Abstract
We assessed the role of [18F]FDG-PET/CT in identifying and managing cancer of unknown primary site (CUP syndrome). We reviewed [18F]FDG-PET/CT scans of individuals with CUP syndrome recorded in clinical referral letters from 2012 to 2019. We evaluated the identification of primary tumor (PT) by [18F]FDG-PET/CT, according to histological subtype, and the impact on clinical management. The median age was 65 years, 36/64 males (56%). PTs were detected in 28/64 (44%) patients. Detection was significantly lower in patients with squamous cell carcinoma (SCC) than with other histologies combined, p = 0.034. Mean age, mean SUVmax (10.6 ± 6.0) and organ involvement were similar between patients with and without discovered PTs; and between patients with SCC and with other histologies combined. However, those with SCC were less likely than the others to present with multi-lesion involvement, p < 0.001. [18F]FDG-PET/CT interpretations apparently affected treatment of 8/28 (29%) patients with PT detected, and in none of the 35 whose PT was not discovered, p < 0.001. [18F]FDG-PET/CT appeared helpful in detecting PT in almost half the patients with CUP syndrome; the lowest rate was for patients with SCC pathology. PET/CT showed limited overall value in guiding clinical management, however benefited those with discovered PT.
Similar content being viewed by others
Introduction
Cancer of unknown primary site (CUP syndrome) is a diverse group of cancers in which the anatomical site of origin remains occult despite detailed investigations. CUP syndrome accounts for 2–5% of cancers worldwide1,2. The median age at presentation is 60–65 years and diagnosis is more common in men than women by a ratio of 3:23. CUP syndrome has a wide variety of clinical presentations and many histological types. Sensitivity to treatment tends to be low and median survival time is 6–10 months4. Due to the difficulty of diagnosis and lengthy investigations, time from initial presentation to treatment is longer and pretreatment costs are higher in patients with CUP syndrome than in patients whose primary tumor site is known5.
CT and conventional MRI enable the detection of only 22–36% of the primary sites of CUP syndrome1,6. These low detection rates have been attributed to functional limitations of these imaging modalities. Both CT and MRI enable the detection of anatomical abnormalities and abnormal contrast enhancement; however, small and non-enhancing lesions in normal sized structures may be missed.
In contrast to conventional imaging modalities, F18-fluorodeoxyglucose PET/CT ([18F]FDG-PET/CT) does not have the abovementioned drawbacks as it leverages the increased glucose metabolism in many malignant cancers (Warburg effect) to detect abnormal uptake of the [18F]FDG7. However, in a head and neck area, CT/MRI is still a better diagnostic tool in assessing abnormalities than other modalities8,9. [18F]FDG-PET/CT is commonly used to search for occult primary tumors undetected by conventional diagnostic methods8,9. While the use of [18F]FDG-PET/CT in the detection of primary tumors (PTs) in CUP syndrome has been suggested for at least two decades3,10,11, its roles in the diagnostic workup of patients with disseminated CUP syndrome remain inconclusive12.
The primary purpose of this study was to assess the role of [18F]FDG-PET/CT in the identification of PTs, and therefore in the management of CUP syndrome in patients with negative conventional diagnostic workup. The secondary objectives were to evaluate the ability of [18F]FDG-PET/CT in discovering PTs according to their histological subtypes, and thus to evaluate the impact on clinical management.
Results
Of 33,679 [18F]FDG-PET/CT scans performed during the study period, 64 [18F]FDG-PET/CT included the term “Unknown Primary”. Demographic and clinical characteristics of the population are presented in Table 1. The study cohort comprised 36 males and 28 females; the median age was 65 years (range: 18–87). The patients had a total number of 145 [18F]FDG–avid lesion sites, with a mean SUVmax of 10.6 (range: 2.2–27.8). The most common sites of [18F]FDG uptake were the lymph nodes: 39/145 (27%), bones: 24 (17%), liver: 17 (12%), lungs: 17 (12%), regions of the head and neck: 7(5%) and brain: 6 (4%). The remaining 35 sites (23%) included the uro-gynecological system, esophagus, peritoneum, skin, colon, thyroid and muscles. The median number of sites/organs involved simultaneously in the same patient was 2 (range: 1–5), as follows: 20 patients (31%) had a finding in one site, 26 (41%) had findings in two sites, 11 (17%) in 3 sites, 5 (8%) in 4 sites, and 2 (3%) had findings in more than five sites. Additionally, most patients, 47/64 (73%), had a multi-lesion metastatic spread disease.
Tumor histologies included: 14 (22%) poorly/undifferentiated carcinomas, 10 (16%) squamous cell carcinoma (SCC), 9 (14%) adenocarcinomas, 1 (2%) neuroendocrine carcinoma and 30 (47%) tumors for which the origin was pathologically and immunohistochemically suggested (including: melanoma, thyroid, peritoneal mesothelioma, sarcoma, thyroid carcinoma, thymoma, ovarian carcinoma, gastrointestinal tumor, pancreatic and biliary carcinoma, carcinoma of breast and lung).
Primary tumors (PTs) discovered by [18F]FDG-PET/CT
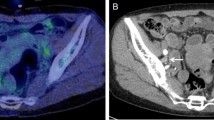
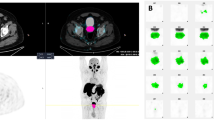
[18F]FDG-PET/CT discovered the PT in 28 patients (44%); while in the remaining 36 (56%), the PTs were not located. Table 2 presents the characteristics of the two groups. Differences in mean patient age) 61.2 vs. 61.9 years, p = 0.72), mean SUVmax of the lesions (10.3 vs 10.8, p = 0.08) and the mean number of organs/sites involved by [18F]FDG-avid lesions (2.4 vs. 1.8, p = 0.47) were not statistically different between patients with and without an identified PT. Variations in anatomic organs and sites involved by [18F]FDG-avid metastatic lesions were also similar between patients with and without discovered PTs. The rates of identification of PTs relative to the histologic groups were: 18/30 (60%) of patients with tumors for which the origin was pathologically and immunohistochemically suggested, 4/9 (44%) of adenocarcinomas, 4/14 (29%) of poorly differentiated carcinomas, 1/10 (10%) of SCC and the sole (100%) neuroendocrine carcinoma. [18F]FDG-PET/CT detection of PTs was significantly worse for the patients with SCC than for the 54 patients with other pathologies, considered as a combined group: 1/10 (10%) vs. 27/54 (50%), p = 0.03. The only patient in whom PT was detected was one of 7 (14.3%) patients with SCC with head and neck findings. Therefore, we decided to examine in more depth the subgroup of patients with SCC pathology and to compare their characteristics to the combined subgroup of patients with pathologies other than SCC (Table 3). Statistically significant differences were not observed between these two subgroups of patients, in mean age (61.7 vs 61.6 years, p = 0.85), mean SUVmax of [18F]FDG -avid lesions (11.3 vs 10.4, p = 0.38) and the mean number of involved organs/sites (1.5 vs 2.2, p = 0.09). Variations in anatomic organs and locations involved by [18F]FDG-avid metastatic lesions were similar between patients with SCC and those with the other histologies combined. However, among the patients with SCC, the proportion with multi-lesion spread was substantially lower than for the rest of the cohort: 2/10 (25%) vs. 45/54 (83%), p < 0.001, see Figs. 1, 2 and 3.
[18F]FDG-PET/CT and treatment management
Clinical data regarding treatment were available for 63 patients; of them, 31 (48%) received specific chemotherapy, 4 (6%) empiric chemotherapy and 6 (9%) palliative radiation; 5 (8%) underwent surgery and 6 (9%) chemo radiation. Eleven (17%) received best supportive care, because of their poor performance status and clinical situation. One patient with SCC histology, whose PT was not detected by PET/CT, was lost to follow up.
The [18F]FDG-PET/CT findings did not appear to affect the clinical management of 55 (87%) of the 63 patients with available data. Treatment was apparently affected in 8/28 (29%) patients with a PT detected by [18F]FDG-PET/CT: seven received chemotherapy that was specific to the diagnosis, and one patient received palliative radiotherapy.
Treatment was apparently not affected by the [18F]FDG-PET/CT scan in any of the 35 for whom the PT was not detected (p < 0.001, Table 2). Therefore, considering the entire cohort, [18F]FDG-PET/CT findings seem to have changed clinical management in 8/63 (13%) patients. Despite the much lower detection rates among patients with SCC, the effect of [18F]FDG-PET/CT findings on clinical management did not appear to differ between these patients and those with other pathologies combined: 1/9 (11%) vs. 7/54 (13%) (p = 1.0, Table 3).
Since tumors for which the origin was pathologically and immunohistochemically suggested comprised the largest subgroup of our cohort, 30 (47%), we compared changes in management between this subgroup of patients and all the other patients combined without a pathologically and immunohistochemically suggested origin. We found similar rates of treatment changes following [18F]FDG-PET/CT for the two subgroups: 4/30 (13%) and 4/33 (12%), respectively.
[18F]FDG-PET/CT detected a higher proportion of PTs among patients with tumors for which the origin was pathologically and immunohistochemically suggested than among all the other patients combined: 18/30 (60%) vs. 10/34 (30%), p = 0.014. However, detection of PTs affected treatment in a smaller proportion of the patients of the former than the latter, 4/18 (22%) vs. 4/10 (40%).
Discussion
More than one decade ago, a multidisciplinary expert panel of oncologists, radiologists and nuclear physicians recommended the use of [18F]FDG PET in the diagnosis of patients with CUP syndrome13. Despite the common use of this imaging technique in this context, data are sparse regarding the characteristics of CUP syndrome for which [18F]FDG-PET/CT is most and least effective. Interestingly, in the current study of patients with CUP syndrome and negative conventional imaging, [18F]FDG-PET/CT detected the PT in only 1 (10%) of the patients with SCC compared to 50% of all those with other pathologies. Nonetheless, the apparent effects of the [18F]FDG-PET/CT findings on clinical management were similar between these two groups: 11% vs. 13%. Thus, surprisingly, the greater detection of PTs in pathologies other than SCC compared to SCC did not have clinical implications.
Our overall rate of tumor detection was 44%, which is within the range of 10%-75% reported in other studies3,11,14,15,16,17,18. While CUP syndrome is a relatively common clinical entity, presentations and histologies are diverse9,19,20 . Notably, consensus has not been reached as to whether CUP syndrome is simply a group of metastatic tumors with an undetected source, or a distinct entity with its own characteristics and behavior17,21,22. Most researchers currently believe that CUP syndrome is a heterogeneous collection of metastatic tumors23. Accordingly, treatment strategies have shifted from empiric cytotoxic therapies to identifying the PT and targeting therapy at the tumor type24. Importantly, detecting PT sites and additional metastases improves disease staging; this helps define prognosis and can better guide surgical intervention with curative intent15. Indeed, several studies have shown longer survival times in patients with CUP syndrome in whom a PT was detected25,26.
Sixteen percent of the patients in the current cohort were with SCC. This is higher than the 5% rate of CUP syndrome that was reported in a number of publications19,27, but substantially lower than the 57% rate that was reported in another study11. Among the reasons for the wide discrepancy in rates are the lack of a standardized definition of CUP syndrome, including the clinical workup and imaging tests required for the diagnosis3,28, and the resultant heterogeneity in selection criteria. Of our 10 patients with SCC, 7 (70%) had [18F]FDG-PET/CT uptake in the head and neck. Similarly, head and neck cancers were reported to represent 75% of patients with CUP syndrome and SCC histology6. In our series, the PT was detected in one of 7 (14.3%) of our patients with SCC who had head and neck findings. Similarly, Majchrzak et al. reported detection of PT in 17% (7/41) of patients with CUP syndrome of cervical lymph nodes with SCC metastases29. This compares with the detection by [18F]FDG-PET/CT of PTs that were not detected by other modalities in 25% of the patients with headand neck metastases in another cohort30. Notably, despite our relatively high proportion of patients with SCC, the age and sex distributions were comparable to those reported in other studies of [18F]FDG-PET/CT in CUP syndrome3. Further, patients’ age, SUVmax of the lesions, and site distribution of [18F]FDG-avid lesions were similar between patients whose PT was and was not detected by [18F]FDG-PET/CT; and between patients with SCC and those with all other pathologies combined. Thus, the distributions of age, involved organ/site and [18F]FDG avidity do not explain the low detection of PTs among our patients with SCC compared to those with other pathologies. Interestingly, our patients with SCC tumors were significantly more likely to present with limited-lesion metastatic spread disease involvement than were patients with the other pathologies combined. We speculate that this finding is due to lower metastatic rates in SCC or to poor [18F]FDG-PET/CT uptake in small SCC metastases, or to a combination of the two. SCC was shown to have a lower ratio of metastases per PT than adenocarcinoma31, while [18F]FDG-PET/CT uptake in SCC was shown to be directly correlated to tumor size, and lower in metastatic tumors than in PTs32.
Changes in treatment were attributed to [18F]FDG-PET/CT detection of primary sites in 29% of our patients with a newly detected PT. However, considering the entire cohort, including patients for whom the PT was not detected, [18F]FDG-PET/CT apparently affected clinical management in only 13%. This is on the lower end of the range of 10–58% (mean 35%) that was reported in a review of 10 studies15. That review found that patients with a planned curative treatment for cancers such as breast, ovary and prostate most benefited from the [18F]FDG-PET/CT scan; thus, differences between studies in the types of cancers may explain the large variability in detection rates15.
While its impact on clinical management may be limited to a subgroup of patients with discovered PT, [18F]FDG-PET/CT may have additional benefits for patients with CUP syndrome. This may explain disparities between studies in the interpretation of the usefulness of [18F]FDG-PET/CT for clinical decisions. Notably, Reinert et al.11 reported a PT detection rate in only 23% of patients with CUP syndrome, but changes in treatment management in twice the number of patients11. [18F]FDG-PET/CT has been recommended for accurate staging, monitoring of treatment response and follow-up in patients with CUP syndrome who undergo active therapy; and as an alternative to contrast CT in patients with severe iodine dye allergy12. Moreover, the use of [18F]FDG-PEFT/CT in place of conventional imaging may lead to earlier diagnosis of the PT and thus facilitate earlier targeted therapy15.
We acknowledge several limitations to this retrospective study. Our database search relied on proper documentation of the disease in the [18F]FDG-PET/CT reports and could therefore present an incomplete sample of patients from our institution. We did not have data regarding the workups that patients underwent according to their clinical presentations. Larger studies of patients with various pathologies and tumor sites are needed to better define the role of [18F]FDG-PET/CT in CUP syndrome and to identify the CUP syndrome subtypes whose management is most influenced by [18F]FDG-PET/CT results.
Conclusion
[18F]FDG-PET/CT appeared helpful in detecting PT in almost half the patients with CUP syndrome; the lowest rate was for patients with SCC pathology. [18F]FDG-PET/CT showed limited overall value in guiding clinical management, however benefited those with discovered PT.
Materials and methods
Study design
We searched the Sheba Medical Center computerized database for [18F]FDG-PET/CT studies that included the term "Unknown Primary" in reports (in the graph of “indication” for the referral) recorded from April 2012 through February 2019. Medical history and tumor histopathology analysis were included in the clinical data. Imaging data were provided from the picture archive and communication system (PACS, Carestream Health 11.0, Rochester, NY), and clinical data from the computerized medical records at Sheba Medical Center.
The study inclusion criterion was an unknown PT according to the clinical referral letter at the performance of the [18F]FDG-PET/CT scan, with or without known tumor histology. For all the patients, the following were performed before [18F]FDG-PET/CT examination: a whole diagnostic workup including a physical examination, CT/MRI or US, and rhino-laryngoscopy in patients with cervical CUP syndrome. Pathological evaluation included immunohistochemical staining for tumor origin.
For this research, tumors were categorized into broad groups based on their histology type: adenocarcinoma, squamous cell carcinoma (SCC), poorly/undifferentiated carcinoma, neuroendocrine carcinoma and tumors in which the origin was pathologically and immunohistochemically suggested. The latter group was relevant when histology results from one of the metastatic lesions indicated a certain tumor origin (including: melanoma, thyroid, peritoneal mesothelioma, sarcoma, thyroid carcinoma, thymoma, ovarian carcinoma, gastrointestinal tumor, pancreatic and biliary carcinoma, carcinoma of breast and lung); however, prior conventional workup did not reveal the primary tumor site. Patients without any available histological data were not included in the study.
Metastatic spread was characterized by variations in anatomic organs or sites involved by [18F]FDG-avid metastatic lesions. Additionally, metastatic spread was evaluated according to the number of [18F]FDG-avid metastatic lesions and classified as a limited-lesion (up to two lesions) or as multi-lesion spread (more than two lesions).
Image assessment
An experienced physician with two specializations (nuclear medicine and radiology) reviewed all the scans of all the patients included in the study. The intensity of [18F]FDG uptake in the lesions was calculated by standardized uptake values max (SUVmax), by manually generating a region of interest over the pathological lesion. The protocol of the [18F]FDG-PET/CT scans was similar to those described in previously reported studies33,34.
We assessed the impact on clinical management, of PT detection by PET/CT, by examining treatment decisions that were made by a referring physician or by a tumor board, and that were influenced by the identification or non-identification of PT. The performance of additional diagnostic procedures after a [18F]FDG-PET/CT study was not considered a change in management.
Statistical analysis
Data are demonstrated as medians with ranges, or as means with standard deviations for continuous variables; and as percentages for categorical parameters. Correlations between subgroups were analyzed using the T-test for continuous variables, and the chi-square test and Fisher's exact test for categorical variables. The SPSS version 25.0 (SPSS, IBM, USA) was used. A P value of less than 0.05 was considered statistically significant.
Ethics
The institutional review board of Sheba Medical Center approved our single-institution study, and informed consent was waived due to the retrospective design. All the methods were performed in accordance with the relevant guidelines and regulations of Sheba Medical Center.
Data availability
All data developed for and used in this study is available upon request of the authors.
Abbreviations
- CUP:
-
Cancer of unknown primary site
- PT:
-
Primary tumor
- SCC:
-
Squamous cell carcinoma
- PET:
-
Positron emission tomography
- [18F]FDG:
-
18-Fluorine-fluorodeoxyglucose
- SUVmax:
-
Maximum standardized uptake values
- MIP:
-
Maximum intensity projection
- MRI:
-
Magnetic resonance imaging
- CT:
-
Computed tomography
- US:
-
Ultrasound
References
Pavlidis, N. & Fizazi, K. Carcinoma of unknown primary (CUP). Crit. Rev. Oncol. Hematol. 69, 271–278 (2009).
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2019. CA. Cancer J. Clin. https://doi.org/10.3322/caac.21551 (2019).
Burglin, S. A., Hess, S., Høilund-Carlsen, P. F. & Gerke, O. 18F-FDG PET/CT for detection of the primary tumor in adults with extracervical metastases from cancer of unknown primary. Medicine (Baltimore) 96, e6713 (2017).
Fizazi, K., Greco, F. A., Pavlidis, N. & Pentheroudakis, G. Cancers of unknown primary site: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. https://doi.org/10.1093/annonc/mdr389 (2011).
Walker, M. S., Weinstein, L., Luo, R. & Marino, I. Pretreatment costs of care and time to initial treatment for patients with cancer of unknown primary. J. Comp. Eff. Res. 7, 523–533 (2018).
Pavlidis, N. & Pentheroudakis, G. Cancer of unknown primary site. Lancet 379, 1428–1435 (2012).
Kwee, T. C., Basu, S., Cheng, G. & Alavi, A. FDG PET/CT in carcinoma of unknown primary. Eur. J. Nucl. Med. Mol. Imaging 37, 635–644 (2010).
Maghami, E. et al. Diagnosis and management of squamous cell carcinoma of unknown primary in the head and neck: ASCO guideline. J. Clin. Oncol. 38, 2570–2596 (2020).
Huasong, H., Shurui, S., Shi, G. & Bin, J. Performance of 18 F-FDG-PET/CT as a next step in the search of occult primary tumors for patients with head and neck squamous cell carcinoma of unknown primary: A systematic review and meta-analysis. Clin. Transl. Imaging 2021, 1–11. https://doi.org/10.1007/S40336-021-00429-W (2021).
Rege, S. et al. Use of positron emission tomography with fluorodeoxyglucose in patients with extracranial head and neck cancers. Cancer https://doi.org/10.1002/1097-0142(19940615)73:12%3c3047::AID-CNCR2820731225%3e3.0.CO;2-# (1994).
Reinert, C. P., Sekler, J., la Fougère, C., Pfannenberg, C. & Gatidis, S. Impact of PET/CT on clinical management in patients with cancer of unknown primary—A PET/CT registry study. Eur. Radiol. 30, 1325–1333 (2020).
Varadhachary, G. R. Carcinoma of unknown primary: Focused evaluation. J. Natl. Compr. Canc. Netw. 9, 1406–1412 (2011).
Fletcher, J. W. et al. Recommendations on the use of 18F-FDG PET in oncology. J. Nucl. Med. https://doi.org/10.2967/jnumed.107.047787 (2008).
Dong, M. et al. Role of fluorodeoxyglucose-PET versus fluorodeoxyglucose-PET/computed tomography in detection of unknown primary tumor: A meta-analysis of the literature. Nucl. Med. Commun. 29, 791–802 (2008).
Sève, P., Billotey, C., Broussolle, C., Dumontet, C. & Mackey, J. R. The role of 2-deoxy-2-[f-18]fluoro-D-glucose positron emission tomography in disseminated carcinoma of unknown primary site. Cancer 109, 292–299 (2007).
Kwee, T. C. & Kwee, R. M. Combined FDG-PET/CT for the detection of unknown primary tumors: Systematic review and meta-analysis. Eur. Radiol. 19, 731–744 (2009).
Greco, F. A. et al. Cancer of unknown primary: Progress in the search for improved and rapid diagnosis leading toward superior patient outcomes. Ann. Oncol. https://doi.org/10.1093/annonc/mdr306 (2012).
Cetin Avci, N., Hatipoglu, F., Alacacıoglu, A., Bayar, E. E. & Bural, G. G. FDG PET/CT and conventional imaging methods in cancer of unknown primary: An approach to overscanning. Nucl. Med. Mol. Imaging 52, 438–444 (2018).
Zytoon, A. A., Elsayed, E. E., Nassar, A. I. & Murakami, K. Pivotal role of PET/CT in characterization of occult metastasis with undetermined origin. Egypt. J. Radiol. Nucl. Med. 51, 1–11 (2020).
Mohamed, D. M. & Kamel, H. A. Diagnostic efficiency of PET/CT in patients with cancer of unknown primary with brain metastasis as initial manifestation and its impact on overall survival. Egypt. J. Radiol. Nucl. Med. 52, 1–8 (2021).
Pentheroudakis, G., Briasoulis, E. & Pavlidis, N. Cancer of unknown primary site: Missing primary or missing biology?. Oncologist 12, 418–425 (2007).
van de Wouw, A. J., Jansen, R. L. H., Speel, E. J. M. & Hillen, H. F. P. The unknown biology of the unknown primary tumour: A literature review. Ann. Oncol. 14, 191–196 (2003).
Varadhachary, G. R. & Raber, M. N. Cancer of unknown primary site. N. Engl. J. Med. 371, 757–765 (2014).
Varadhachary, G. & Abbruzzese, J. L. Carcinoma of unknown primary. Abeloff’s Clin. Oncol. 2020, 1694–1702. https://doi.org/10.1016/B978-0-323-47674-4.00091-8 (2020).
Haas, I., Hoffmann, T. K., Engers, R. & Ganzer, U. Diagnostic strategies in cervical carcinoma of an unknown primary (CUP). Eur. Arch. Otorhinolaryngol. 259, 325–333 (2002).
Raber, M. N., Faintuch, J., Abbruzzese, J. L., Sumrall, C. & Frost, P. Continuous infusion 5-fluorouracil, etoposide and cis-diamminedichloro platinum in patients with metastatic carcinoma of unknown primary origin. Ann. Oncol. 2, 519–520 (1991).
Greco, F. A. & John, D. H. Cancer of unknown primary site. in Cancer: Principles & Practice of Oncology 10th edn (eds DeVita, V. T. et al. 1720–1739 (Lippincott Williams & Wilkins, 2014).
Taylor, M. B., Bromham, N. R. & Arnold, S. E. Carcinoma of unknown primary: key radiological issues from the recent National Institute for Health and Clinical Excellence guidelines. Br. J. Radiol. 85, 661–671 (2012).
Majchrzak, E., Cholewiński, W. & Golusiński, W. Carcinoma of unknown primary in the head and neck: The evaluation of the effectiveness of 18F-FDG-PET/CT, own experience. Rep. Pract. Oncol. Radiother. 20, 393 (2015).
Rusthoven, K. E., Koshy, M. & Paulino, A. C. The role of fluorodeoxyglucose positron emission tomography in cervical lymph node metastases from an unknown primary tumor. Cancer 101, 2641–2649 (2004).
Budczies, J. et al. The landscape of metastatic progression patterns across major human cancers. Oncotarget 6, 570–583 (2015).
Yen, T.-C. et al. 18F-FDG uptake in squamous cell carcinoma of the cervix is correlated with glucose transporter 1 expression. J. Nucl. Med. 45, 22–29 (2004).
Davidson, T. et al. Fat necrosis after abdominal surgery: A pitfall in interpretation of FDG-PET/CT. Eur. Radiol. 28, 2264–2272 (2018).
Ben Shimol, J. et al. The utility of PET/CT in large vessel vasculitis. Sci. Rep. 10, 1–10 (2020).
Funding
No specific funding was received from any bodies in the public, commercial or not-for-profit sectors to carry out the work described in this article.
Author information
Authors and Affiliations
Contributions
E.N. and T.D. designed the study. A.Z. provided statistical advice for this manuscript. E.N. and T.D. gathered the imaging data. T.D. interpreted the data, I.B. designed the work and revised the article, U.A., L.B., A.Z., D.U., I.B. and T.D. wrote the manuscript. T.D. prepared the figures and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nissan, E., Amit, U., Baron, L. et al. The usefulness of [18F]FDG-PET/CT in detecting and managing cancers with unknown primary site depends on histological subtype. Sci Rep 11, 17732 (2021). https://doi.org/10.1038/s41598-021-96451-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-96451-z
This article is cited by
-
Diagnostic accuracy of contrast-enhanced computed tomography in assessing cervical lymph node status in patients with oral squamous cell carcinoma
Journal of Cancer Research and Clinical Oncology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.