Abstract
This study explored the dosimetric difference between hypofractionated whole-breast irradiation (HFWBI) with sequential boost (SEB) and simultaneous integrated boost (SIB) based on supine and prone positions to identify the superior boost mode and superior position. Thirty breast cancer patients eligible for HFWBI after breast-conserving surgery were enrolled. All patients underwent 3DCT simulation scanning in both supine and prone positions. For the SEB-HFWBI plan, the dose prescribed for the planning target volume (PTV) of whole breast (WB) was 2.67 Gy per fraction with a total of 15 fractions, followed by a sequential boost of 3.2 Gy per fraction to the PTV of tumor bed (TB) in 3 fractions. For the SIB-HFWBI plan, the dose prescribed for the PTV of WB was 2.67 Gy per fraction with a total of 15 fractions, with a simultaneously integrated boost of 3.2 Gy per fraction to the PTV of TB with a total of 15 fractions. Regardless of the position, for the PTV of TB, the conformal index (CI) in the SIB-HFWBI plans was greater than those in the SEB-HFWBI plans (T = − 8.114, − 8.114; both P < 0.05). The CI for the PTV of WB increased significantly in the prone position relative to the supine position in both two plans(Z = − 3.340, − 3.501; all P < 0.05). The study suggested that prone SIB-HFWBI might be more suitable for postoperative radiotherapy after breast-conserving surgery for early-stage breast cancer patients.
Similar content being viewed by others
Currently, breast-conserving surgery (BCS) followed by whole-breast irradiation (WBI) is widely accepted as the standard of care for early breast cancer1,2,3. Although conventional fractionation WBI (CFWBI) has remained the main treatment model in China, hypofractionated WBI (HFWBI) has arisen after proposing that the α/β ratio of breast cancer might be as low as approximately 4 in 19894. Two randomized studies found that HFWBI after BCS showed equivalent therapeutic effects and lower acute radiation-induced reactions than CFWBI5,6. Moreover, HFWBI provides a shortened time of treatment and improves patient convenience7. Hence, HFWBI as a valid alternative for early-stage breast cancer patients after BCS is rapidly replacing CFWBI worldwide.
Both CFWBI and HFWBI are involved in tumor bed (TB) boost since an additional boost after WBI is indispensable for the vast majority of patients after BCS8. Furthermore, a boost dose to the TB can improve local control, particularly in young patients with negative prognostic factors for local relapse9,10. Frequently, a sequential boost (SEB) to the TB was used, but when the simultaneously integrated boost (SIB) to the TB was used, there was more convenience and superior tolerance for the patient due to shorter treatment time and better dosimetric distribution11,12. However, there is no uniform standard for the way (SEB or SIB) and radiation dose fractionation of the tumor bed boost for hypofractionated radiotherapy13,14,15.
Comparative studies on WBI have demonstrated a better dose conformance to the treatment target and a lower dose to the lung in the prone position than in the supine position16,17,18. However, the advantages of HFWBI in different TB boost methods in the supine or prone position have not been established. Therefore, in this study, we performed dosimetric comparisons of the targets and organs at risk (OARs) for HFWBI with the same method of TB boost in the two positions or with different methods of TB boost in the same position, to seek the superior plan and position.
Methods
Patient selection
Breast cancer patients eligible for HFWBI19 following BCS were enrolled for this study. The oncoplastic BCS was one of the exclusion criterions because of the risk of inconsistent boost delineation and all enrolled patients had ≥ 5 surgical clips fixed to the central bottom and lateral edges of the surgical cavity to mark the TB boundaries. Regional lymph node irradiation was not required for all the enrolled patients. All patients underwent 3DCT simulation scanning both in the supine and prone positions with free breathing on the same day. Moreover, no seroma was observed in the operative cavity during simulation scanning. Full informed consent was obtained for all patients and/or their legal guardians, and the study was approved by the institutional research ethics board of the Shandong Cancer Hospital Ethics Committee and was performed in accordance with relevant guidelines/regulations.
CT simulation
Patients were scanned for three-dimensional computed tomography (3DCT) simulation under supine and prone positions on a 16-slice computed tomography (CT) scanner (Philips Brilliance Bores CT, Netherlands) with free breathing. For the supine position, the patients were immobilized on a breast board using arm support (with both arms abducted and raised overhead) and knee support. The clinically palpable ipsilateral breast was demarcated with metal wires. The CT simulation in the prone position on a specifically dedicated treatment board was performed in all patients with both arms above their head. The board contained an open aperture on one side to allow for the ipsilateral breast to hang freely away from the chest wall. The CT images were acquired in 3 mm slices from the cricothyroid membrane to 5 cm below the diaphragm. The CT dataset was exported to the Eclipse treatment planning system (Eclipse 15.5, Varian Medical Systems, Palo Alto, CA, USA) for target and OAR delineation and to formulate treatment plans.
Target definition
The delineation of the target volume and OARs was performed by the same radiation oncologist with over 5 years of experience in breast radiotherapy. On both supine and prone scanning images, the TB was delineated based only on the surgical clips and defined as TBSupine and TBProne, respectively. The clinical target volume (CTV) and planning target volume (PTV) for the TB were created by 5-mm and 10-mm expansion of the TB, respectively, and defined as CTVSupine-TB, CTVProne-TB, PTVSupine-TB, and PTVProne-TB. The CTV for the whole breast (WB) included the glandular breast tissue of the ipsilateral breast and was defined as CTVSupine-WB and CTVProne-WB. The PTV for the WB was the CTV for the WB plus a 5-mm margin and defined as PTVSupine-WB and PTVProne-WB, respectively. Moreover, the CTV for the WB was limited to the glandular-pectoral muscle wall interface and 5 mm from the skin surface, including the CTV for the TB. The target volume, as well as all organs at risk, such as heart, lung and contralateral breast were contoured according to the Radiation Therapy Oncology Group (RTOG) delineation guidelines for adjuvant radiotherapy of early breast cancer20. All delineation of the targets was determined using the same clinical criteria, whether in the supine or prone position.
Treatment planning
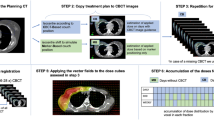
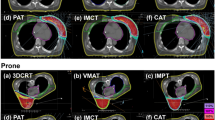
For each patient, four different plans, SIB-HFWBI and SEB-HFWBI in both supine and prone positions, were generated. All treatment plans were performed in VARIAN’s ECLIPSE TPS Version 15.5 (Anisotropic Analytical Algorithm calculation model) using field in field technique with a 6-MV photon beam. For the SIB-HFWBI plan, the dose prescribed for the PTV of the WB was 2.67 Gy per fraction with a total of 15 fractions, with a simultaneously integrated boost of 3.2 Gy per fraction to the PTV of the TB with a total of 15 fractions (Fig. 1). For the SEB-HFWBI plan, the dose prescribed for the PTV of the WB was 2.67 Gy per fraction with a total of 15 fractions, followed by a sequential boost of 3.2 Gy per fraction to the PTV of the TB in 3 fractions (Fig. 2). In addition, the criteria of the plans were to ensure that at least 95% of the PTV received the prescription dose. Optimization was addressed to reduce both the dose for the IPSL and the heart. In the supine treatment planning, patients were treated with two opposing tangential fields for the prescribed dose to be delivered to the WB. To reduce the IPSL volume as much as possible, 4–6 segmented fields were set up to adjust the homogeneity of the target volume. The field angle of the TB was the same in both the SEB-HFWBI and SIB-HFWBI plans. In the prone treatment planning, two opposing tangential fields were also set up for the prescribed dose to be delivered to the WB. The field angle was chosen to avoid exposure to the contralateral breast as the primary consideration and to minimize the ipsilateral irradiated lung volume. Moreover, 4–6 segmented fields were also added to adjust the homogeneity of the target volume in the prone setup. In the SIB-HFWBI plan, the entire breast and TB were simultaneously irradiated, while in the SEB setup, the treatment plan for total breast and TB were superimposed and calculated.
Dosimetric evaluation
Dose-volume histogram (DVH) parameters for the PTV, heart, IPSL, and contralateral breast were calculated for each plan in all patients. The conformal index (CI) and homogeneity index (HI) were evaluated for the PTV. The V100% means the PTV coverage percent of the 100% prescribed dose line in the treatment plan.
CI was defined as follows:
where Ref. isodose volume of the PTV represents the absolute volume of the PTV that is covered by the prescribed dose and Ref. isodose volume represents the absolute volume covered by the prescribed dose21.
HI was defined as follows:
where D2 and D98 represent the doses covering 2% and 98% of the PTV, respectively22.
The IPSL and heart were evaluated using the mean dose (Dmean) and the volumes that received ≥ 5 Gy, 10 Gy, 20 Gy, 30 Gy and 40 Gy (V5, V10, V20, V30 and V40, respectively). The contralateral breast was evaluated using the Dmean and D2. The MU represented the monitor units in the treatment plan.
Statistical methods
Statistical analysis was performed with SPSS 19.0 software (IBM Corporation, Armonk, NY, USA). The data that did not follow a normal distribution were analyzed by the Wilcoxon signed-rank test and are described using medians and ranges. Data that followed a normal distribution were analyzed by paired-samples t-tests and are described using means and standard deviations. The Wilcoxon signed-rank test was used to compare the dosimetric parameters of the targets and IPSL for HFWBI with different methods of TB boost in the same position. Our study performed dosimetric comparisons of the heart between the SEB-HFWBI and SIB-HFWBI regimens in the same position via paired-samples t-tests. Data were considered statistically significant at P < 0.05.
Results
Patient characteristics
Our study analyzed thirty patients treated with HFWBI following BCS for early-stage breast cancer between July 2018 and December 2019. The median age was 46 (ranging from 30 to 60). Patients had stage I or II (TIN0M0-T2N0M0) breast cancer according to the 2009 7th edition of the American Joint Committee on Cancer. Fourteen of the 30 patients had left-sided breast cancer, and the remaining sixteen had right-sided breast cancer. Patients underwent lumpectomy with sentinel lymph node dissection (SLND) or axillary lymph node dissection (ALND) and had ensured tumor-negative margins during a single operation. The characteristics of the study population are displayed in Table 1.
Dosimetric comparisons of the targets between the SEB-HFWBI and SIB-HFWBI plans on the same position
Table 2 shows that the dosimetric parameters, including D2, D98 and V100%for PTVTB and V100% for PTVWB were all significantly higher for the SEB-HFWBI plan than for the SIB-HFWBI plan based on the same position (all P < 0.05). The CIs for PTVTB and PTVWB were significantly lower in the SEB-HFWBI plan than in the SIB-HFWBI plan in both the supine and prone positions (supine: T = − 8.114, − 13.356; prone: T = − 8.114, − 13.356; all P < 0.05, see Table 3 for details). Furthermore, regardless of patient position, the HI of PTVTB was significantly better with the SIB approach than with the SEB approach (supine: Z = − 6.552, P = 0.000; prone: Z = − 6.552; P = 0.000).
Comparison of dosimetric parameters of the OARs between the SEB-HFWBI and SIB-HFWBI plans in the same position
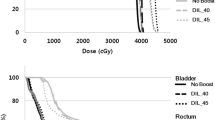
The IPSL dose parameters (Dmean, V5, V10, V20, V30, V40) showed significantly lower averages for the SIB-HFWBI plan than for the SEB-HFWBI plan in both the supine and prone positions (all P < 0.05, see Table 4 for details). The values for heart dose parameters, including Dmean, V5, V10, V20, V30, and V40, in left-sided breast cancer patients treated with the SEB-HFWBI plan were significantly higher than in those treated with the SIB-HFWBI plan in the same position (all P < 0.05, see Table 4 for details). In both the supine and prone position, the Dmean to the heart showed no statistically significant differences between the SIB-HFWBI and SEB-HFWBI plans in the right-sided breast cancer patients (Z = − 1.518, − 1.741, P = 0.067, 0.076). In the SIB-HFWBI regimen, D2 and Dmean to the contralateral breast was significantly lower than that in the SEB-HFWBI regimen in both the supine and prone positions (S: Z = − 3.252, − 3.658; P = 0.001, 0.000; P: Z = − 3.252, − 3.658; P = 0.001, 0.000). The SIB setup indeed revealed fewer MUs than the SEB setup in both the supine and prone positions, and the differences were statistically significant (Z = − 4.783, 4.783; P = 0.000, 0.000, see Table 5 for details).
Dosimetric comparison of the targets and OARs for HFWBI with the same TB boost in two different positions
For both the SIB-HFWBI and SEB-HFWBI plans, the CI for the PTVWB increased slightly in the prone position relative to the supine position (Z = − 3.340, − 3.501; all P < 0.05, see Table 6 for details). Moreover, for the IPSL, the Dmean, V5, V10, and V20 obtained in the prone position were all significantly lower than those obtained in the supine position in both the SIB-HFWBI plan and SEB-HFWBI plan (SIB: Z = − 4.782, − 4.704, − 4.782, − 4.783; SEB: Z = − 4.782, − 4.782, − 4.782, − 4.782; all P = 0.000). Regardless of the SEB or SIB approach, no significant differences in the Dmean to the heart were evident between the supine and prone positions in the left-sided breast cancer patients (T = 0.278, 0.393; P = 0.786, 0.701).
Discussion
A commonly used regimen involves WBI after BCS to a dose of 45–50 Gy over 5 weeks, with a sequential boost delivered to the TB, which again prolongs the overall treatment time by 1–2 weeks. Although the normal fractionation scheme of WBI is widely accepted, approximately 15–20% of BCS patients eventually choose to give up on radiotherapy due to a lengthy treatment course23,24. As the results from multiple randomized studies have been gradually published25,26,27, HFWBI in 15 or 16 fractions is slowly replacing normal fractionation schemes for WBI worldwide. Indeed, HFWBI as an alternative to the CFWBI regimen has become a superior choice for early breast cancer patients after BCS, which substantially increases patient convenience because of shortened treatment duration and a reduction in cost. Furthermore, randomized controlled trials comparing HFWBI with CFWBI showed a slight reduction in acute toxicity and significantly better cosmetic outcomes19,28.
A TB boost should also be an essential part of the standard setup for HFWBI. However, the relevant studies25,26,27 that laid the foundation for the safety and equivalence of HFWBI had no uniform agreement regarding the TB boost after HFWBI. The optimal dose, fractionation schedule, delivery method, and timing of the boost remain undefined. A Canadian multicenter, prospective, randomized trial of early-stage breast cancer patients reported the equivalence of HFWBI (a dose of 42.5 Gy in 16 fractions) to CFWBI (a dose of 50 Gy in 25 fractions)27. However, a boost to the TB was not included in this trial. The UK START Trial A delivered 10 Gy to the TB in five daily fractions after HFWBI sequentially25,26. Recent studies have explored the regimen of TB boost in HFWBI14,29. Gupta et al.14 reported the results of a phase 2 HFWBI randomized trial with a follow-up of 5 years. They delivered a WB dose of 36.63 Gy in 11 fractions of 3.33 Gy, followed by a TB boost of 13.32 Gy in 4 fractions of 3.33 Gy. The results of the 5-year follow-up showed that the locoregional control reached 97.7%, the rate of excellent breast cosmesis reached 95% and the acute and late toxicity rates were relatively low. A randomized controlled trial comparing CF-WBI with HFWBI verified that the overall rates of any physician-assessed acute toxic effects of grade 2 or higher or grade 3 or higher were lower with HFWBI than with CFWBI (47% and 78%, P < 0.001)29. Schmeel et al.30 reported similar results in their randomized controlled trial. In terms of the comparison between sequential and simultaneous integrated boost, the results of a phase III randomized study conducted by Paelinck et al.15 demonstrated that grade 2/3 dermatitis was significantly more frequent in the SEB-HFWBI arm and that the incidence of edema was lower in the SIB-HFWBI arm. But the latest data showed the physician-assessed two-years toxicity and photographic analysis were not significantly different between SIB and SEB treatment arms31. Nevertheless, Onal et al.32 demonstrated that the SIB technique showed better target-volume dose distribution and better sparing heart in volumetric-modulated arc therapy and helical-tomotherapy compared to the SEB technique. Consequently, even though the above studies have shown that HFWBI is superior to CFWBI, the controversy between the SIB and SEB still remained. Afterwards, in our study, when comparing the values for OAR dose parameters including the IPSL, the contralateral breast and heart in left-sided breast cancer patients, significantly lower averages were found for the SIB-HFWBI plan.
To the best of our knowledge, our study on the comparison of dosimetric parameters between SEB-HFWBI and SIB-HFWBI in prone and supine positions is the first to address this topic using FIMRT. In this study, we first compared the differences between SEB and SIB plans in the same position. The results showed that the CI of PTVTB and PTVWB in SIB plans were better than that in the SEB plans, and the PTVTB in the SIB plan had better dose homogeneity compared to the SEB plan. For organs at risk, the SIB plans significantly reduced the dose to the ipsilateral lung, heart and contralateral breast. The SIB plans significantly reduced MUs, which could reduce machine wastage. In conclusion, the SIB plans showed better dosimetric advantages over the SEB plans in both supine and prone position. Van Parijs et al.33 compared SEB and SIB plans of 10 patients with breast cancer in supine position, the results confirmed the dosimetric advantages of SIB for breast irradiation, even when compared to an advanced and highly conformal sequential technique. The result was the same as our study.
Then, we compared the treatment plans for different positions. The results showed that, for both the SIB-HFWBI and SEB-HFWBI plans, the CI for the PTVWB was superior in the prone position than in the supine position. Furthermore, for the IPSL, the dose parameters obtained in the prone position were all significantly lower than those obtained in the supine position. However, for the left-sided breast cancer patients, we verified that the variance in the heart dose parameters between supine and prone positions was not statistically significant with either the SEB-HFWBI or SIB-HFWBI approach. This may be because in the prone position, the heart droops and enters the irradiation field. In fact, several studies have clarified the dosimetric advantages of CFWBI in prone position13,34,35,36. Bergom et al.36 and Alonso-Basanta et al.37 reported that the dose homogeneity in prone WBI was improved and that the high dose distribution to the target was also reduced accordingly. Osa et al.38 also indicated that the advantages of prone SIB-HFWBI were the significantly reduced in-field volume of the IPSL and heart. Controversy exists regarding supine and prone positions in terms of the irradiated dose to the heart. Lymberis et al.39 indicated that prone positioning reduced the in-field heart volume in the majority (87%) of left-sided breast cancer patients. However, the results of previous study38 concluded that no significant difference in the in-field volume of the heart was observed between the supine and prone positions, which was consistent with our findings. Furthermore, Kim et al.40 suggested that the breast target volume for patients with small breasts (< 750 cm3) were no difference between the supine and prone positions. However, Kirby et al.35 also found that about two-thirds of breast cancer patients could benefit from the prone irradiation, especially for the protection to the heart and the left coronary artery. And further analysis showed that only a whole breast CTV > 1000 cm3 was associated with improved cardiac dosimetry under the prone position35. In our study, the enrolled patients with breast volumes less than 750 cm3 comprised 76% of all patients. Meanwhile, only 10% of the women had breast volumes > 1000 cm3. Therefore, even though the heart droops in the prone position, the irradiation field entered in heart was similar to the supine position.
In our study, deep inspiration breath hold (DIBH) was not used. To further reduce the dose to the heart and lung, DIBH could be used. Mulliez et al.41 verified that the ability and feasibility of prone deep inspiration breath hold to decrease the in-field volume of heart and lung for left-sided WBI. For prone positioning, there is a problem about postural repeatability. Although Deseyne et al.42 demonstrated that a newly developed crawl couch could improve precision and comfort and reduce set-up errors compared to the standard prone breast board in prone-WBI. Lakosi et al.43 analyzed respiratory motion of surgical clips, chest wall (CW) and the anterior displacement of the heart, results showed that prone position significantly reduced respiration related CW and surgical clip movements but increased anterior heart displacement. The study recommended daily online correction to maximize the heart protection effect in prone position.
Conclusion
Regardless of the supine or prone position, SIB-HFWBI offered more appropriate target coverage and lower doses to OARs, especially the IPSL, contralateral breast and heart, in left breast cancer patients. For both the SEB-HFWBI plan and SIB-HFWBI plans, the prone treatment showed a better dose conformance to the treatment target and a lower dose to the lung in the prone position than in the supine position. In summary, our study suggested that prone SIB-HFWBI might be more suitable for postoperative radiotherapy after breast-conserving surgery for early-stage breast cancer patients.
Abbreviations
- HF-WBI:
-
Hypofractionated whole-breast irradiation
- CFWBI:
-
Conventional fractionation whole-breast irradiation
- WBI:
-
Whole breast radiation
- 3DCT:
-
Three-dimensional computed tomography
- BCS:
-
Breast-conserving surgery
- FIMRT:
-
Forward intensity modulated radiation therapy
- SIB:
-
Simultaneous integrated boost
- SEB:
-
Sequential integrated boost
- TB:
-
Tumor bed
- CTV:
-
Clinical target volume
- PTV:
-
Planning target volume
- PTVTB :
-
Planning target volume for the TB
- WB:
-
Whole breast
- PTVWB :
-
Planning target volume for the WB
- OARs:
-
Organs at risk
- SLND:
-
Sentinel lymph node dissection
- ALND:
-
Axillary lymph node dissection
- UOQ:
-
Upper outer quadrant
- LOQ:
-
Lower outer quadrant
- UIQ:
-
Upper inner quadrant
- LIQ:
-
Lower inner quadrant
- CI:
-
Conformal index
- HI:
-
Homogeneity index
- V5:
-
The volumes that received ≥ 5 Gy
- V10:
-
The volumes that received ≥ 10 Gy
- V20:
-
The volumes that received ≥ 20 Gy
- V30:
-
The volumes that received ≥ 30 Gy
- V40:
-
The volumes that received ≥ 40 Gy
- Dmean:
-
The mean dose
- D2:
-
The doses covering 2% of the PTV
- D98:
-
The doses covering 98% of the PTV
- V100%:
-
The PTV coverage percent of the 100% prescribed line in the treatment plan
- MU:
-
Monitor unit
- DVH:
-
Dose-volume histogram
- CW:
-
Chest wall
- DIBH:
-
Deep inspiration breath hold
- PTVWB-PTVTB :
-
The target volume obtained by subtracting the PTVTB from the PTVWB
References
Clarke, M. et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: An overview of the randomised trials. Lancet 366(9503), 2087–2106. https://doi.org/10.1016/S0140-6736(05)67887-7 (2005).
Fisher, B. et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N. Engl. J. Med. 347(16), 1233–1241. https://doi.org/10.1056/NEJMoa022152 (2002).
Holli, K. et al. Radiotherapy after segmental resection of breast cancer with favorable prognostic features: 12-year follow-up results of a randomized trial. J. Clin. Oncol. 27(6), 927–932. https://doi.org/10.1200/JCO.2008.19.7129 (2009).
Fowler, J. F. The linear-quadratic formula and progress in fractionated radiotherapy. Br. J. Radiol. 62(740), 679–694. https://doi.org/10.1259/0007-1285-62-740-679 (1989).
Haviland, J. S. et al. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomized controlled trials. Lancet Oncol. 14(11), 1086–1094. https://doi.org/10.1016/S1470-2045(13)70386-3 (2013).
Andrade, T. R. M. et al. Meta-analysis of long-term efficacy and safety of hypofractionated radiotherapy in the treatment of early breast cancer. Breast 48, 24–31. https://doi.org/10.1016/j.breast.2019.08.001 (2019).
Dwyer, P., Hickey, B., Burmeister, E. & Burmeister, B. Hypofractionated whole-breast radiotherapy: Impact on departmental waiting times and cost. J. Med. Imag. Radiat. Oncol. 54(3), 229–234. https://doi.org/10.1111/j.1754-9485.2010.02163.x (2010).
Bartelink, H. et al. Whole-breast irradiation with or without a boost for patients treated with breast-conserving surgery for early breast cancer: 20-year follow-up of a randomised phase 3 trial. Lancet Oncol. 16(1), 47–56. https://doi.org/10.1016/S1470-2045(14)71156-8 (2015).
Whelan, T. et al. Randomized trial of breast irradiation schedules after lumpectomy for women with lymph node-negative breast cancer. J. Natl. Cancer Inst. 94(15), 1143–1150. https://doi.org/10.1093/jnci/94.15.1143 (2002).
Vrieling, C. et al. Prognostic factors for local control in breast cancer after long-term follow-up in the EORTC boost vs no boost trial: A randomized clinical trial. JAMA Oncol. 3(1), 42–48. https://doi.org/10.1001/jamaoncol.2016.3031 (2017).
Alford, S. L., Prassas, G. N., Vogelesang, C. R., Leggett, H. J. & Hamilton, C. S. Adjuvant breast radiotherapy using a simultaneous integrated boost: Clinical and dosimetric perspectives. J. Med. Imag. Radiat. Oncol. 57(2), 222–229. https://doi.org/10.1111/j.1754-9485.2012.02473.x (2013).
Bantema-Joppe, E. J. et al. Simultaneous integrated boost irradiation after breast-conserving surgery: Physician-rated toxicity and cosmetic outcome at 30 months’ follow-up. Int. J. Radiat. Oncol. Biol. Phys. 83(4), e471–e477. https://doi.org/10.1016/j.ijrobp.2012.01.050 (2012).
Teh, A. Y. et al. Concomitant intensity modulated boost during whole breast hypofractionated radiotherapy–a feasibility and toxicity study. Radiother. Oncol. 102(1), 89–95. https://doi.org/10.1016/j.radonc.2011.10.015 (2012).
Gupta, A. et al. 5-Year results of a prospective phase 2 trial evaluating 3-week hypofractionated whole breast radiation therapy inclusive of a sequential boost. Int. J. Radiat. Oncol. Biol. Phys. 105(2), 267–274. https://doi.org/10.1016/j.ijrobp.2019.05.063 (2019).
Paelinck, L. et al. Does an integrated boost increase acute toxicity in prone hypofractionated breast irradiation? A randomized controlled trial. Radiother. Oncol. 122(1), 30–36. https://doi.org/10.1016/j.radonc.2016.12.023 (2017).
Griem, K. L. et al. Three-dimensional photon dosimetry: A comparison of treatment of the intact breast in the supine and prone position. Int. J. Radiat. Oncol. Biol. Phys. 57(3), 891–899. https://doi.org/10.1016/s0360-3016(03)00723-5 (2003).
McKinnon, R. et al. The prone technique for breast irradiation - is it ready for clinical trials?. Breast 18(1), 30–34. https://doi.org/10.1016/j.breast.2008.09.006 (2009).
Veldeman, L. et al. Preliminary results on setup precision of prone-lateral patient positioning for whole breast irradiation. Int. J. Radiat. Oncol. Biol. Phys. 78(1), 111–118. https://doi.org/10.1016/j.ijrobp.2009.07.1749 (2010).
Smith, B. D. et al. Radiation therapy for the whole breast: Executive summary of an American Society for Radiation Oncology (ASTRO) evidence-based guideline. Pract. Radiat. Oncol. 8(3), 145–152. https://doi.org/10.1016/j.prro.2018.01.012 (2018).
Radiation therapy oncology group. Breast cancer atlas for radiation therapyplanning: consensus definitions. http://www.rtog.org/CoreLab/ContouringAtlases/BreastCancerAtlas.aspx (2009).
Wang, W. et al. Correlation between target motion and the dosimetric variance of breast and organ at risk during whole breast radiotherapy using 4DCT. Radiat. Oncol. 8, 111. https://doi.org/10.1186/1748-717X-8-111 (2013).
International Commission on Radiation Units and Measurements. Prescribing, recording, and reporting photon-beam intensity-modulated radiation therapy (IMRT). J. ICRU 10, 27–38 (2010).
Morrow, M. et al. Factors predicting the use of breast-conserving therapy in stage I and II breast carcinoma. J. Clin. Oncol. 19(8), 2254–2262. https://doi.org/10.1200/JCO.2001.19.8.2254 (2001).
Polednak, A. P. Trends in and predictors of breast-conserving surgery and radiotherapy for breast cancer in Connecti cut, 1988–1997[J]. Int. J. Radiat. Oncol. Biol. Phys. 53(1), 157–163. https://doi.org/10.1016/S0360-3016(01)02829-2 (2002).
START Trialists’ Group et al. The UK Standardisation of Breast Radiotherapy (START) Trial A of radiotherapy hypofractionation for treatment of early breast cancer: A randomised trial. Lancet Oncol. 9(4), 331–341. https://doi.org/10.1016/S1470-2045(08)70077-9 (2008).
START Trialists’ Group et al. The UK Standardisation of Breast Radiotherapy (START) Trial B of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet 371(9618), 1098–1107. https://doi.org/10.1016/S0140-6736(08)60348-7 (2008).
Whelan, T. J. et al. Long-term results of hypofractionated radiation therapy for breast cancer. N. Engl. J. Med. 362(6), 513–520. https://doi.org/10.1056/NEJMoa0906260 (2010).
Speers, C. & Pierce, L. J. Postoperative radiotherapy after breast-conserving surgery for early-stage breast cancer: A review. JAMA Oncol. 2(8), 1075–1082. https://doi.org/10.1001/jamaoncol.2015.5805 (2016).
Shaitelman, S. F. et al. Acute and short-term toxic effects of conventionally fractionated vs hypofractionated whole-breast irradiation: A randomized clinical trial. JAMA Oncol. 1(7), 931–941. https://doi.org/10.1001/jamaoncol.2015.2666 (2015).
Schmeel, L. C. et al. Acute radiation-induced skin toxicity in hypofractionated vs. conventional whole-breast irradiation: An objective, randomized multicenter assessment using spectrophotometry. Radiother. Oncol. 146, 172–179. https://doi.org/10.1016/j.radonc.2020.02.018 (2020).
Van Hulle, H. et al. Two-year toxicity of simultaneous integrated boost in hypofractionated prone breast cancer irradiation: Comparison with sequential boost in a randomized trial. Radiother. Oncol. 158, 62–66. https://doi.org/10.1016/j.radonc.2021.02.010 (2021).
Onal, C., Efe, E., Guler, O. C. & Yildirim, B. A. Dosimetric comparison of sequential versus simultaneous-integrated boost in early-stage breast cancer patients treated with breast-conserving surgery. In Vivo 33(6), 2181–2189. https://doi.org/10.21873/invivo.11720 (2019).
Van Parijs, H. et al. Breast conserving treatment for breast cancer: dosimetric comparison of sequential versus simultaneous integrated photon boost. Biomed. Res. Int. 2014, 827475. https://doi.org/10.1155/2014/827475 (2014).
Formenti, S. C., DeWyngaert, J. K., Jozsef, G. & Goldberg, J. D. Prone vs supine positioning for breast cancer radiotherapy. JAMA 308(9), 861–863. https://doi.org/10.1001/2012.jama.10759 (2012).
Kirby, A. M. et al. Prone versus supine positioning for whole and partial-breast radiotherapy: A comparison of non-target tissue dosimetry. Radiother. Oncol. 96(2), 178–184. https://doi.org/10.1016/j.radonc.2010.05.014 (2010).
Bergom, C. et al. Prone whole-breast irradiation using three-dimensional conformal radiotherapy in women undergoing breast conservation for early disease yields high rates of excellent to good cosmetic outcomes in patients with large and/or pendulous breasts. Int. J. Radiat. Oncol. Biol. Phys. 83(3), 821–828. https://doi.org/10.1016/j.ijrobp.2011.08.020 (2012).
Alonso-Basanta, M., Ko, J., Babcock, M., Dewyngaert, J. K. & Formenti, S. C. Coverage of axillary lymph nodes in supine vs. prone breast radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 73(3), 745–751. https://doi.org/10.1016/j.ijrobp.2008.04.040 (2009).
Osa, E. O. et al. Prone breast intensity modulated radiation therapy: 5-year results. Int. J. Radiat. Oncol. Biol. Phys. 89(4), 899–906. https://doi.org/10.1016/j.ijrobp.2014.03.036 (2014).
Lymberis, S. C. et al. Prospective assessment of optimal individual position (prone versus supine) for breast radiotherapy: volumetric and dosimetric correlations in 100 patients. Int. J. Radiat. Oncol. Biol. Phys. 84(4), 902–909. https://doi.org/10.1016/j.ijrobp.2012.01.040 (2012).
Kim, H. & Kim, J. Evaluation of the anatomical parameters for normal tissue sparing in the prone position radiotherapy with small sized left breasts. Oncotarget 7(44), 72211–72218. https://doi.org/10.18632/oncotarget.12662 (2016).
Mulliez, T. et al. Heart dose reduction by prone deep inspiration breath hold in left-sided breast irradiation. Radiother. Oncol. 114(1), 79–84. https://doi.org/10.1016/j.radonc.2014.11.038 (2015).
Deseyne, P. et al. Crawl positioning improves set-up precision and patient comfort in prone whole breast irradiation. Sci. Rep. 10(1), 16376. https://doi.org/10.1038/s41598-020-72702-3 (2020).
Lakosi, F. et al. Respiratory motion, anterior heart displacement and heart dosimetry: Comparison between prone (Pr) and supine (Su) whole breast irradiation. Pathol Oncol. Res. 21(4), 1051–1058. https://doi.org/10.1007/s12253-015-9932-9 (2015).
Acknowledgements
This manuscript was edited by American Journal Experts (AJE).
Funding
The National Key Research Program of China (No. 2016YFC0904700). National Natural Science Foundation of China (No. 81703038). Natural Science Foundation of Shandong Provence (No. ZR2017PH006). The Key Research Development Program of Shandong Province (No. 2017GSF18102). The Innovation Project of Shandong Academy of Medical Sciences (2019-04). The Academic Promotion Program of Shandong First Medical University (2019ZL002). The foundation of National Natural Science Foundation of China (81972863, 81627901 and 82030082). The National Key Research and Development Projects of China (2018YFC1312201) Radiation Oncology Innovate Unit, Chinese Academy of Medical Sciences (2019RU071). Taishan Scholars Program of Shandong Province (NO.ts 20190982).
Author information
Authors and Affiliations
Contributions
T.Y. and Y.L. contributed to the patients collection, data statistics and analysis and the manuscript writing. J.L. and J.Y. participated in the study design. T.S. made contributions in creating the HFWBI plan. M.X. contributed to review the delineation. W.W., Q.S., Y.Z. made important contributions in collecting data and revising the content.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yu, T., Li, Y., Sun, T. et al. A comparative study on hypofractionated whole-breast irradiation with sequential or simultaneous integrated boost on different positions after breast-conserving surgery. Sci Rep 11, 18017 (2021). https://doi.org/10.1038/s41598-021-97520-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-97520-z
This article is cited by
-
Prone versus supine free-breathing for right-sided whole breast radiotherapy
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.