene (PE) microspheres to the cleansing creams. After removing cosmetic ingredients from the creams using chemical digestion, damage to the PE microspheres was identified using Fourier transform infrared (FT-IR) spectroscopy. Field emission scanning electron microscopy (FE-SEM) images were obtained before and after digestion and used to characterize the morphology of the PE microspheres. The highest digestion efficiency was obtained using a chemical digestion method consisting of heating and stirring a sample in a 10 wt% KOH solution at 55 °C and 300 rpm for 5 days and did not damage the PE microspheres. The Nile red (9-diethylamino-5H-benzo[α]phenoxazine-5-one) staining method was effective in identifying small microplastics (< 106 μm). The optimal staining conditions are 5 μg/ml Nile red in n-hexane for green wavelengths.
Similar content being viewed by others
Introduction
Microplastics are pieces of synthetic polymer compounds with sizes of 5 mm or lower that; can be intentionally or unintentionally produced1 and exposed to the environment through various routes. Recently, countries with advanced cosmetics technologies, such as the United States, the United Kingdom, France, and South Korea, have legally prohibited the intentional use of microplastics in cosmetics2,3,4,5,6,7,8. Several studies have been performed on the detection of microplastics in cosmetics but were conducted before the ban on the intentional use of microplastics in cosmetics and applied an analysis method developed for intentionally used microplastics9,10,11,12. These studies were carried out using cosmetic products containing microbeads, and the pretreatment method was heating and stirring the products with only purified water until complete dissolution. After pretreatment was completed, the color, shape, polymer composition, size, density, and weight of microplastics were analyzed. Currently, most microplastics research is focused on detection in the ocean, sand, and living organisms, and pretreatment using strong acids is mostly performed for the purpose of digesting organic matters13,14,15,16,17,18,19,20.
Microplastics, as defined by the Ministry of Food and Drug Safety (MFDS) of South Korea, which has the 9th largest cosmetics market in the world21, refer to “solid plastics of 5 mm or less that are ‘intentionally’ added into products for the purpose of exfoliating and cleansing7.” Following the MFDS guidelines for the analysis of prohibited ingredients in cosmetics, the following test method was used: 3 g of a product were mixed with 100 ml of purified water and 100 ml of ethanol, and the mixture was homogenized by stirring for at least 10 min. Then, the mixture was passed through a metal filter, and the filter and retained particles were oven-dried at 50 °C. The retained particles were identified using an FT-IR microscope. Microplastics were identified by comparing the spectrum of the sample particles with the spectra of various plastics recommended by the MFDS.
The MFDS test method has limitations: (1) the cosmetic ingredients in all formulations are not completely removed by pretreatment with only purified water and ethanol; (2) semi-solid substances, such as sucrose or wax in cosmetics, can be misidentified as solids; (3) as was recently reported for bottled water22,23,24,25, the method is limited to analyzing microplastics that may have been unintentionally added to cosmetics; and (4) the expensive FT-IR microscope and metal filters required for analysis require specialized fabrication, preventing universal applications.
The objective of this study is to find an optimal digestion solution for the pretreatment of cosmetics in cream formulations and develop an analysis method for cosmetics that can detect all intentionally and unintentionally added microplastics with good accessibility and accuracy using the fluorescence of the plastic coloring reagent Nile red (NR).
Results
Determination of the optimal chemical digestion solution
The cosmetic products used in this study were cleansing creams in a viscous formulation. Polyethylene (PE) microspheres were used as the target materials and spiked into the cleansing creams on which experiments were conducted. PE is a plastic that has been widely used as a cosmetic ingredient for exfoliating purposes, as well as a material for cosmetic containers.
Experiments were conducted on different solutions by using cleansing cream B, which was the richest formulation. The solutions used in this experiment were HNO3 (60%), Acid mix (HNO3:HClO4 = 4:1, v:v), H2O2 (35%), KOH (10%), and D.I water. Among those, acid mix is a digestion solution recommended by the Oslo-Paris Commision (OSPAR) where the mechanism by which 15 Governments & the EU cooperate to protect the marine environment of the North-East Atlantic26.
Vacuum filtration was performed after chemical digestion, and the filter was completely dried. The PE microsphere particles retained on the filter were counted and stored in a glass vial. Subsequently, the weight of the filter from which the particles were removed was measured. After filtration, the digestion efficiency (%) was calculated using Eq. (1)27:
where Wi is the initial weight of the sample (g), Wa is the weight of the dry filter after filtration (g) and Wb is the weight of the dry filter before filtration (g).
The filtered PE microsphere particles were counted, and the recovery rate (%) was calculated using Eq. (2):
where Na is the number of microplastics (MPs) added to the sample and Nb is the number of MPs retained on the dry filter after filtration.
The following digestion efficiencies were determined using the different solutions (Fig. 1): HNO3; 90.87 ± 4.81%, Acid Mix; 96.09 ± 1.79%, H2O2; 90.34 ± 4.26%, KOH; 98.65 ± 1.14%, and D.I water; 88.01 ± 1.87%. D.I water produced the lowest digestion efficiency among the solutions of 90% or lower (Fig. 2a).
A digestion efficiency > 95% is considered to be significant, which was met using the acid mix and KOH. As the acid mix is a mixture of HNO3 and HClO4, KOH was determined to be the optimal digestion solution considering of cost and convenience of use.
In the filter images obtained after filtration (Supplementary Fig. S2), filtrates with a lumpy cream formulation were observed in solutions other than KOH. All the solutions used in this study can be effectively used to detect microplastics in ocean environmental samples10,28,29,30,31,32,33. In the successful chemical digestion of cosmetic products, the cosmetic ingredients are removed, and only the PE spiked into the product remains on the filter paper after filtration. However, lumpy cream formulations were observed in all the samples digested with acidic solutions. This result suggests that the conventional method of using acidic solutions to detect microplastics in the ocean is not suitable for cosmetic products.
After being confirmed as the optimal digestion solution, KOH was used to digest cleansing creams A–C. A high digestion efficiency > 95% was obtained. Monitoring the loss of 20 PEs added to the creams during filtration showed a recovery rate > 95% (Fig. 2b).
The experiments to determine the digestion solution were performed 5 times per solution, and the experiment using KOH was performed 10 times per cream, for a total of 30 experiments were carried out (Table 1).
Chemical damage test
Chemical weathering of microplastics occurs under environmental conditions, such as in oceans and sand because of UV exposure and abrasion, but does not occur in cosmetics. Thus, chemical damage occurs under inappropriate pretreatment.
Damage to the PE mocrospheres was determined by comparing the spectra before chemical digestion and drying and storage following KOH digestion. The measurement results confirmed that the peaks of the PE microspheres after and before pretreatment and the reference peak of the PE microspheres presented by MFDS were consistent (Fig. 3 and Supplementary Fig. S3). This result suggests that no ingredients in the cosmetic formulation remained on the surface because KOH pretreatment was effectively performed without destroying the PE chemical structure.
Physical damage test
FE-SEM was used to compare the morphology and surface characteristics of PE microspheres before and after pretreatment. First, it was observed whether the overall spherical shape was maintained at low magnification, and the surface properties were then identified at high magnification.
As the pretreatment on PE microspheres using KOH solution was effective, no cosmetic formulation ingredients remained on the surface; thus, the images of the PE microspheres obtained after digesting were similar to those obtained before chemical digestion (Fig. 4a–d). By contrast, the ingredients were not properly removed from samples using an acid solution, resulting in irregular and uneven surfaces, and the overall shape had a cut shape, rather than a spherical one (Fig. 4e,f). These results suggest that physical weathering occurred and the acid solution is not suitable for the pretreatment of cosmetic formulations.
Solvent test for fluorescence analysis
A solvent test is the most basic step in fluorescence analysis using NR. As NR produces different colors depending on the solvent, a solvent should be selected that does not damage the black PC filter used for fluorescence analysis. Three solvents were considered: methanol, n-hexane, and chloroform.
Solvent tests were performed using 5 μg/ml working solutions of NR in each solvent. The black PC filter was significantly damaged by chloroform and bleached by methanol (Supplementary Fig. S7).
A dyed black PC filter was placed on a glass slide, and a small quantity of PE microspheres was placed on the filter and covered with a cover glass. The edge of the cover glass was fixed with grease to prepare the sample for fluorescence analysis (Fig. 5). The analysis using chloroform failed because the black PC filter melted, such that failed to act as a background, and methanol was not visualized by fluorescence. Only n-hexane was clearly observed. Thus, n-hexane is the least decomposed of the investigated solvents and was selected for fluorescence analysis.
Determination of excitation and emission wavelengths
To observe the dyed PE microspheres, experiments were conducted at three wavelengths commonly used in fluorescence analysis34. First, a shadow image was obtained to identify the shape and location of the PE microspheres and used to determine whether the PE microspheres could be identified from fluorescence at different wavelengths (Supplementary Fig. S8). The PE microspheres could be visualized at both red and green wavelengths but not at blue wavelengths. Thus, experiments were conducted using different concentrations of PE microspheres determine the optimal conditions at red and green wavelengths.
Optimization of staining concentrations
A stock solution of 1000 μg/ml NR was prepared and diluted with n-hexane to prepare 1, 5, 10, and 20 μg/ml solutions. Figure 6 shows the observed images for each concentration. Very weak fluorescence was emitted by the 1 μg/ml solution, making it difficult to visualize the PE microspheres. For green wavelengths, PE microspheres were clearly observed at NR concentration of 5 μg/ml or higher whereas for red wavelengths, PE microspheres could be observed at 10 μg/ml or higher. For green wavelengths, there was no significant difference in visualizing PE microspheres at 5 μg/ml or higher, whereas fluorescence appeared in the background and a blurring phenomenon was observed with increasing concentration for red wavelengths. Thus, the optimal NR staining condition for observing the microspheres was determined to be 5 μg/ml NR for green wavelengths, considering cost efficiency and the fluorescence intensity.
PE microspheres dyed under optimal conditions can be clearly identified and counted using a tile mode that can capture the entire filter shape (Supplementary Fig. S9).
Discussion
The objective of this study was to develop an accessible and accurate method to detect unintentionally added microplastics in cream formulations. Cosmetic products come in various formulations ranging from toners to creams. In dilute formulations with a high moisture content, chemical digestion can be successfully performed using only purified water, whereas rich formulations have the disadvantage of a high oil content, resulting in the complete removal of all ingredients. Although it would be interesting to apply the proposed method to cosmetic products with real microbeads, but the sale and manufacturing of cosmetics containing plastic microbeads is currently prohibited by law and/or reguations, such that samples cannot be obtained. Therefore, experiments were performed using cleansing creams in rich and viscous formulations and PE which was widely used in the form of microbeads in cosmetics.
In this study, PE microspheres were added to viscous cleansing creams, and the resulting mixtures were chemically digested with diggerent solutions to pretreat organic matter to determine the optimal solution. The highest digestion efficiency was obtained by heating and stirring the sample with a 10% KOH solution at 55 °C and 300 rpm for 5 days. The results of FT-IR and FE-SEM analyses confirmed that chemical digestion did not cause physical or chemical damage to the added PE microspheres. When chemical digestion was performed with acidic solutions used to detect microplastics in the ocean35,36,37,38,39, ineffective pretreatment resulted in cosmetics ingredients remaining on the surfaces of the PE microspheres. This result suggests that the conventional method using acidic solutions to detect microplastics in the ocean is not suitable for cosmetic products.
The NR staining method selectively stains microplastics40,41,42,43 that can then be visually analyzed using microscopy; thus, this method facilitates more accessible and accurate analysis than the conventional method without requiring a specialized instrument, such as an FT-IR microscope. Among the investigated solvents, fluorescence was clearly observed without damaging the black PC filter in n-hexane. As n-hexane has a low solubility for NR powder, a 1000 μg/ml standard stock solution of NR was prepared with acetone34, and a working solution was prepared by dilution with n-hexane for use in the experiments. The dyed PE microspheres were most clearly identified at green wavelengths, and the optimal staining concentration was 5 μg/ml NR. Fluorescence analysis using NR was particularly effective in identifying small microplastics (< 106 μm) and could therefore improve the accuracy of the analysis by identifying microplastics that could be missed or lost by filtration because of their small size.
As only PE was used in this study, further study is necessary using other polymers, such as polypropylene (PP) and methyl methacrylate (PMMA). Using purified water to rinse the filter paper used to perform an initial filtration after chemical digestion using KOH solution, followed by performing a second filtration using a black PC filter would be reduce microplastics loss and increase the accuracy of detection, identification, and counting using the proposed analysis method.
Methods
Materials
The target microplastics used in this study were clear polyethylene microspheres (Cospheric LLC, Santa Barbara, CA, USA). Hydrogen peroxide (35%, DAEJUNG, Siheung-si, South Korea), nitric acid (60%, DAEJUNG, Siheung-si, South Korea), perchloric acid (70%, DAEJUNG, Siheung-si, South Korea), sodium hypochlorite (9%, DAEJUNG, Siheung-si, South Korea), and potassium hydroxide (84%, DAEJUNG, Siheung-si, South Korea) were used as pretreatment solvents.Filtration was performed using 90-mm diameter Whatman Grade 40, Ashless filter paper, and 25-mm diameter black polycarbonate (PC) filter paper (0.8 μm) was used for fluorescence analysis. The dyeing reagents used in the fluorescence analysis were Nile red (Sigma-Aldrich, Saint Louis, USA), acetone (99.5%, DAEJUNG, Siheung-si, South Korea), n-hexane (95%, DAEJUNG, Siheung- si, South Korea), methyl alcohol (99.8%, JT Baker, Phillipsburg, NJ, USA), and chloroform (99.8%, DAEJUNG, Siheung-si, South Korea).
Sample preparation
Three viscous creams from the cleansing product group were used in the experiments. As this study is not affiliated with a specific brand name, the products were labeled Cleansing Creams A-C to avoid any legal issues. Cleansing Creams A-C were selected from the three most sold products in skin care-cleansing-cleansing cream/milk in domestic drug stores.
Chemical digestion
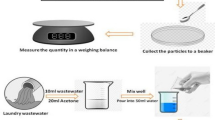
To determine the solution for use in chemical digestion, 2 g of a cleansing cream was placed into a 250 ml beaker, to which 20 PE microspheres (850–1000 μm) were added. Then, 50 ml of each digestion solution was added to the beaker, and the mixture was stirred at 50–55 °C for 5 days at 300 rpm. The beaker was sealed to prevent evaporation and contamination.
Filtration
After chemical digestion was completed, the sample was cooled approximately 30 °C and filtered under reduced pressure. The filter was then transferred to a glass petri dish (90 mm in diameter) using tweezers, covered with a lid, and dried at 40 °C to completely remove moisture. Using a plastic petri dish generates static electricity that can result in the loss of the filtered PE microsphere particles. To prevent this loss, a glass petri dish should be used instead of a plastic dish. The PE microsphere particles on the completely dried filter were counted and stored in a glass vial. To calculate the digestion efficiency, the weights of the filter and petri dish were measured. This step enabled the percentage of undigested cleansing cream remaining in the filter to be calculated, indicating whether digestion was successful.
Spectroscopy
After chemical digestion, FT-IR spectroscopy was used to check for damage to the PE microspheres. The spectra of the PE microspheres before and after digestion and the reference PE spectra provided by the MFDS were compared. The FT-IR spectra were obtained in transmittance mode by using a Vertex 70 FT-IR spectrometer. The FT-IR spectra were analyzed from 500 to 4000 cm−1, and 16 scans were performed to obtain each spectrum.
Field emission scanning electron microscopy
To identify the shape and surface characteristics of the PE microspheres before and after pretreatment, images were obtained by using FE-SEM. An FE-SEM IT-500HR was used, and the operating voltage was 5.0 kV. Before observation, the samples were coated with Pt for 240 s by using a sputter coater. The samples compared under the same conditions, that is, the overall spherical shape was observed at × 80 magnification, and the surface characteristics were observed at × 1000 magnification.
Fluorescence analysis using Nile red staining
Laser scanning confocal microscopy was performed using a Zeiss LSM 980 for fluorescence analysis. The operation laser power was 2–5%, the pinhole size was 100 μm, the master gain value was 550–600 V, and a digital offset was not used.
Solvent test for fluorescence analysis
The solvent used in the experiments should not damage the black PC filter used for fluorescence analysis. Three solvents were considered: methanol, n-hexane, and chloroform. A few drops of each solvent were dropped onto the filter, and the color change and the degree of damage to the filter were observed.
Excitation and emission wavelengths
There is a small difference in the excitation/emission wavelengths of NR depending on the polarity of the solvent and dyeing conditions. To observe dyed organic matter, experiments were conducted with three types of wavelengths commonly used in fluorescence microscopy: red: excitation wavelength (ex.) 565 nm and emission wavelength (em.) 753 nm; green: (ex.) 460 nm and (em.) 568 nm; and blue: (ex.) 410 nm and (em.) 597 nm.
Staining of microplastics using various concentrations of Nile red solution
NR powder was dissolved in each solvent to prepare stock solutions of 1000 μg/ml that were further diluted with solvent to prepare working solutions of various concentrations. All the prepared solutions were syringe filtered before use. A small quantity of PE microspheres (90–106 μm) were placed on a black PC filter, the filter was dyed with 400 μl of a working solution, air-dried for 5 min, and a preparat was fabricated and analyzed using a Zeiss LSM 980. Observation was performed immediately after production to prevent reduction in the NR fluorescence.
Data availability
The data that support the findings of this study are available in the paper and its Supplementary Information or from the corresponding author upon request.
References
Cole, M., Lindeque, P., Halsband, C. & Galloway, T. S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 62, 2588–2597 (2011).
Environmental Protection (Microbeads) (England) Regulations. 2017 No. 1312. https://www.legislation.gov.uk/uksi/2017/1312/contents/made (2017).
Environmental Protection (Microbeads) (Northern Ireland) Regulations. 2019 No 18. Northern Ireland. https://www.legislation.gov.uk/nisr/2019/18/contents/made (2019).
Environmental Protection (Microbeads) (Scotland) Regulations. 2018 No. 162. Scotland. https://www.legislation.gov.uk/ssi/2018/162/introduction/made (2018).
Environmental Protection (Microbeads) (Wales) Regulations. 2018 No. 760 (W. 151). Wales. http://www.legislation.gov.uk/wsi/2018/760/contents/made (2018).17.
France. Décret n° 2017–291 du 6 mars 2017. https://www.legifrance.gouv.fr/loda/id/JORFTEXT000034154540 (2017).
Ministry of Food and Drug Safety of Republic of South Korea. Regulations on the Safety Standards, etc. of Cosmetics. Notice No. 2017-30. https://mfds.go.kr/brd/m_211/view.do?seq=11563&srchFr=&srchTo=&srchWord=%EB%8C%80%ED%95%9C%EB%AF%BC%EA%B5%AD%EC%95%BD%EC%A0%84&srchTp=0&itm_seq_1=0&itm_seq_2=0&multi_itm_seq=0&company_cd=&company_nm=&page=1 (2017).
United Nations Environmental Programme (UNEP). Legal Limits on Single-Use Plastics and Microplastics: A Global Review of National Laws and Regulations. https://www.unep.org/resources/publication/legal-limits-single-use-plastics-and-microplastics-global-review-national (2018).
Cheung, P. K. & Fok, L. Characterisation of plastic microbeads in facial scrubs and their estimated emissions in Mainland China. Water Res. 122, 53–61 (2017).
Guerranti, C., Martellini, T., Perra, G., Scopetani, C. & Cincinelli, A. Microplastics in cosmetics: Environmental issues and needs for global bans. Environ. Toxicol. Pharmacol. 68, 75–79 (2019).
Lei, K. et al. Microplastics releasing from personal care and cosmetic products in China. Mar. Pollut. Bull. 123, 122–126 (2017).
Napper, I. E., Bakir, A., Rowland, S. J. & Thompson, R. C. Characterisation, quantity and sorptive properties of microplastics extracted from cosmetics. Mar. Pollut. Bull. 99, 178–185 (2015).
Besley, A., Vijver, M. G., Behrens, P. & Bosker, T. A standardized method for sampling and extraction methods for quantifying microplastics in beach sand. Mar. Pollut. Bull. 114, 77–83 (2017).
Cole, M. et al. Isolation of microplastics in biota-rich seawater samples and marine organisms. Sci. Rep. 4, 4528 (2014).
Dehaut, A. et al. Microplastics in seafood: Benchmark protocol for their extraction and characterization. Environ. Pollut. 215, 223–233 (2016).
Hall, N., Berry, K., Rintoul, L. & Hoogenboom, M. Microplastic ingestion by scleractinian corals. Mar. Biol. 162, 725–732 (2015).
Lusher, A., Welden, N., Sobral, P. & Cole, M. Sampling, isolating and identifying microplastics ingested by fish and invertebrates. Anal. Methods 9, 1346–1360 (2017).
Rezania, S. et al. Microplastics pollution in different aquatic environments and biota: A review of recent studies. Mar. Pollut. Bull. 133, 191–208 (2018).
Shim, W. J., Hong, S. H. & Eo, S. E. Identification methods in microplastic analysis: A review. Anal. Methods 9, 1384–1391 (2017).
Van Cauwenberghe, L., Devriese, L., Galgani, F., Robbens, J. & Janssen, C. R. Microplastics in sediments: A review of techniques, occurrence and effects. Mar. Environ. Res. 111, 5–17 (2015).
International Trade Administration. https://www.trade.gov/knowledge-product/korea-cosmetics (2020).
Kosuth, M., Mason, S. A. & Wattenberg, E. V. Anthropogenic contamination of tap water, beer, and sea salt. PLoS ONE 13, e0194970 (2018).
Liebezeit, G. & Liebezeit, E. Synthetic particles as contaminants in German beers. Food Addit. Contam. Part A-Chem. 31, 1574–1578 (2014).
Mason, S. A., Welch, V. G. & Neratko, J. Synthetic polymer contamination in bottled water. Front. Chem. 6, 407 (2018).
Schymanski, D., Goldbeck, C., Humpf, H.-U. & Fürst, P. Analysis of microplastics in water by micro-Raman spectroscopy: Release of plastic particles from different packaging into mineral water. Water Res. 129, 154–162 (2018).
Enders, K., Lenz, R., Beer, S. & Stedmon, C. A. Extraction of microplastic from biota: Recommended acidic digestion destroys common plastic polymers. ICES J. Mar. Sci. 74, 326–331 (2017).
Karami, A. et al. A high-performance protocol for extraction of microplastics in fish. Sci. Total Environ. 578, 485–494 (2017).
Avio, C. G., Gorbi, S. & Regoli, F. Experimental development of a new protocol for extraction and characterization of microplastics in fish tissues: First observations in commercial species from Adriatic Sea. Mar. Environ. Res. 111, 18–26 (2015).
Claessens, M., Van Cauwenberghe, L., Vandegehuchte, M. B. & Janssen, C. R. New techniques for the detection of microplastics in sediments and field collected organisms. Mar. Pollut. Bull. 70, 227–233 (2013).
De Witte, B. et al. Quality assessment of the blue mussel (Mytilus edulis): Comparison between commercial and wild types. Mar. Pollut. Bull. 85, 146–155 (2014).
Kühn, S. et al. The use of potassium hydroxide (KOH) solution as a suitable approach to isolate plastics ingested by marine organisms. Mar. Pollut. Bull. 115, 86–90 (2017).
Santana, M., Ascer, L., Custódio, M., Moreira, F. & Turra, A. Microplastic contamination in natural mussel beds from a Brazilian urbanized coastal region: Rapid evaluation through bioassessment. Mar. Pollut. Bull. 106, 183–189 (2016).
Thiele, C. J., Hudson, M. D. & Russell, A. E. Evaluation of existing methods to extract microplastics from bivalve tissue: Adapted KOH digestion protocol improves filtration at single-digit pore size. Mar. Pollut. Bull. 142, 384–393 (2019).
Shim, W. J., Song, Y. K., Hong, S. H. & Jang, M. Identification and quantification of microplastics using Nile Red staining. Mar. Pollut. Bull. 113, 469–476 (2016).
Davidson, K. & Dudas, S. E. Microplastic ingestion by wild and cultured Manila clams (Venerupis philippinarum) from Baynes Sound, British Columbia. Arch. Environ. Contam. Toxicol. 71, 147–156 (2016).
Desforges, J.-P.W., Galbraith, M. & Ross, P. S. Ingestion of microplastics by zooplankton in the Northeast Pacific Ocean. Arch. Environ. Contam. Toxicol. 69, 320–330 (2015).
Devriese, L. I. et al. Microplastic contamination in brown shrimp (Crangon crangon, Linnaeus 1758) from coastal waters of the Southern North Sea and Channel area. Mar. Pollut. Bull. 98, 179–187 (2015).
Nuelle, M.-T., Dekiff, J. H., Remy, D. & Fries, E. A new analytical approach for monitoring microplastics in marine sediments. Environ. Pollut. 184, 161–169 (2014).
Roch, S. & Brinker, A. Rapid and efficient method for the detection of microplastic in the gastrointestinal tract of fishes. Environ. Sci. Technol. 51, 4522–4530 (2017).
Erni-Cassola, G., Gibson, M. I., Thompson, R. C. & Christie-Oleza, J. A. Lost, but found with Nile red: A novel method for detecting and quantifying small microplastics (1 mm to 20 μm) in environmental samples. Environ. Sci. Technol. 51, 13641–13648 (2017).
Hengstmann, E. & Fischer, E. K. Nile red staining in microplastic analysis—proposal for a reliable and fast identification approach for large microplastics. Environ. Monit. Assess. 191, 612 (2019).
Jee, A.-Y., Park, S., Kwon, H. & Lee, M. Excited state dynamics of Nile Red in polymers. Chem. Phys. Lett. 477, 112–115 (2009).
Maes, T., Jessop, R., Wellner, N., Haupt, K. & Mayes, A. G. A rapid-screening approach to detect and quantify microplastics based on fluorescent tagging with Nile Red. Sci. Rep. 7, 1–10 (2017).
Acknowledgements
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2020R1F1A107197911) and Korea Ministry of Environment (MOE) Graduate School specialized in Integrated Pollution Prevention and Control Project.
Author information
Authors and Affiliations
Contributions
T.G.L. managed the whole project, designed all the experiments and jointly wrote the manuscript; S.L. conducted the experiments, analyzed the data, and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lee, S., Lee, T.G. A novel method for extraction, quantification, and identification of microplastics in CreamType of cosmetic products. Sci Rep 11, 18074 (2021). https://doi.org/10.1038/s41598-021-97557-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-97557-0
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.