Abstract
Prognosis of patients with HER2+ breast-to-brain-metastasis (BBM) is dismal even after current standard-of-care treatments, including surgical resection, whole-brain radiation, and systemic chemotherapy. Radiation and systemic chemotherapies can also induce cytotoxicity, leading to significant side effects. Studies indicate that donor-derived platelets can serve as immune-compatible drug carriers that interact with and deliver drugs to cancer cells with fewer side effects, making them a promising therapeutic option with enhanced antitumor activity. Moreover, human induced pluripotent stem cells (hiPSCs) provide a potentially renewable source of clinical-grade transfusable platelets that can be drug-loaded to complement the supply of donor-derived platelets. Here, we describe methods for ex vivo generation of megakaryocytes (MKs) and functional platelets from hiPSCs (hiPSC-platelets) in a scalable fashion. We then loaded hiPSC-platelets with lapatinib and infused them into BBM tumor-bearing NOD/SCID mouse models. Such treatment significantly increased intracellular lapatinib accumulation in BBMs in vivo, potentially via tumor cell-induced activation/aggregation. Lapatinib-loaded hiPSC-platelets exhibited normal morphology and function and released lapatinib pH-dependently. Importantly, lapatinib delivery to BBM cells via hiPSC-platelets inhibited tumor growth and prolonged survival of tumor-bearing mice. Overall, use of lapatinib-loaded hiPSC-platelets effectively reduced adverse effects of free lapatinib and enhanced its therapeutic efficacy, suggesting that they represent a novel means to deliver chemotherapeutic drugs as treatment for BBM.
Similar content being viewed by others
Introduction
The survival of patients with primary breast cancer has increased substantially due to the development of therapies targeting systemic disease1,2. Nonetheless, metastases, which are responsible for 90% of all cancer deaths3, remain a major challenge in breast cancer4. Patients diagnosed with brain metastases originating from primary breast tumors have a dismal 1-year survival rate of ~ 20%2,5. Among patients with metastatic breast cancer, ~ 30% develop brain metastases, and those with HER2 + breast-to-brain metastases (BBMs) have significantly worse outcomes5,6,7,8,9.
The current standard-of-care treatment for BBMs includes surgical removal, whole-brain radiation therapy, and systemic chemotherapy with lapatinib (a selective small-molecule dual-tyrosine kinase inhibitor of HER2 and EGFR)10,11,12,13,14. Studies show that lapatinib treatment after whole-brain radiation therapy can improve survival of patients with HER2-positive breast cancer with multiple brain metastasis but can at the same time promote significant neurological symptoms15. Monotherapy with lapatinib has shown partial and stable responses in 8% and 16%, respectively, of BBM patients16. Lapatinib treatment also improves overall survival by preventing development of brain metastasis16. Nonetheless, the relatively poor therapeutic effects of lapatinib are likely due to low bioavailability following oral administration as well as to drug efflux caused by activity of P-glycoprotein (P-GP) and breast cancer resistance protein (BCRP) at the BBB (blood brain barrier), which limits lapatinib delivery to the brain17,18,19,20. Interestingly, clinical use of lapatinib is further restricted because of its extensive albumin binding capacity, and poor aqueous solubility. Therefore, there is an urgent need for a new therapeutic strategy to enhance brain delivery of lapatinib and improve its efficacy against early brain metastasis. Towards this various groups have developed lapatinib bound into lipoprotein like nanoparticles20,21,22,23,24. Since HER2+ BBM treatments, including lapatinib, are associated with limited availability and significant cytotoxicity associated with neurological symptoms25, several research groups have developed more targeted therapy using liposomes, polymeric nanoparticles, or polymeric micelles26 to deliver chemotherapeutic drugs directly to cancer cells or cancer tissues20,21,23,24,25,27,28,29,30,31,32. However, these systems face several limitations, including nanotoxicity, low biodegradability, low clearance, adverse immune responses, and short in vivo circulation time33.
Platelets are anucleate, cellular fragments derived from megakaryocyte membranes predominantly present in bone marrow34,35,36,37. Novel drug delivery systems have been designed to mimic various properties possessed by platelets, such as their ability to adhere to and deliver toxic drugs to tumor cells38,39. Most of these types of delivery systems utilize platelet membranes and require a complex production process. Platelet membrane-cloaked nanoparticles have also been considered but are still less biocompatible as they induce an immune response in vivo40,41,42,43. Unlike nanoparticle-based drug delivery systems, platelets are naturally cleared from the body by reticuloendothelial cells in liver and spleen44,45,46,47,48,49. Platelets harboring encapsulated agents have demonstrated systemic clearance similar to donor-derived platelets28,39. Previous studies report that platelets are activated by tumor cells and adhere to tumor cells, a phenomenon referred to as tumor cell-induced platelet aggregation (TCIPA), which can also increase metastasis 50. Activated platelets release contents from their granules, and platelets loaded with drugs (such as doxorubicin 51) release those drugs in the proximity of tumor site50. However, cancer patients undergoing chemotherapy can be thrombocytopenic, and cancer patients who receive multiple allogenic drug-loaded platelet transfusions could develop refractoriness to platelets due to HLA alloreactivity and subsequently require transfusions with HLA-matched donor platelets loaded with drugs52,53,54. Finding alternative sources of non-immunogenic, high-quality autologous platelets could reduce these risks.
Since human induced pluripotent stem cells (hiPSCs) are a potentially replenishable source of transfusable drug-loaded HLA negative (Universal) or autologous or HLA matched platelets, our primary objectives were to develop a clinically adaptable protocol to generate hiPSC-derived megakaryocytes and platelets in vitro and then determine whether those hiPSC-platelets could serve as drug carriers to target HER2+ BBMs in vitro and in mouse models. In vivo, lapatinib-loaded hiPSC-platelets targeted BBMs and achieved a longer retention time than synthetic drug delivery systems or free drugs. Encapsulated lapatinib appeared to escape immunosurveillance and delivered drug in the vicinity of tumor cells via TCIPA, apparently circumventing the blood–brain barrier (BBB), which can interfere with treatment of brain metastases55. We conclude that drug-loaded hiPSC-platelets exhibit enhanced therapeutic efficacy at potentially reduced drug dosages, limiting damage to normal tissues, and could serve as an efficient drug carrier to treat HER2+ BBM tumors in brain.
Results
Differentiation of human hiPSC lines into megakaryocytes and functional characterization of hiPSC-platelets
Feeder-free hiPSCs can reportedly be differentiated into megakaryocytes and platelets through hemogenic endothelial-like cell and hematopoietic progenitor intermediates56,57,58,59,60,61. Using published methodologies with modifications, we generated a population containing ~ 60–80% CD41a+ CD42b+ (double-positive) platelets from hiPSC-derived megakaryocytes in serum- and feeder-free conditions in a scalable PBS biotech bioreactor, using KP457 (ADM17 inhibitor)62, GNF451 (AHR antagonist)56,63, Y27632 (ROCK inhibitor)57,60, and barasertib (aurora kinase inhibitor)64, as shown in scheme Fig. 1A,B and described in detail in the Methods and Materials section. HiPSC lines used for megakaryocyte and platelet derivation expressed stemness markers, exhibited canonical hiPSC colony morphology (Supplementary Fig. S1B and F), and showed a normal karyotype (Supplementary Fig. S1C).
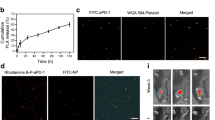
Differentiation of human hiPSC cell lines into megakaryocytes and functional characterization of hiPSC-platelets. (A) Schematic showing the stepwise differentiation of hiPSCs into immature CD41+ megakaryocytes. (B) Schematic showing the stepwise maturation of hiPSC-derived immature CD41+ megakaryocytes and terminal differentiation to produce pro-platelets (hiPSC-platelets). (C) Flow cytometry-based size profile of adult human donor-derived platelets and Day 6 and Day 7 hiPSC-platelets (top), and the percentage of CD41a+CD42b+ donor-derived platelets and hiPSC-platelets (bottom). (D) Time-dependent increase in the CD41a+CD42b+ platelet-generating ability of hiPSC-derived megakaryocytes from Day 5 to Day 7 (mean ± SD, n = 3). * indicates p < 0.001 compared to Day 5 hiPSC-platelets. (E) Transmission electron micrographs of adult human donor-derived platelets and Day 7 hiPSC-platelets. Colored arrows indicate organelles: blue, granules; green, glycogen granules; red, mitochondria; and violet, OCS (open canalicular system). Scale bar = 400 nm. (F) LTA-based aggregation assays of donor-derived platelets and hiPSC-platelets stimulated with 20 µM ADP and 20 µM TRAP, showing time-dependent variability. (mean ± SD, n = 3). (G) Surface PAC1 and P-Sel activation of donor-derived platelets and hiPSC-platelets by 20 µM ADP and 20 µM TRAP, measured via flow cytometry (Fortessa). Orange bars represent unstimulated platelets, and blue bars represent platelets activated upon exposure to ADP and TRAP (mean ± SD, n = 3). (H) Representative flow cytometry (Fortessa) plots showing surface PAC1 and P-Sel activation of donor-derived platelets and hiPSC-platelets by 20 µM ADP and 20 µM TRAP. Orange contour plots represent unstimulated platelets, and blue contour plots represent platelets activated upon exposure to ADP and TRAP.
Culturing of Day 0 immature megakaryocytes (Supplementary Fig. S2A (Gating Strategy)) in STEMspan Megakaryocyte Expansion Supplement (STEMCELL Technologies) and STEMspan ACF medium, along with addition of barasertib starting on Day 2, increased the ploidy of immature CD41a+ CD42b+ megakaryocytes (Supplementary Fig. S2A (Gating Strategy)) by Day 4 (Supplementary Fig. S1A (Scheme) and D), which was used as a surrogate marker for megakaryocyte maturation (“ ≥ 8n ploidy” served as a marker of maturation). Addition of barasertib has been previously shown to promote generation of polyploid megakaryocytes (Supplementary Fig. S1D and S2B) and increased terminal differentiation to matured megakaryocytes to platelets64. Similarly, megakaryocytes cultured in ultra-low adherent culture plates along with barasertib produced ~ 30 Calcein Blue AM+ Annexin-V- CD41a+ CD42b+ platelets per megakaryocyte cell by day 7 (Fig. 1C,D) (Fig. 1C, Supplementary Fig. S2C), indicating that our protocol can successfully differentiate hiPSC cells through the stages of immature diploid megakaryocytes (Supplementary Fig. S2A), to maturation (Supplementary Fig. S2B) and to terminal differentiation of platelets (Supplementary Fig. S2C).
Large, highly polyploid (Supplementary Fig. S1D) megakaryocytes (Supplementary Fig. S1E) contained demarcation membrane systems and organelles, including mitochondria, granules, and multiple nuclei, and were capable of generating distinct pro-platelets (Supplementary Fig. S1G) by culture days 5–7 (Fig. 1D). Platelets can be damaged by extracellular metalloproteinases, which shorten their time in circulation and promote loss of surface CD42b expression relative to CD41 (αIIb)65,66. To determine if pro-platelets were differentiated and intact, we examined CD41a and CD42b expression in living (Calcein Blue AM+) and non-apoptotic (Annexin V−) hiPSC platelets purified by BSA gradient segregation (Fig. 1C,D and Supplementary Fig. S2C) and compared expression to infused donor platelets (Supplementary Fig. S2C–E). We observed no or minimal loss of surface CD42b on iPS-derived relative to donor platelets. Approximately 80% of CD41+ hiPSC-derived platelets were CD42b+. Another ~ 10% of CD41+ hiPSC-derived platelets were smaller in size and granularity relative to the larger proportion of CD41+CD42b+ hiPSC-derived platelets. We conclude that this 10% was non-functional and did not count them as platelets (Supplementary Fig. S2E). Using platelets from human blood donors to establish size gating (Fig. 1C and Supplementary Fig. S2C), we observed that the ~ 80% of hiPSC-platelets generated above expressed CD41a and CD42b comparably to donor platelets. We then examined the kinetics of platelet production from maturing megakaryocytes by flow cytometry-based counting of CD41+CD42b+ platelet-sized particles and found that platelet levels began to increase on day 5 and peaked at day 7 (Fig. 1D).
Transmission electron micrography (TEM) analysis demonstrated that hiPSC-platelets were ultrastructurally identical to human donor platelets (Fig. 1E): hiPSC-platelets were anucleate and possessed organelles seen in adult human blood donor platelets, including mitochondria, OCSs (open canalicular systems), and granules. We then performed light transmission aggregometry (LTA), a method used clinically to evaluate platelet function in vitro67,68,69, on hiPSC-platelets generated in vitro from hiPSCs and showed that 3 × 107 resting hiPSC-platelets resuspended in human plasma reached 50–60% aggregation at all time points analyzed after exposure to 20 µM ADP and TRAP (Fig. 1F). Donor platelets exhibited stronger aggregation and reached 85–80% aggregation under the same conditions. To assess platelet activation, we performed activation assays using a FITC-conjugated PAC1 antibody, which binds to the activated conformation of αIIbβ3 integrin, and a PE-conjugated P-Selectin monoclonal antibody, which binds to P-selectin (P-Sel) on the surface of granules in activated platelets. In response to ADP and TRAP treatment, hiPSC-platelets showed stronger PAC1 binding than unstimulated controls (Fig. 1G,H and Supplementary Fig. S3A). PAC1 and P-Sel activation profiles of hiPSC-platelets were similar to those of donor platelets, although some hiPSC-platelets showed pre-activation (Fig. 1G,H), as has been reported by others 66.
Functional comparison of lapatinib-loaded and non-loaded hiPSC-derived platelets
We next confirmed that our hiPSC-platelets could encapsulate drugs by assessing uptake of the fluorescent probe coumarin-6 (C6, 0.1%) by fluorescence imaging (Fig. 2A). We then demonstrated that doxorubicin could be encapsulated into hiPSC-derived platelets using the same protocol, suggesting that lapatinib could be similarly encapsulated (Supplementary Fig. S4A). We and others51,70,71,72 previously demonstrated that donor platelets can take up drug via passive transfer (Supplementary Fig. S4A). We calculated the encapsulation efficiency (a number that describes the extent of drug encapsulation) to be 93% in hiPSC-platelets, while drug loading capability (which indicates the amount of drug loaded per unit weight of hiPSC-platelets) to be 49% (Fig. 2B), the latter suggesting that the volume ratio of hiPSC-platelets to lapatinib was ~ 1:2. Interestingly, pH modulated lapatinib release in vitro: release was slower at pH 7.4 but more rapid at pH 6.5. Accordingly, at pH 6.5, ~ 80% of loaded lapatinib was released into the buffer within 40 h, as compared to ~ 40% release at pH 7.4 within 40 h (Fig. 2C).
Functionality characterization and comparison of lapatinib-loaded hiPSC-platelets to non-loaded hiPSC-platelets. (A) Fluorescence image of hiPSC-platelets under an oil immersion lens (×100), demonstrating the in vitro cellular uptake of fluorescent C6 (green). Scale bars = 10 µm. Inset zoomed in images of individual C6-loaded hiPSC-platelets are shown on the right. (B) Encapsulation efficiency and drug loading capability of lapatinib-loaded hiPSC-platelets with different concentrations of lapatinib (means ± SD, n = 3). ** indicates p < 0.01. (C) Analysis of lapatinib release kinetics from hiPSC- and donor-derived platelets in PBS at pH values of 6.5 and 7.4 at 37 °C over 40 h. (D) Transmission electron micrograph of non-loaded hiPSC and donor-derived platelets and platelets loaded with 25 µM lapatinib. Colored arrows indicate organelles: blue, granules; green, glycogen granules; red, mitochondria; and violet, OCS. Scale bar = 400 nm. (E) LTA-based aggregation assays of non-loaded and 25 µM lapatinib-loaded hiPSC- and donor-derived platelets stimulated with 20 µM ADP and 20 µM TRAP, showing time-dependent variability (mean ± SD, n = 3). (F) Representative flow cytometry (Fortessa) plots showing the surface PAC1 and P-Sel activation of non-loaded and 25 µM lapatinib-loaded hiPSC- and donor-derived platelets by 20 µM ADP and 20 µM TRAP. Orange contour plots represent unstimulated platelets, and blue contour plots represent platelets activated upon exposure to ADP and TRAP.
Additional TEM analysis indicated that both lapatinib-loaded and non-loaded platelets showed organelles such as mitochondria, granules and glycogen granules, and that lapatinib-loaded platelets exhibited normal morphology (Fig. 2D). Lapatinib-loaded hiPSC-platelets and donor platelets showed comparable activation capacity in vitro based on surface expression of PAC1 and P-Sel (Fig. 2F and Supplementary Fig. S3A), as well as a similar ability to aggregate in vitro after ADP and TRAP exposure (Fig. 2E). These analyses confirm that hiPSC-platelets maintain integrity and biological function after lapatinib loading and retain properties of bona fide human platelets.
Anti-tumor effects of lapatinib-loaded hiPSC-platelets against BBM cells
We next analyzed viability of BBM1 lines established from HER2+ patient-derived BBM tumors6,41,73 after treating cultures with hiPSC-platelets loaded with varying lapatinib concentrations (20 to 100 µM). At 24 h Lapatinib IC50 was 24.6 µM, a value that progressively decreased to 7.9 and 0.49 µM at 48 h to 72 h, respectively (Fig. 3A). Given this finding plus the fact that the optimal drug loading capability of hiPSC-platelets was ~ 49% (or a 1:2 volume ratio), (Fig. 2B), we chose to load 24.6 µM lapatinib into hiPSC-platelets by incubating them in twice the desired concentration, namely, 49.0 µM lapatinib. We then used Boyden chambers to measure cytotoxicity of lapatinib-loaded hiPSC- and donor-platelets, as well as that of free lapatinib, against BBM1 or BBM2 lines (Fig. 3B). After incubation for 24, 48, and 72 h, free lapatinib reduced cell viability to 45.306%, 38.14%, and 27.17%, respectively, whereas lapatinib-loaded hiPSC- or donor-platelets reduced cell viability to 25.06%, 21.38%, and 7.18%, respectively, relative to unloaded platelet controls (Fig. 3B). Subsequent analyses performed in Boyden chambers of surface Annexin-V on BBM cells exposed 72 h to lapatinib-loaded hiPSC- or donor-platelets versus non-loaded controls indicated that exposure to both types of drug-loaded platelets significantly increased surface Annexin-V levels, while exposure to non-loaded controls had little or no effect Annexin-V positivity (Fig. 3C,D). RT-qPCR analysis of FACS-sorted CD326+ BBM cells revealed significant upregulation of the pro-apoptotic genes BAD, BAK, BAX, and p53 in BBM cells exposed to lapatinib-loaded hiPSC-platelets relative to those exposed to non-loaded control hiPSC-platelets (Fig. 3E), indicative of apoptosis. To determine whether BBM cells contained lapatinib delivered by hiPSC-platelets, we first loaded hiPSC-platelets with saline, the fluorescent probe C6, or a 1:1 mixture of lapatinib and C6 and analyzed C6 accumulation in BBM cells by flow cytometry (Fig. 3F and Supplementary Fig. S5A). We then isolated C6-positive BBM cells by FACS and analyzed lapatinib concentrations in these cells via LC–MS/MS (Supplementary Fig. S5B) as well as viability using CCK8 assays (Fig. 3G). Lapatinib levels were significantly elevated in BBM cells exposed to lapatinib-loaded hiPSC- or donor-platelets compared to BBM cells exposed to C6 only-loaded hiPSC- or donor-platelets or with non-loaded platelets (Controls) (Fig. 3F,G and Supplementary Fig. S5A and S5B). We also observed significantly reduced viability of BBM cells exposed to a 1:1 mixture of lapatinib and C6, as compared to non-loaded or C6-loaded platelets (Fig. 3G).
Toxicity studies in BBM cells. (A) The effect of lapatinib-loaded hiPSC-platelets against BBM cells at 24, 48, and 72 h, with various concentrations of lapatinib loaded into the hiPSC-platelets. The inset table (bottom) shows the IC50 (µM) of lapatinib-loaded hiPSC-platelets against BBM cells at 24, 48, and 72 h (mean ± SD, n = 3). (B) Cytotoxic effects of non-loaded hiPSC- and donor-derived platelets, free lapatinib, and lapatinib-loaded hiPSC- and donor-derived platelets on BBM1 and BBM2 cells at 24, 48, and 72 h. ** indicates p < 0.001. (C) Representative flow cytometry plots demonstrating the surface expression of Annexin-V on CD326 + BBM cells exposed to non-loaded and lapatinib-loaded hiPSC- and donor-derived platelets. (D) Surface Annexin-V quantification of BBM cells exposed to non-loaded and lapatinib-loaded hiPSC- and donor-derived platelets (mean ± SD, n = 3). ** indicates p < 0.001. (E) mRNA expression of apoptosis-associated genes in BBM cells under various treatment conditions, evaluated by TaqMan RT-qPCR. * indicates p < 0.001. (F) Representative flow cytometry plot showing BBM cells exposed to non-loaded, C6-loaded, and 1:1 lapatinib + C6-loaded hiPSC- and donor-derived platelets for 48 h. (G) CCK8 viability assay results showing the percentage of viable BBM cells after exposure to non-loaded, C6-loaded, and 1:1 lapatinib + C6-loaded hiPSC- and donor-derived platelets for 48 h (mean ± SD, n = 3). ** indicates p < 0.001.
Analysis of therapeutic efficacy of lapatinib-loaded hiPSC-platelets in vivo
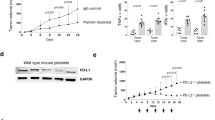
We next assessed potential anti-tumor effects of lapatinib-loaded hiPSC-platelets in vivo using female NOD/SCID mice bearing xenograft BBM tumors, which we developed41,74 (Fig. 4A). To do so, we injected BBM cells (100 K) expressing ZsGreen1 Renilla luciferase intracranially in the subcortical region of mice via the cisterna magna on day 0 and allowed tumor development to occur over 7 days. Once tumors were detectable by bioluminescence imaging (BLI) lapatinib-loaded hiPSC or donor platelets, free lapatinib, or non-loaded control hiPSC and donor platelets were intravenously administered via the tail vein every 3 days. Tumor progression was then monitored by BLI every 2 days up to day 30 (Fig. 4A,B,E). That analysis revealed significantly reduced BLI counts in mice infused with lapatinib-loaded hiPSC- or donor-platelets relative to cohorts that received non-loaded hiPSC- or donor-platelets or free lapatinib (Fig. 4A,B). We monitored mice for up to 60 days and observed that BLI intensity decreased to undetectable levels in mice treated with lapatinib-loaded hiPSC-platelets (Fig. 4A) relative to vehicle- or non-loaded platelet-treated controls. Moreover, overall survival of mice bearing BBM tumors was significantly higher in animals treated with lapatinib-loaded hiPSC- or donor-platelets relative to vehicle- or non-loaded platelet-treated controls (Fig. 4C,E and Supplementary Fig. S6B and C). Pre-treatment of BBM1 cells with lapatinib-loaded hiPSC-platelets also reduced tumor seeding capacity of BBM1 cells by 1,000-fold relative pre-treatment with non-loaded hiPSC-platelets (Fig. 4D). As expected, tumor size (as determined using HE-stained horizontal brain sections and AMIRA-based area quantification) also decreased significantly in tumor-bearing mice infused with lapatinib-loaded hiPSC- or donor-platelets relative to free lapatinib or untreated controls (Fig. 4E and Supplementary Fig. S7C). Also, after 24 days of treatment with free lapatinib, mice had a final average body weight of ~ 18.9 g, indicative of significant weight loss, compared to animals treated with non-loaded or lapatinib-loaded hiPSC-platelets (Fig. 5A), suggesting that lapatinib toxicity to normal tissue is reduced by encapsulation in hiPSC- or donor-platelets. Interestingly, histopathological analysis using hematoxylin and eosin (H&E) staining of heart, liver, spleen, lung, and kidney did not reveal significant abnormalities in any of the treatment groups (Supplementary Fig. S6A).
Therapeutic efficacy of lapatinib-loaded hiPSC-platelets in vivo.(A) Representative BLI images of xenografted BBM1-derived tumor-bearing female NOD/SCID mice treated with vehicle (control), free lapatinib, and non-loaded and lapatinib-loaded hiPSC- and donor-derived platelets every 3 days. (B) Bioluminescence was quantified for BBM1-derived tumor-bearing female NOD/SCID mice throughout the experiment, as indicated. Mice were injected with vehicle (control), free lapatinib, and non-loaded and lapatinib-loaded hiPSC- and donor-derived platelets every 3 days. * indicates p < 0.05, ** indicates p < 0.001. Each group contains n = 7 mice. (C) Overall survival of variously treated BBM1-derived tumor-bearing female NOD/SCID mice. Control vs. non-loaded hiPSC- and donor-derived platelets, ns (non-significant); control vs. lapatinib, p < 0.05; control vs. lapatinib-loaded hiPSC- and donor-derived platelets, p < 0.001. (D) Tumor-seeding capability of BBM1 cells in female NOD/SCID mice pre-treated with vehicle (control), and non-loaded and lapatinib-loaded hiPSC and donor-derived platelets. (E) Tumor volume of BBM-derived tumor-bearing NOD/SCID mice treated with vehicle, free lapatinib, and non-loaded and lapatinib-loaded hiPSC- and donor-derived platelets, measured at 40 days post-implant (n = 7 per group). Control vs. lapatinib, p < 0.05; control vs. free lapatinib, * p < 0.05; non-loaded platelets vs. lapatinib-loaded platelets, ** p < 0.001.
In vivo distribution of lapatinib-loaded hiPSC-platelets. (A) Body weights of variously treated BBM1-derived tumor-bearing female NOD/SCID mice (n = 7) over a 30-day period. ** indicates p < 0.001. (B) Plasma concentrations of lapatinib, measured by LC–MS/MS after injection with free lapatinib or lapatinib-loaded hiPSC- and donor-derived platelets in NOD/SCID mice (n = 7). Lapatinib-loaded hiPSC- and donor-derived platelets vs. free lapatinib, * p < 0.001. (C) Lapatinib concentrations in various tissues of female NOD/SCID mice (n = 7), collected 4 h after infusion with free lapatinib or lapatinib-loaded hiPSC- and donor-derived platelets. * indicates p < 0.001.
We also measured plasma lapatinib concentrations in mice bearing BBM derived tumors at various timepoints after treatment (Fig. 5B). Whereas free lapatinib was rapidly cleared with a short half-life (t1/2 = 2.1 ± 0.6 h), lapatinib released from lapatinib-loaded hiPSC- or donor platelets remained at a higher level with a longer half-life (t1/2 = 31.3 ± 0.9 h; Fig. 5B). Analysis of tissue distribution showed that, compared to levels of lapatinib released from lapatinib-loaded hiPSC- or donor-platelets, concentrations of free lapatinib were significantly higher in heart, kidney and liver tissues but relatively lower in tumor tissue (Fig. 5C). However, we observed an increase in lapatinib concentration in lungs of tumor-bearing mice treated with lapatinib-loaded hiPSC- and donor-platelets relative to free lapatinib. Furthermore, lapatinib levels were higher in kidney and heart in free lapatinib-administered animals relative to animals treated with lapatinib-loaded hiPSC- and donor-derived platelets, suggesting decreased toxicity (Fig. 5C). To confirm minimal adverse effects in animals treated with lapatinib-loaded donor- or hiPSC-platelets, we performed histopathological analyses of all major organs including lung, kidney, and heart from BBM tumor-bearing mice treated with both. Relative to non-loaded platelet controls, we observed no significant changes in morphology in any group analyzed (Supplementary Fig. S6A). To confirm that elevated lapatinib levels did not alter heart function we also quantified levels of cardiac troponin I (cTnI) in sera of variously treated tumor-bearing mice and found that levels were lower in all test groups than those seen in positive control mice exposed to isoproterenol, which induces higher troponin I levels, strongly suggesting that heart function is normal in mice treated with lapatinib-loaded or non-loaded donor or hiPSC-platelets (Supplementary Fig. S7A). Circulation or clearance kinetics of 5 × 108 intravenously infused non-loaded or lapatinib-loaded human iPSC- and donor-platelets was comparable in macrophage-depleted NOD/SCID mice, with a time to reach maximal accumulation (Tmax) of 1 h (Supplementary Fig. S7B and S8A-B). HiPSC-platelets, like human blood platelets, circulated for at least 24–48 h, indicating that lapatinib-loaded hiPSC-platelets possess a lower rate of clearance from blood circulation, which may allow them to accumulate and release lapatinib in the vicinity of the tumor tissues, potentially sparing normal tissues.
Discussion
Breast cancer patients can develop BBMs years or even decades after their initial diagnosis, indicative of a long latency period, despite the presence of circulating tumor cells12,75,76. Metastases are responsible for 90% of all cancer deaths3,4,7,8. Patients diagnosed with brain metastases have a dismal probability of 1-year survival1. Furthermore, the advancement and improved efficacy of treatments for primary breast cancer have led to an increased propensity to develop metastatic breast tumors6,10. Although all breast cancer subtypes can metastasize to the brain, patients with HER2+ primary breast tumors have a higher risk of developing brain metastasis9. ~ 40% of patients with HER2+ primary breast cancer develops brain metastasis1,6. Major treatment modalities for HER2+ BBMs have been radiation therapy, surgical resection of BBM tumors, and systemic chemotherapy treatment with various drugs77,78,79,80,81,82. However, the benefits of radiation therapy are limited to short-term palliation of distressing symptoms and this modality cause neurocognitive dysfunction83. Additionally, a major limitation in developing effective chemotherapeutic treatments for HER2+ BBMs is the poor activity of anti-neoplastic agents against HER2+ BBMs and poor penetration of these agents into brain tissue3,7,8.
Previous studies reported modest activity of lapatinib against BBM tumors in the brain22,84,85,86. Therefore, development of drug delivery systems that can augment BBB clearance to reach the tumors residing in the brain, reduce side effects and simultaneously improve the efficacy, therapeutic index, and biocompatibility of promising drugs treatments is needed27,28,29,30,33,42,87. Although various drug delivery systems have been developed, poor biocompatibility has limited their application in clinical settings. Some studies have demonstrated the utility of natural donor-derived blood platelets as drug carriers51. For example, a recent study demonstrated the use of doxorubicin-loaded blood-platelets as a delivery system to treat lymphoma and achieved a longer retention time, relative to free drug retention times. However, allogenic drug-loaded platelet transfusions run the risk of alloimmunization to HLA52,53,61. Patients who receive multiple platelet transfusions, such as those with various types of cancer, often develop platelet refractoriness due to HLA alloreactivity and subsequently require additional transfusions with HLA-matched donor platelets88,89,90,91. Therefore, finding alternative sources of non-immunogenic, high-quality platelets might decrease risks associated with allogeneic drug-loaded platelet transfusions.
Therefore, our main objectives were to develop a clinically adaptable protocol to generate hiPSC-derived megakaryocytes that can efficiently mature and terminally differentiate into highly functional platelets and to determine whether hiPSC-platelets possess properties of natural platelets and could serve as an alternative to allogeneic donor-derived platelets as drug carriers for targeting and treating BBMs. Here, we report a serum and feeder-free protocol to differentiate human hiPSCs into megakaryocytes, which in turn mature and terminally differentiate to generate functional platelets. Using this method, we generated hiPSC-platelets on a large scale, allowing us to perform flow cytometry-based activation assays and LTA-based aggregation assays, in vitro. The latter demonstrated that donor-derived platelets do aggregate more readily than hiPSC-derived platelets, potentially due to the method used to enrich them. Specifically, we performed differential centrifugation to remove the naïve megakaryocytes from platelet medium followed by BSA density gradient-based centrifugation to generate purer platelet populations. These methods efficiently remove large megakaryocytes but cannot remove small-sized debris materials (e.g., CD42b- or CD41a- or Annexin-V+ particles) that does not aggregate and can lead to higher background. This purification step does not occur in the preparation of fresh donor platelets. Furthermore, hiPSC derived platelets are fetal in nature showed better aggregation profiles compared to platelets derived from CD34+ umbilical cord blood cells, which show no or minimal aggregation based on LTA representing significant improvement61. Subsequently, we used these hiPSC-platelets to encapsulate lapatinib and found that they can be loaded with an encapsulation efficiency of ~ 90% without any alteration in platelet morphology and functionality (Fig. 2). Using Boyden chambers, we observed that lapatinib-loaded hiPSC- and donor-derived platelets significantly reduced the viability of BBM1 and BBM2 cells compared to free lapatinib (Fig. 3B). We attribute this increased cytotoxicity to the rapid migration of the lapatinib-loaded platelets from the top chamber to the bottom chamber, where the BBM1 and BBM2 tumor cells were cultured in a monolayer; however, this remains to be validated through time-lapse imaging based analyses of binding kinetics of variously stained platelets binding to the tumor cells.
We also showed that the in vitro release of lapatinib from lapatinib-loaded hiPSC- and donor-derived platelets was pH-sensitive. This is critical, as the tumor microenvironment is acidic compared to normal tissues, reflecting the hypoxic conditions induced by rapid tumor cell proliferation92,93. However, it remains unclear how lapatinib release from hiPSC- and donor-derived platelets is accelerated under acidic conditions. The lower pH could induce the generation of platelet-derived extracellular vesicles containing lapatinib, as has been reported for cancer cell-derived vesicles94. Loaded platelets can also respond to thrombin-mediated activation and generate extracellular vesicles, which could augment lapatinib release. Such vesicles, which are ~ 200 nm in diameter, could be important secondary drug delivery systems for lapatinib with greater potential to infiltrate BBM tumors and fuse with cancer cells. In addition, an acidic environment can promote the generation of mixed platelet-leukocyte aggregates and increased chemotaxis of neutrophils mediated by platelets in a P-Sel-dependent manner95; and P-Sel, in turn, is exposed on the platelet surface under acidic conditions. Finally, alterations in platelet structure have been reported under acidic conditions96, which may increase OCS “permeability” 97. Overall, these findings suggest that drug-loaded hiPSC- and donor-derived platelets are more likely to release their contents in or around the acidic environment established by BBM tumor cells rather than the normal physiological environment, reducing potential toxicity to surrounding tissues.
After treatment with free lapatinib and lapatinib-loaded hiPSC-platelets, the growth inhibition and apoptosis of BBM cells were comparable in vitro. However, the effective treatment of brain metastases is generally hindered by the BBB55. Thus, our more clinically significant finding was that hiPSC-platelets can act as an efficient drug carrier for the treatment of HER2+ BBMs tumors residing in the brain. We found that, compared to the infusion of free lapatinib, the infusion of lapatinib-loaded hiPSC-platelets significantly decreased tumor progression and tumor size and more potently reduced the tumor-seeding capability of BBM cells. Plasma analyses also indicated that lapatinib concentrations remained higher in mice treated with lapatinib-loaded hiPSC-platelets, with a much longer half-life than the free drug. These results suggest that lapatinib-loaded hiPSC-platelets could be more effective against BBM than free lapatinib. Furthermore, following infusion of lapatinib-loaded hiPSC-platelets, lapatinib levels were significantly higher in tumors relative to the major organs evaluated, which was not the case in mice infused with free lapatinib. Consistent with these findings, our histological assessment of various tissues in treated mice revealed no overt signs of injury; however, we did observe body weight loss in tumor-bearing mice treated with free lapatinib, which is an early indicator of toxicity. Finally, cTnI levels were low and comparable among mice treated with non-loaded and lapatinib-loaded hiPSC- and donor-derived platelets, indicating the absence of cardiac injury in all of the groups tested. These results demonstrate that hiPSC lines can be safely used to generate functional platelets that are morphologically and functionally similar to donor-derived platelets to serve as drug carriers. In summary, our work supports a promising new strategy to significantly improve the efficacy of chemotherapeutic drugs such as lapatinib against BBM, while reducing the potential side effects frequently induced by their direct systemic administration.
Methods and materials
Ethics statements
The use of human specimens was approved by the City of Hope (COH) Institutional Review Board (IRB; protocols #07047 and #16015) 11,41,74. Written informed consent was obtained from all patients under protocol #07047, and the study was conducted in accordance with the Declaration of Helsinki, institutional guidelines, and all local, state, and federal regulations. This study was carried out in compliance with the ARRIVE guidelines. All NOD-scid IL2Rgammanull (NOD/SCID) mouse studies were approved by the COH Institutional Animal Care and Use Committee (protocol #10044)98 and carried out in accordance with the relevant guidelines and regulations.
Reagents
STEMdiff APEL Media, STEMspan-ACF, STEMspan megakaryocyte expansion supplement, mTeSR1, and RelesR were procured from STEMCELL Technologies. All cytokines used were obtained from R&D systems. Y-27632 was purchased from Stemgent. Human Collagen IV was from Advanced BioMatrix. Matrigel and the antibodies for flow cytometry were obtained from BD Biosciences. StemPro Accutase was from Life Technologies. Heparin was purchased from Sigma.
Human pluripotent stem cell (hiPSC) cultures
The hiPSC lines hiPSC-DF-19-9-7 T and hiPSC-DF-6-9-9 T were obtained from WiCell and reprogrammed with episomal vectors. All human pluripotent stem cells were cultured on a Matrigel-coated surface with mTeSR1 medium. Confluent pluripotent stem cells were dissociated either with RelesR (STEMCELL Technologies). All pluripotent stem cells used in this study had normal karyotypes. Cell line authentication was performed by short tandem repeat profiling by IDEXX BioAnalytics, and the cells were determined to be Mycoplasma-negative by PCR (Agilent Mycosenser Mycoplasma Assay Kit) as recently as 1 month before the final experiments.
Breast-to-brain metastasis (BBM) cell cultures
BBM tissue specimens (HER2+) were collected and propagated as previously described 73. Briefly, the cell lines COH-BBM1 (BBM1) and COH-BBM2 (BBM2) were established by validation of phenotypic markers (HER2, pan-cytokeratin) and exclusion of brain cells (astrocytes, microglia, and endothelial cells), and cultured in DMEM/F12 media (Thermo Fisher Scientific) supplemented with 10% fetal bovine serum (FBS; Sigma Aldrich), 1% Glutamax (Life Technologies), and 1% antibiotic–antimycotic (Life Technologies), at 37 °C and 5% CO2. Cell line authentication was performed by short tandem repeat profiling by IDEXX BioAnalytics, and the cell lines were determined to be Mycoplasma-negative by PCR (Agilent Mycosenser Mycoplasma Assay Kit) as recently as 1 month before the final experiments.
In vivo treatment of BBM xenograft tumors with free lapatinib and lapatinib-loaded hiPSC-platelets
All NOD/SCID mice were maintained under veterinary supervision and housed under standard living conditions, with a 12-h light/dark cycle and access to food and water ad libitium. Investigators were blinded to treatment group for the analysis of mice. The experiments were not randomized. No statistical methods were used to predetermine sample size. No strategy was used to eliminate or identify confounders. Female NOD/SCID mice were randomized to treatment and control groups of n = 7, giving 80% power to detect a treatment effect size of 65% compared to a baseline response of 5% at a significance level of 0.05. Throughout the course of our experiments, no animals were excluded from the study. Macrophages were depleted in all NOD/SCID mice by intravenous injection of liposome-encapsulated clodronate, as shown previously 61,99.
To evaluate tumor growth and survival in vivo, BBM1 cells (100 K in 20 μL PBS) were transduced with mCherry and firefly luciferase (mCherry:LUC, Addgene)100,101,102 and injected into the brains of 6-week-old NOD/SCID female mice (7 mice/group, 24 total; 2 mm right and 1 mm anterior to the bregma suture). Only NOD/SCID female mice were used for these studies because BBM tumors occur predominantly in females. Tumor progression was monitored every 48 h for 40 days using BLI on a Xenogen Imaging System (Xenogen Corp). At the conclusion of the experiments, mice were euthanized, and their brains and other tissues collected and fixed in formalin (Thermo Fisher Scientific) for downstream lapatinib concentration analyses.
The tumor-bearing female NOD/SCID mice were randomly divided into six groups to receive treatment with: (1) normal saline; (2) non-loaded hiPSC-platelets; (3) free lapatinib; (4) lapatinib-loaded hiPSC-platelets; (5) non-loaded donor-derived platelets; (6) lapatinib-loaded donor-derived platelets. Each group had n = 7 female NOD/SCID mice, for a total of 42 animals. The dose of lapatinib for each injection was 6 mg/kg and based on the effective drug loading rate of 48.5%, the dose of lapatinib-loaded hiPSC-platelets was 12 mg/kg. Treatments were intravenously administered via the tail vein, and the volumes of tumors were measured every 3 days.
Statistical analyses
Data are presented as mean ± standard deviation (SD), using data generated from n = 3 biological replicates with n = 2 technical replicates present for each biological replicate. The statistical significance of differences between groups was determined (unless otherwise noted) using one- or two-way analysis of variance (ANOVA) with Bonferroni correction for multiple comparisons (GraphPad Prism 8.4.1). Other statistical evaluations were performed using Student’s t tests. Kaplan–Meier curves were used to model overall survival. A P value of < 0.05 was considered statistically significant. In the figures, statistically significant P values are represented by * < 0.05, ** < 0.01, and *** < 0.001.
All additional methods are described in the Supplemental Material and Methods section.
Ethics statement
All the experiments in this study were carried out in compliance with the ARRIVE guidelines. All NOD-scid IL2Rgammanull (NOD/SCID) mouse studies were approved by City of Hope’s Institutional Animal Care and Use Committee (protocol #10044) and carried out in accordance with the guidelines and regulations highlighted in the approved protocol.
Change history
20 September 2023
Editor’s Note: Readers are alerted that concerns have been raised about discrepancies in some of the data reported in this manuscript. Appropriate editorial action will be taken once this matter is resolved.
12 March 2024
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1038/s41598-024-56291-z
References
Zimmer, A. S., Van Swearingen, A. E. D. & Anders, C. K. HER2-positive breast cancer brain metastasis: A new and exciting landscape. Cancer Rep. https://doi.org/10.1002/cnr2.1274 (2020).
Wolff, A. C. et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch. Pathol. Lab. Med. 131, 18–43. https://doi.org/10.1043/1543-2165(2007)131[18:ASOCCO]2.0.CO;2 (2007).
Gupta, G. P. & Massague, J. Cancer metastasis: Building a framework. Cell 127, 679–695. https://doi.org/10.1016/j.cell.2006.11.001 (2006).
Dillekås, H., Rogers, M. S. & Straume, O. Are 90% of deaths from cancer caused by metastases?. Cancer Med. 8, 5574–5576. https://doi.org/10.1002/cam4.2474 (2019).
Lin, N. U. & Winer, E. P. Brain metastases: The HER2 paradigm. Clin. Cancer Res. 13, 1648–1655. https://doi.org/10.1158/1078-0432.CCR-06-2478 (2007).
Neman, J. et al. Human breast cancer metastases to the brain display GABAergic properties in the neural niche. Proc. Natl. Acad. Sci. 111, 984. https://doi.org/10.1073/pnas.1322098111 (2014).
Lowery, F. J. & Yu, D. Brain metastasis: Unique challenges and open opportunities. Biochim. Biophys. Acta Rev. Cancer 49–57, 2017. https://doi.org/10.1016/j.bbcan.2016.12.001 (1867).
Witzel, I., Oliveira-Ferrer, L., Pantel, K., Müller, V. & Wikman, H. Breast cancer brain metastases: Biology and new clinical perspectives. Breast Cancer Res. 18, 8–8. https://doi.org/10.1186/s13058-015-0665-1 (2016).
Moo, T.-A., Sanford, R., Dang, C. & Morrow, M. Overview of breast cancer therapy. PET Clin. 13, 339–354. https://doi.org/10.1016/j.cpet.2018.02.006 (2018).
Markman, M. Lapatinib as a therapeutic option in brain metastases from HER2+ breast cancer. Ann. Palliat. Med. 2, 35–36 (2013).
Choy, C. et al. Cooperation of neurotrophin receptor TrkB and Her2 in breast cancer cells facilitates brain metastases. Breast Cancer Res. 19, 51. https://doi.org/10.1186/s13058-017-0844-3 (2017).
Lang, F. F. & Sawaya, R. Surgical treatment of metastatic brain tumors. Semin. Surg. Oncol. 14, 53–63. https://doi.org/10.1002/(sici)1098-2388(199801/02)14:1%3c53::aid-ssu7%3e3.0.co;2-1 (1998).
Cihan, Y. B. Lapatinib? or Radiotherapy? In cranial metastasis of breast cancer. Eur. J. Breast Health 15, 205–206. https://doi.org/10.5152/ejbh.2019.4874 (2019).
Tevaarwerk, A. J. & Kolesar, J. M. Lapatinib: A small-molecule inhibitor of epidermal growth factor receptor and human epidermal growth factor receptor-2 tyrosine kinases used in the treatment of breast cancer. Clin. Ther. 31(Pt 2), 2332–2348. https://doi.org/10.1016/j.clinthera.2009.11.029 (2009).
Fontanella, C. et al. Central nervous system involvement in breast cancer patients: Is the therapeutic landscape changing too slowly?. Cancer Treat. Rev. 46, 80–88. https://doi.org/10.1016/j.ctrv.2016.03.014 (2016).
Lin, N. U. et al. Multicenter phase II study of lapatinib in patients with brain metastases from HER2-positive breast cancer. Clin. Cancer Res. 15, 1452–1459. https://doi.org/10.1158/1078-0432.Ccr-08-1080 (2009).
Burris, H. A. 3rd. et al. A phase I and pharmacokinetic study of oral lapatinib administered once or twice daily in patients with solid malignancies. Clin. Cancer Res. 15, 6702–6708. https://doi.org/10.1158/1078-0432.Ccr-09-0369 (2009).
Polli, J. W. et al. The role of efflux and uptake transporters in [N-{3-chloro-4-[(3-fluorobenzyl)oxy]phenyl}-6-[5-({[2-(methylsulfonyl)ethyl]amino}methyl)-2-furyl]-4-quinazolinamine (GW572016, lapatinib) disposition and drug interactions. Drug Metab. Disposit. Biol. Fate Chem. 36, 695–701. https://doi.org/10.1124/dmd.107.018374 (2008).
Polli, J. W. et al. An unexpected synergist role of P-glycoprotein and breast cancer resistance protein on the central nervous system penetration of the tyrosine kinase inhibitor lapatinib (N-{3-chloro-4-[(3-fluorobenzyl)oxy]phenyl}-6-[5-({[2-(methylsulfonyl)ethyl]amino}methyl)-2-furyl]-4-quinazolinamine; GW572016). Drug Metab. Disposit. Biol. Fate Chem. 37, 439–442. https://doi.org/10.1124/dmd.108.024646 (2009).
Wan, X. et al. Lapatinib-loaded human serum albumin nanoparticles for the prevention and treatment of triple-negative breast cancer metastasis to the brain. Oncotarget 7, 34038–34051. https://doi.org/10.18632/oncotarget.8697 (2016).
Bonde, G. V. et al. Lapatinib nano-delivery systems: A promising future for breast cancer treatment. Expert Opin. Drug Deliv. 15, 495–507. https://doi.org/10.1080/17425247.2018.1449832 (2018).
Chintalaramulu, N., Vadivelu, R., Nguyen, N. T. & Cock, I. E. Lapatinib inhibits doxorubicin induced migration of HER2-positive breast cancer cells. Inflammopharmacology 28, 1375–1386. https://doi.org/10.1007/s10787-020-00711-9 (2020).
Huo, Z.-J. et al. Novel nanosystem to enhance the antitumor activity of lapatinib in breast cancer treatment: Therapeutic efficacy evaluation. Cancer Sci 106, 1429–1437. https://doi.org/10.1111/cas.12737 (2015).
Wang, H. et al. Doxorubicin and lapatinib combination nanomedicine for treating resistant breast cancer. Mol. Pharm. 11, 2600–2611. https://doi.org/10.1021/mp400687w (2014).
Venditto, V. J. & Szoka, F. C. Cancer nanomedicines: So many papers and so few drugs!. Adv. Drug Deliv. Rev. 65, 80–88. https://doi.org/10.1016/j.addr.2012.09.038 (2013).
Peters, D. et al. Targeting atherosclerosis by using modular, multifunctional micelles. Proc. Natl. Acad. Sci. 106, 9815. https://doi.org/10.1073/pnas.0903369106 (2009).
Kim, H. S. & Yoo, H. S. Targeted nanoparticles for smarter cancer therapy: Original research article: Folate receptor targeted biodegradable polymeric doxorubicin micelles, 2004. J. Control Release 190, 64–66 (2014).
Molavi, O. et al. Anti-CD30 antibody conjugated liposomal doxorubicin with significantly improved therapeutic efficacy against anaplastic large cell lymphoma. Biomaterials 34, 8718–8725. https://doi.org/10.1016/j.biomaterials.2013.07.068 (2013).
Cheng, W. W. & Allen, T. M. Targeted delivery of anti-CD19 liposomal doxorubicin in B-cell lymphoma: A comparison of whole monoclonal antibody, Fab’ fragments and single chain Fv. J. Control Release 126, 50–58. https://doi.org/10.1016/j.jconrel.2007.11.005 (2008).
Park, J. et al. PEGylated PLGA nanoparticles for the improved delivery of doxorubicin. Nanomedicine 5, 410–418. https://doi.org/10.1016/j.nano.2009.02.002 (2009).
Cedrone, E. et al. Anticoagulants influence the performance of in vitro assays intended for characterization of nanotechnology-based formulations. Molecules https://doi.org/10.3390/molecules23010012 (2017).
Lee, J. H. & Nan, A. Combination drug delivery approaches in metastatic breast cancer. J. Drug Deliv. 2012, 915375. https://doi.org/10.1155/2012/915375 (2012).
Patra, J. K. et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 16, 71. https://doi.org/10.1186/s12951-018-0392-8 (2018).
Lu, Y., Hu, Q., Jiang, C. & Gu, Z. Platelet for drug delivery. Curr. Opin. Biotechnol. 58, 81–91. https://doi.org/10.1016/j.copbio.2018.11.010 (2019).
Patel, S. R., Hartwig, J. H. & Italiano, J. E. Jr. The biogenesis of platelets from megakaryocyte proplatelets. J Clin Invest 115, 3348–3354. https://doi.org/10.1172/JCI26891 (2005).
Hartwig, J. & Italiano, J. Jr. The birth of the platelet. J. Thromb. Haemost. 1, 1580–1586. https://doi.org/10.1046/j.1538-7836.2003.00331.x (2003).
Machlus, K. R. & Italiano, J. E. Jr. The incredible journey: From megakaryocyte development to platelet formation. J. Cell Biol. 201, 785–796. https://doi.org/10.1083/jcb.201304054 (2013).
Jurasz, P., Alonso-Escolano, D. & Radomski, M. W. Platelet–cancer interactions: Mechanisms and pharmacology of tumour cell-induced platelet aggregation. Br. J. Pharmacol. 143, 819–826. https://doi.org/10.1038/sj.bjp.0706013 (2004).
Shi, Q. & Montgomery, R. R. Platelets as delivery systems for disease treatments. Adv. Drug Deliv. Rev. 62, 1196–1203. https://doi.org/10.1016/j.addr.2010.06.007 (2010).
Jin, K., Luo, Z., Zhang, B. & Pang, Z. Biomimetic nanoparticles for inflammation targeting. Acta Pharm. Sin. B 8, 23–33. https://doi.org/10.1016/j.apsb.2017.12.002 (2018).
Ansari, K. I., Bhan, A., Liu, X., Chen, M. Y. & Jandial, R. Astrocytic IGFBP2 and CHI3L1 in cerebrospinal fluid drive cortical metastasis of HER2+breast cancer. Clin. Exp. Metastasis 37, 401–412. https://doi.org/10.1007/s10585-020-10032-4 (2020).
Hu, C. M. et al. Nanoparticle biointerfacing by platelet membrane cloaking. Nature 526, 118–121. https://doi.org/10.1038/nature15373 (2015).
Mei, D. et al. Platelet membrane-cloaked paclitaxel-nanocrystals augment postoperative chemotherapeutical efficacy. J. Control Release 324, 341–353. https://doi.org/10.1016/j.jconrel.2020.05.016 (2020).
Barbieri, S. S. et al. Abnormal megakaryopoiesis and platelet function in cyclooxygenase-2-deficient mice. Thromb. Haemost. 114, 1218–1229. https://doi.org/10.1160/TH14-10-0872 (2015).
Johnson, K. E. et al. Aspirin inhibits platelets from reprogramming breast tumor cells and promoting metastasis. Blood Adv. 3, 198–211. https://doi.org/10.1182/bloodadvances.2018026161 (2019).
Matsuura, S. et al. Platelet dysfunction and thrombosis in JAK2(V617F)-mutated primary myelofibrotic mice. Arterioscler. Thromb. Vasc. Biol. 40, e262–e272. https://doi.org/10.1161/ATVBAHA.120.314760 (2020).
French, S. L. et al. Platelet-derived extracellular vesicles infiltrate and modify the bone marrow during inflammation. Blood Adv. 4, 3011–3023. https://doi.org/10.1182/bloodadvances.2020001758 (2020).
Cloutier, N. et al. Platelets release pathogenic serotonin and return to circulation after immune complex-mediated sequestration. Proc. Natl. Acad. Sci. U S A 115, E1550–E1559. https://doi.org/10.1073/pnas.1720553115 (2018).
Machlus, K. R. et al. Selinexor-induced thrombocytopenia results from inhibition of thrombopoietin signaling in early megakaryopoiesis. Blood 130, 1132–1143. https://doi.org/10.1182/blood-2016-11-752840 (2017).
Singla, T., Singla, G., Ranga, S., Singla, S. & Arora, R. Role of platelet aggregation in metastatic breast cancer patients. Indian J. Pathol. Microbiol. 63, 564–569. https://doi.org/10.4103/IJPM.IJPM_817_19 (2020).
Xu, P. et al. Doxorubicin-loaded platelets as a smart drug delivery system: An improved therapy for lymphoma. Sci. Rep. 7, 42632. https://doi.org/10.1038/srep42632 (2017).
Pena, J. R. et al. Anti-HLA alloantibodies in surgical patients refractory to platelet transfusion. Am. J. Hematol. 89, E133-137. https://doi.org/10.1002/ajh.23757 (2014).
Geddis, A. E. & Kaushansky, K. Immunology. The root of platelet production. Science 317, 1689–1691. https://doi.org/10.1126/science.1148946 (2007).
Fasano, R. M. et al. Persistence of recipient human leucocyte antigen (HLA) antibodies and production of donor HLA antibodies following reduced intensity allogeneic haematopoietic stem cell transplantation. Br. J. Haematol. 166, 425–434. https://doi.org/10.1111/bjh.12890 (2014).
Arvanitis, C. D., Ferraro, G. B. & Jain, R. K. The blood–brain barrier and blood–tumour barrier in brain tumours and metastases. Nat. Rev. Cancer 20, 26–41. https://doi.org/10.1038/s41568-019-0205-x (2020).
Nakamura, S. et al. Expandable megakaryocyte cell lines enable clinically applicable generation of platelets from human induced pluripotent stem cells. Cell Stem Cell 14, 535–548. https://doi.org/10.1016/j.stem.2014.01.011 (2014).
Nakamura, S., Sugimoto, N. & Eto, K. Ex vivo generation of platelet products from human iPS cells. Inflamm. Regen. 40, 30. https://doi.org/10.1186/s41232-020-00139-2 (2020).
Moreau, T. et al. Corrigendum: Large-scale production of megakaryocytes from human pluripotent stem cells by chemically defined forward programming. Nat. Commun. 8, 15076. https://doi.org/10.1038/ncomms15076 (2017).
Moreau, T. et al. Large-scale production of megakaryocytes from human pluripotent stem cells by chemically defined forward programming. Nat. Commun. 7, 11208. https://doi.org/10.1038/ncomms11208 (2016).
Ito, Y. et al. Turbulence activates platelet biogenesis to enable clinical scale ex vivo production. Cell 174, 636-648e618. https://doi.org/10.1016/j.cell.2018.06.011 (2018).
Feng, Q. et al. Scalable generation of universal platelets from human induced pluripotent stem cells. Stem Cell Rep. 3, 817–831. https://doi.org/10.1016/j.stemcr.2014.09.010 (2014).
Hirata, S. et al. Selective inhibition of ADAM17 efficiently mediates glycoprotein Ibalpha retention during ex vivo generation of human induced pluripotent stem cell-derived platelets. Stem Cells Transl. Med. 6, 720–730. https://doi.org/10.5966/sctm.2016-0104 (2017).
Seo, H. et al. A β1-tubulin-based megakaryocyte maturation reporter system identifies novel drugs that promote platelet production. Blood Adv. 2, 2262–2272. https://doi.org/10.1182/bloodadvances.2018019547 (2018).
Jarocha, D. et al. Enhancing functional platelet release in vivo from in vitro-grown megakaryocytes using small molecule inhibitors. Blood Adv. 2, 597–606. https://doi.org/10.1182/bloodadvances.2017010975 (2018).
Nishikii, H. et al. Metalloproteinase regulation improves in vitro generation of efficacious platelets from mouse embryonic stem cells. J. Exp. Med. 205, 1917–1927. https://doi.org/10.1084/jem.20071482 (2008).
Wang, Y. et al. Comparative analysis of human ex vivo-generated platelets vs megakaryocyte-generated platelets in mice: A cautionary tale. Blood 125, 3627–3636. https://doi.org/10.1182/blood-2014-08-593053 (2015).
Le Blanc, J., Mullier, F., Vayne, C. & Lordkipanidze, M. Advances in platelet function testing-light transmission aggregometry and beyond. J. Clin. Med. https://doi.org/10.3390/jcm9082636 (2020).
Alberelli, M. A., Innocenti, I., Autore, F., Laurenti, L. & De Candia, E. Ibrutinib does not affect ristocetin-induced platelet aggregation evaluated by light transmission aggregometry in chronic lymphocytic leukemia patients. Haematologica 103, e119–e122. https://doi.org/10.3324/haematol.2017.179044 (2018).
Khan, H. et al. Personalization of aspirin therapy ex vivo in patients with atherosclerosis using light transmission aggregometry. Diagnostics (Basel). https://doi.org/10.3390/diagnostics10110871 (2020).
Sarkar, S., Alam, M. A., Shaw, J. & Dasgupta, A. K. Drug delivery using platelet cancer cell interaction. Pharm. Res. 30, 2785–2794. https://doi.org/10.1007/s11095-013-1097-1 (2013).
Wu, Y.-W. et al. Clinical-grade cryopreserved doxorubicin-loaded platelets: Role of cancer cells and platelet extracellular vesicles activation loop. J. Biomed. Sci. 27, 45. https://doi.org/10.1186/s12929-020-00633-2 (2020).
Xu, P. et al. Doxorubicin-loaded platelets conjugated with anti-CD22 mAbs: A novel targeted delivery system for lymphoma treatment with cardiopulmonary avoidance. Oncotarget 8, 58322–58337. https://doi.org/10.18632/oncotarget.16871 (2017).
Neman, J. et al. Co-evolution of breast-to-brain metastasis and neural progenitor cells. Clin. Exp. Metastasis 30, 753–768. https://doi.org/10.1007/s10585-013-9576-7 (2013).
Jandial, R., Choy, C., Levy, D. M., Chen, M. Y. & Ansari, K. I. Astrocyte-induced Reelin expression drives proliferation of Her2(+) breast cancer metastases. Clin. Exp. Metastasis 34, 185–196. https://doi.org/10.1007/s10585-017-9839-9 (2017).
Eccles, S. A. & Welch, D. R. Metastasis: Recent discoveries and novel treatment strategies. Lancet 369, 1742–1757. https://doi.org/10.1016/S0140-6736(07)60781-8 (2007).
Nakasu, Y., Mitsuya, K., Deguchi, S., Hayashi, N. & Harada, H. Surgical resection of metastatic brain tumors: Risk of local recurrence and leptomeningeal dissemination, and implications for individualized treatment. No Shinkei Geka 46, 185–195. https://doi.org/10.11477/mf.1436203703 (2018).
Semba, R. et al. Difficulty diagnosing a brain tumor during clinical maintenance of a complete response to anti-HER2 treatments for metastatic breast cancer: A case report. Case Rep. Oncol. 13, 1311–1316. https://doi.org/10.1159/000511051 (2020).
Zimmer, A. S., Van Swearingen, A. E. D. & Anders, C. K. HER2-positive breast cancer brain metastasis: A new and exciting landscape. Cancer Rep. (Hoboken) https://doi.org/10.1002/cnr2.1274 (2020).
Nayak, L., Abrey, L. E. & Iwamoto, F. M. Intracranial dural metastases. Cancer 115, 1947–1953. https://doi.org/10.1002/cncr.24203 (2009).
Tarantino, P., Prat, A. & Curigliano, G. New anti-HER2 agents for brain metastasis: Histology-agnostic weapons?. Breast Cancer Res. Treat. https://doi.org/10.1007/s10549-020-05982-y (2020).
Hou, Y. et al. PHF20L1 as a H3K27me2 reader coordinates with transcriptional repressors to promote breast tumorigenesis. Sci. Adv. 6, eaaz0356. https://doi.org/10.1126/sciadv.aaz0356 (2020).
Serafim Junior, V., Fernandes, G. M. M., Oliveira-Cucolo, J. G., Pavarino, E. C. & Goloni-Bertollo, E. M. Role of tropomyosin-related kinase B receptor and brain-derived neurotrophic factor in cancer. Cytokine 136, 155270. https://doi.org/10.1016/j.cyto.2020.155270 (2020).
Huang, Z. et al. Brain metastasis reirradiation in patients with advanced breast cancer. J. Radiat. Res. 58, 142–148. https://doi.org/10.1093/jrr/rrw087 (2017).
Bartsch, R. et al. Activity of T-DM1 in Her2-positive breast cancer brain metastases. Clin. Exp. Metastasis 32, 729–737. https://doi.org/10.1007/s10585-015-9740-3 (2015).
Saleem, A. et al. Lapatinib access into normal brain and brain metastases in patients with Her-2 overexpressing breast cancer. EJNMMI Res. 5, 30. https://doi.org/10.1186/s13550-015-0103-5 (2015).
Li, Y. et al. Real-world data of triplet combination of trastuzumab, lapatinib, and chemotherapy in HER2-positive metastatic breast cancer: A multicenter retrospective study. Front. Oncol. 10, 271. https://doi.org/10.3389/fonc.2020.00271 (2020).
Shi, S. et al. The use of cationic MPEG-PCL-g-PEI micelles for co-delivery of Msurvivin T34A gene and doxorubicin. Biomaterials 35, 4536–4547. https://doi.org/10.1016/j.biomaterials.2014.02.010 (2014).
Robinet, E. et al. Blood versus marrow hematopoietic allogeneic graft. Transfus. Apher. Sci. 29, 53–59. https://doi.org/10.1016/S1473-0502(03)00104-6 (2003).
Gilson, C. R., Patel, S. R. & Zimring, J. C. CTLA4-Ig prevents alloantibody production and BMT rejection in response to platelet transfusions in mice. Transfusion 52, 2209–2219. https://doi.org/10.1111/j.1537-2995.2011.03550.x (2012).
Reittie, J. E. et al. Endogenously generated activated killer cells circulate after autologous and allogeneic marrow transplantation but not after chemotherapy. Blood 73, 1351–1358 (1989).
Grijzenhout, M. A., Aarts-Riemens, M. I., de Gruijl, F. R., van Weelden, H. & van Prooijen, H. C. UVB irradiation of human platelet concentrates does not prevent HLA alloimmunization in recipients. Blood 84, 3524–3531 (1994).
Dai, G., Jia, W., Hu, X. & Xu, L. X. Study of thermal effect on breast tumor metabolism and growth using metabonomics. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 1899–1902, 2013. https://doi.org/10.1109/EMBC.2013.6609896 (2013).
Zhuang, W. et al. Two-photon AIE luminogen labeled multifunctional polymeric micelles for theranostics. Theranostics 9, 6618–6630. https://doi.org/10.7150/thno.33901 (2019).
Taraboletti, G. et al. Bioavailability of VEGF in tumor-shed vesicles depends on vesicle burst induced by acidic pH. Neoplasia (New York) 8, 96–103. https://doi.org/10.1593/neo.05583 (2006).
Etulain, J. et al. Acidosis downregulates platelet haemostatic functions and promotes neutrophil proinflammatory responses mediated by platelets. Thromb. Haemost. 107, 99–110. https://doi.org/10.1160/th11-06-0443 (2012).
Djaldetti, M., Fishman, P., Bessler, H. & Chaimoff, C. pH-induced platelet ultrastructural alterations. A possible mechanism for impaired platelet aggregation. Arch. Surg. (Chicago, Ill) 114, 707–710. https://doi.org/10.1001/archsurg.1979.01370300061009 (1979).
Escolar, G. & White, J. G. The platelet open canalicular system: A final common pathway. Blood Cells 17, 467–485 (1991) ((discussion 486-495)).
Ansari, K. I. et al. Autocrine GM-CSF signaling contributes to growth of HER2+ breast leptomeningeal carcinomatosis. Cancer Res. https://doi.org/10.1158/0008-5472.Can-21-0259 (2021).
Hu, Z. & Yang, Y. G. Full reconstitution of human platelets in humanized mice after macrophage depletion. Blood 120, 1713–1716. https://doi.org/10.1182/blood-2012-01-407890 (2012).
Keramati, A. R. et al. Genome sequencing unveils a regulatory landscape of platelet reactivity. Nat. Commun. 12, 3626. https://doi.org/10.1038/s41467-021-23470-9 (2021).
Rodriguez, B. A. T. et al. A platelet function modulator of thrombin activation is causally linked to cardiovascular disease and affects PAR4 receptor signaling. Am. J. Hum. Genet. 107, 211–221. https://doi.org/10.1016/j.ajhg.2020.06.008 (2020).
Lee, D. H. et al. Integrative genomic analysis reveals four protein biomarkers for platelet traits. Circ. Res. 127, 1182–1194. https://doi.org/10.1161/circresaha.119.316447 (2020).
Acknowledgements
The authors express their gratitude to the City of Hope Analytical Cytometry Core. This work was made possible by the generous support of the City of Hope Department of Surgery and a grant from the United States Department of Defense Breast Cancer Research Program (W81XWH-19-1-0310).
Author information
Authors and Affiliations
Contributions
R.J. and M.Y.C. provided the tumor biopsy samples. K.A. aided in BBM1 and 2 cell line derivation, subcloning, and in vivo characterization experiments and analyzed the data. A.B. performed the hiPSC culture and differentiation experiments and analyzed the data. A.B. and R.J. wrote the manuscript. R.J. and A.B. designed and supervised the experiments and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
Rahul Jandial is a principal investigator on a grant to City of Hope National Medical Center from Department of Defense. City of Hope National medical center has pending patent application related to findings in this manuscript. The authors Arunoday Bhan, and Rahul Jandial are named as inventors in these applications.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article has been retracted. Please see the retraction notice for more detail:https://doi.org/10.1038/s41598-024-56291-z
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bhan, A., Ansari, K., Chen, M.Y. et al. RETRACTED ARTICLE: Human induced pluripotent stem cell-derived platelets loaded with lapatinib effectively target HER2+ breast cancer metastasis to the brain. Sci Rep 11, 16866 (2021). https://doi.org/10.1038/s41598-021-96351-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-96351-2
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.