Abstract
CD226 is an activating receptor expressed on the cell surface of natural killer cells and T cells. Although CD226 polymorphism is known to be involved in systemic lupus erythematosus (SLE), the involvement of soluble CD226 (sCD226) in SLE is still unknown. In the present study, we measured serum sCD226 levels using an enzyme-linked immunosorbent assay in 58 SLE patients and 33 healthy controls (HCs) and evaluated their associations with SLE Disease Activity Index 2000 (SLEDAI-2K), clinical manifestations, laboratory data, and the cumulative probability of flare. Serum sCD226 levels showed no significant differences between SLE patients and HCs. However, sCD226 levels were significantly elevated in active SLE patients with a SLEDAI-2K score of ≥ 20 compared with HCs. In SLE patients, sCD226 levels were significantly correlated with SLEDAI-2K scores and anti-dsDNA antibody titers. Moreover, the cumulative probability of flare was markedly higher in patients with high sCD226 than in those with low sCD226. In patients with neuropsychiatric involvement, sCD226 levels were elevated and reflected neuropsychiatric disease activity. These findings indicate that serum sCD226 levels are associated with disease activity and flares of SLE. Thus, it may be a useful biomarker for SLE, and its monitoring allows for more precise SLE management.
Similar content being viewed by others
Introduction
Systemic lupus erythematosus (SLE) is a multi-systemic autoimmune disease with diverse clinical manifestations1,2, commonly with renal and neuropsychiatric involvement, and shows variable severities2,3. Because the clinical course of SLE varies and flares occur several times, it is important to diagnose clinical manifestations and monitor the disease activity4. Biomarkers are valuable for assessing disease activities and predicting flares, but useful biomarkers have not been established yet.
The pathogenesis of SLE is multifactorial and includes genetic factors2,5,6,7. Genome-wide association studies have reported an association between nonsynonymous rs763361 polymorphism in CD226 and SLE in multiple ancestries8,9,10,11. CD226 is a transmembrane glycoprotein mainly expressed on T cells and natural killer (NK) cells, which acts as an activating receptor and mediates cytotoxicity12,13,14. CD226 plays an important role in the immune system, and a previous study showed that the proportion of CD226 on NK cells was decreased in active SLE patients and that CD226+ NK cells may be involved in the immunopathogenesis of SLE15.
A soluble form of CD226 (sCD226), which is shed from the membrane type of CD226 (mCD226) in human serum, has been identified. The utility of sCD226 as a biomarker has been reported in acute graft-versus-host disease (aGVHD)16,17 and some types of cancers18,19,20. As for autoimmune diseases, a more recent study found that serum sCD226 levels were associated with disease activity in rheumatoid arthritis (RA)21. Although several findings suggest that CD226 is involved in the pathogenesis of SLE8,9,10,11,15, the association between sCD226 and SLE is still unknown.
This study aimed to reveal the association of sCD226 with SLE by measuring serum sCD226 levels using an enzyme-linked immunosorbent assay (ELISA) in SLE patients, as well as by evaluating the associations between sCD226 levels and the disease activity, clinical manifestations, and flares of SLE.
Results
Serum sCD226 levels are increased in active SLE patients and reflect disease activity
To study the association between sCD226 and SLE, we first measured serum sCD226 levels using ELISA in 58 SLE patients (mean age, 41.0 years; 53 females) and 33 healthy controls (HCs) (mean age, 36.2 years; 28 females). No significant differences were found between SLE patients and HCs in terms of age and gender. The baseline characteristics of the SLE patients are shown in Table 1. There were 42 patients receiving treatment. Thus, we first confirmed that serum sCD226 levels were almost the same between SLE patients with medication, such as corticosteroids and immunosuppressive agents, and those without medication and were not significantly correlated with prednisolone equivalent dose (see Supplementary Fig. S1). Although there was no significant difference between SLE patients and HCs regarding median levels of serum sCD226, these still had a wide range of values and the SLE patients had higher values than had HCs (Fig. 1). Because of this, we classified the SLE patients into three groups on the basis of SLE Disease Activity Index 2000 (SLEDAI-2K) scores, and then compared sCD226 levels between HCs and these groups. Serum sCD226 levels were found to be significantly elevated in active SLE patients than in other SLE patients and HCs (Fig. 2a). Similarly, serum sCD226 levels were increased in an active SLE patient receiving no treatment than in other 15 SLE patients receiving no treatment [20.0 ng/ml vs 0.22 ng/ml (0.10–2.65); P = 0.15].
Serum sCD226 levels in SLE patients and HCs. Serum sCD226 levels were compared between SLE patients and HCs. Data are shown as box plots. The boxes represent the upper and lower IQRs; lines inside the boxes represent the median; whiskers represent 1.5 times the upper and lower IQRs; points outside the whiskers represent outliers. Statistical differences among groups were evaluated using the Mann–Whitney U test. sCD226 soluble CD226, SLE systemic lupus erythematosus, HCs healthy controls, IQRs interquartile ranges.
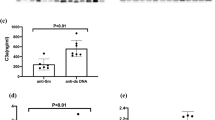
Associations between serum sCD226 levels and SLEDAI-2K scores. (a) Serum sCD226 levels were compared between SLE patients with SLEDAI-2K scores of 0, 1–19, and ≥ 20, as well as HCs. (b) Correlations between serum sCD226 levels and SLEDAI-2K scores in SLE patients. (c) Serum sCD226 levels before and after treatment in 11 SLE patients. (d) SLEDAI-2K scores before and after treatment in 11 SLE patients. (e) Correlations between the ΔsCD226 and the ΔSLEDAI-2K scores in SLE patients. Each data point represents a single subject. Horizontal lines show the median. Statistical differences among groups were evaluated using the Steel test, setting HCs as a control. Correlation analyses were evaluated using Spearman’s rank correlation. sCD226 soluble CD226, SLE systemic lupus erythematosus, SLEDAI-2K SLE Disease Activity Index 2000, HCs healthy controls, ΔsCD226 changes in sCD226, ΔSLEDAI-2K changes in SLEDAI-2K.
We next studied the relationship between sCD226 levels and SLE disease activity. Serum sCD226 levels had a significantly positive correlation with SLEDAI-2K (ρ = 0.39; P = 0.003) (Fig. 2b). When compared with conventional biomarkers, sCD226 levels were significantly correlated with anti-dsDNA antibody titers (ρ = 0.28; P = 0.035) and inversely correlated with serum levels of C3 (ρ = − 0.17; P = 0.19) and C4 (ρ = − 0.20; P = 0.14) (see Supplementary Fig. S2). In comparison with laboratory findings, serum sCD226 levels had no obvious correlation to white blood cell count, platelet count, erythrocyte sedimentation rate, and C-reactive protein (see Supplementary Fig. S3).
Furthermore, we also examined sCD226 levels in 11 SLE patients before and after treatment. Among the 11 SLE patients, serum sCD226 levels were decreased or remained low after treatment in 9 SLE patients with improvement in SLEDAI-2K scores. In contrast, serum sCD226 levels were increased in two SLE patients: one with no improvement in SLEDAI-2K scores and the other with newly neuropsychiatric manifestation after treatment (Fig. 2c,d). The changes in sCD226 had a significant correlation with the changes in SLEDAI-2K scores (ρ = 0.67; P = 0.024) (Fig. 2e).
sCD226 levels can predict disease flare
In this study, flares occurred in 15 out of 58 SLE patients examined. Because the highest sCD226 level in HCs was 10.0 ng/ml, we defined this as the cut-off value for classifying SLE patients as having high and low sCD226 levels. The cumulative probability of flare for patients with high sCD226 (Fig. 3, shown in solid lines) was significantly higher than that for those with low sCD226 (Fig. 3, shown in dashed lines) (P = 0.016).
sCD226 levels are increased in SLE patients with neuropsychiatric manifestation and reflect neuropsychiatric disease activity
We then assessed the association between sCD226 levels and clinical manifestations of SLE. Serum sCD226 levels were elevated in patients with mucocutaneous, hematological, musculoskeletal, and/or neuropsychiatric manifestations classified using the British Isles Lupus Assessment Group (BILAG) 2004 index (Table 2). In clinical descriptors of SLEDAI-2K, serum sCD226 levels were significantly increased in patients with psychosis, visual disturbance, arthritis, myositis, rash, and/or mucosal ulcers (see Supplementary Table S1).
Because neuropsychiatric systemic lupus erythematosus (NPSLE) is a major vital organ manifestation of SLE3, we further studied the relationships between sCD226 levels and neuropsychiatric manifestation. Serum sCD226 levels were elevated in patients with active neuropsychiatric manifestation (Table 2). Serum sCD226 levels were also significantly higher in active NPSLE patients than in previous NPSLE patients (Fig. 4a). Moreover, among active NPSLE patients, sCD226 levels were significantly higher in patients with BILAG category A, which is defined as severe disease activity, than in those with BILAG category B (Fig. 4b).
Serum sCD226 levels in SLE patients with neuropsychiatric manifestation. (a) Serum sCD226 levels were compared between active NPSLE patients and previous NPSLE patients. (b) Serum sCD226 levels were compared between neuropsychiatric SLE patients with BILAG category A and those with BILAG category B. Each data point represents a single subject. Horizontal lines show the median. Statistical differences among groups were evaluated using the Mann–Whitney U test. sCD226 soluble CD226, SLE Systemic lupus erythematosus, NPSLE neuropsychiatric systemic lupus erythematosus, BILAG British Isles Lupus Assessment Group.
Discussion
In this study, we demonstrated that serum sCD226 levels were significantly increased in active SLE patients and were associated with disease activity and neuropsychiatric manifestation. We also showed that SLE patients with high sCD226 levels had a high probability of experiencing disease flares.
CD226 is an immunoglobulin superfamily expressed on the cell membrane of NK cells, T cells, B cells, monocytes, and platelets12,13,14. A soluble form of CD226 has been identified and was reported to be shed from the cell membrane maybe by a certain protease16. CD226 is a costimulatory adhesion molecule involved in certain immune functions such as mediating cytotoxic signals12,13,14, the ligands of which are CD112 and CD15513. This costimulatory molecule has a paired receptor, T cell immunoreceptor with immunoglobulin and immunoreceptor tyrosine-based inhibitory motif domain (TIGIT), which is a coinhibitory receptor that inhibits the interaction between CD155 and CD226, in turn also inhibits the activation of T cells and NK cells22,23. On the basis of the results of genome-wide association studies and functional analyses of mCD226 on T cells or NK cells, CD226 is thought to play an important role in the pathogenesis of autoimmune diseases such as SLE8,9,10,11,15, RA11,24,25,26,27, and systemic sclerosis28,29,30. However, the utility of sCD226 as a biomarker and its functions in these diseases remain unknown.
In this study, we aimed to investigate the association between sCD226 and SLE, and found that serum sCD226 levels were significantly elevated in active SLE patients than in HCs and were associated with disease activity. Moreover, we also found that the cumulative probability of flare was significantly higher in patients with high sCD226 than in patients with low sCD226, indicating that sCD226 may be predictive of flares. According to the 2019 EULAR recommendation, SLE treatment should aim for remission or low disease activity and prevention of flares4. Although many useful biomarkers for monitoring disease activity have been reported31,32, no proper biomarkers for predicting flares were found. In our study, serum sCD226 levels were associated with not only disease activity but also with SLE flares. Therefore, sCD226 may be a useful biomarker for SLE.
In this study, we further investigated the association between sCD226 levels and neuropsychiatric manifestation, which is a frequent major organ manifestation in SLE3,4. Diverse neuropsychiatric symptoms make it difficult to distinguish from other diseases and hinder a proper diagnosis3. Additionally, its management is challenging because of the variable severity3. Useful biomarkers for both diagnosing and monitoring disease activity are required to manage NPSLE properly, but none have been established yet33,34. Although cerebrospinal fluid (CSF) tests from lumbar puncture are valuable for the exclusion of infectious diseases and some CSF biomarkers, such as anti-ribosomal P protein antibodies, IgG index, and IL-6, may be useful for assessing disease activity, the procedure is invasive and difficult to repeat for monitoring3,33. In our study, serum sCD226 levels were elevated in patients with neuropsychiatric involvement and reflected neuropsychiatric disease activity. The findings indicate that serum sCD226 may be a useful biomarker for both diagnosing and monitoring neuropsychiatric manifestation of SLE.
Regarding the function of sCD226 in cancer, several studies reported that sCD226 may block the cytotoxicity of NK cells by blocking CD155 or CD11218, and that sCD226 could directly inhibit the proliferation of cancer cells in vitro19. In aGVHD, Kanaya et al. explained that binding of sCD226 to CD155 may cancel the inhibitory signals by TIGIT in T cells16. In mouse SLE models, treatment with TIGIT-Ig fusion protein reduced autoantibody production and improved survival rate35. These findings indicate the binding of sCD226 to CD155 may cancel the inhibitory signals by TIGIT in SLE as well; this interaction is likely involved in the pathogenesis of SLE. Although we showed the possible utility of sCD226 as a biomarker for SLE in this study, we did not study the immune functions of sCD226 in SLE. Therefore, further analyses are required to reveal the functions of sCD226 in SLE.
This study had some limitations. First, our study had a small sample size and was conducted at a single center. This study needs to be replicated with a larger sample size in a multicenter setting. Second, the functions of sCD226 are still unknown; further analyses are required to reveal this. Lastly, our study was a retrospective study. We could not measure sCD226 levels longitudinally in all SLE patients, and changes in the levels at several points or over a short period are not apparent. To ensure the association of sCD226 levels with the disease activity and prognosis of SLE, a prospective study with longitudinal assessments should be performed.
In conclusion, we showed that serum sCD226 levels were elevated in active SLE patients and were associated with disease activity and prognosis. Serum sCD226 may be a useful biomarker for SLE, and its monitoring allows for more precise SLE management.
Methods
Study population
We studied 58 Japanese patients who were treated for SLE at the Kyushu University Hospital between 2014 and 2020. We enrolled patients who met at least four of the American College of Rheumatology revised criteria for SLE36 and had no other autoimmune disease, infection, or cancer. Many of these patients were treated with corticosteroids, hydroxychloroquine, and immunosuppressive drugs, either as monotherapy or in combination. Among these 58 SLE patients, we were able to assess 11 patients both before and after treatment. We studied 33 HCs as well.
This study was approved by the ethics committee of Kyushu University Hospital (approval number 2019-481) in accordance with the Helsinki Declaration. All participants gave written informed consent.
Data collection
We obtained the following information from the medical records of the patients: demographic data, clinical manifestations, laboratory findings, and medications at baseline and after treatment. Disease activity was evaluated using SLEDAI-2K37, with active SLE defined as having a SLEDAI-2K score of ≥ 2038. Clinical manifestations were classified using the BILAG 2004 index39, and patients with BILAG category A or category B at the time of sCD226 level measurement were defined as those with each clinical manifestation. Neuropsychiatric disease activity was assessed by BILAG 2004 index39. A flare was defined as a measurable increase in disease activity usually leading to a change of treatment4,40.
Enzyme-linked immunosorbent assay
Serum sCD226 levels were measured via sandwich ELISA, in accordance with a previous report16. In summary, 96-well plates were coated with purified anti-human CD226 (DNAM-1) antibody (TX25; BioLegend, San Diego, CA, USA) (8 μg/ml, 100 μl/well) for 2 h at room temperature and then washed with washing buffer (0.05% Tween 20). The plates were blocked using a blocking buffer (1% BSA in PBS, 100 μl/well) for 2 h at room temperature and then washed. Recombinant human DNAM-1/CD226 Fc chimera protein (as a standard) (R&D Systems, Minneapolis, MN, USA) and serum samples were added at 100 μl/well and incubated overnight at 4 °C. The plates were washed and then incubated with human DNAM-1/CD226 biotinylated antibody (R&D Systems) (0.6 μg/ml, 100 μl/well) for 1 h at room temperature. After washing, streptavidin–horseradish peroxidase (R&D Systems) (1:200 in a washing buffer, 100 μl/well) was added and incubated for 30 min at room temperature. The plates were washed and then reacted with 3,3′,5,5′-tetramethylbenzidine substrate reagent set (BD Biosciences, San Jose, CA, USA) (100 μl/well) for 20 min at room temperature. The reaction was stopped by H2SO4 (2 N) (50 μl/well), and then absorbance was measured at 450 nm using a microtiter plate reader (Thermo Fisher Scientific, Waltham, MA, USA). All values were determined in duplicate. The assay range was 0.1–20.0 ng/ml.
Statistical analysis
Results are expressed as the median and interquartile range unless otherwise stated. Comparisons between two groups were done using the Student’s t-test for normally distributed continuous variables or using the Mann–Whitney U test for non-normally distributed variables. When making multiple group comparisons, the Steel test was used for non-normally distributed variables, setting HCs as a control. The correlations between two continuous variables were analyzed using Spearman’s rank correlation. Flaring episodes were represented via the Kaplan–Meier method and compared using log-rank tests. All tests were two-tailed and P-values < 0.05 were considered statistically significant. All analyses were performed using the JMP software, version 15 (SAS Institute, Cary, NC, USA).
Data availability
All data generated or analyzed during this study are included in this published article and its Supplementary Information files.
References
Tsokos, G. C. Systemic lupus erythematosus. N. Engl. J. Med. 365, 2110–2121 (2011).
Tsokos, G. C. Autoimmunity and organ damage in systemic lupus erythematosus. Nat. Immunol. 21, 605–614 (2020).
Schwartz, N., Stock, A. D. & Putterman, C. Neuropsychiatric lupus: New mechanistic insights and future treatment directions. Nat. Rev. Rheumatol. 15, 137–152 (2019).
Fanouriakis, A. et al. 2019 Update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann. Rheum. Dis. 78, 736–745 (2019).
Tsokos, G. C., Lo, M. S., Reis, P. C. & Sullivan, K. E. New insights into the immunopathogenesis of systemic lupus erythematosus. Nat. Rev. Rheumatol. 12, 716–730 (2016).
Harley, I. T. W., Kaufman, K. M., Langefeld, C. D., Harley, J. B. & Kelly, J. A. Genetic susceptibility to SLE: New insights from fine mapping and genome-wide association studies. Nat. Rev. Genet. 10, 285–290 (2009).
Bentham, J. et al. Genetic association analyses implicate aberrant regulation of innate and adaptive immunity genes in the pathogenesis of systemic lupus erythematosus. Nat. Genet. 47, 1457–1464 (2015).
Sun, C. et al. High-density genotyping of immune-related loci identifies new SLE risk variants in individuals with Asian ancestry. Nat. Genet. 48, 323–330 (2016).
Nie, D. et al. Gene–gene interaction between CD40 and CD226 gene on systemic lupus erythematosus in the Chinese Han population. Rheumatol. Int. 36, 1657–1662 (2016).
Wang, Y. F. et al. Identification of ST3AGL4, MFHAS1, CSNK2A2 and CD226 as loci associated with systemic lupus erythematosus (SLE) and evaluation of SLE genetics in drug repositioning. Ann. Rheum. Dis. 77, 1078–1084 (2018).
Maiti, A. K. et al. Non-synonymous variant (Gly307Ser) in CD226 is associated with susceptibility to multiple autoimmune diseases. Rheumatology (Oxford) 49, 1239–1244 (2010).
Shibuya, A. et al. DNAM-1, a novel adhesion molecule involved in the cytolytic function of T lymphocytes. Immunity 4, 573–581 (1996).
Xu, Z. & Jin, B. A novel interface consisting of homologous immunoglobulin superfamily members with multiple functions. Cell. Mol. Immunol. 7, 11–19 (2010).
Martinet, L. & Smyth, M. J. Balancing natural killer cell activation through paired receptors. Nat. Rev. Immunol. 15, 243–254 (2015).
Huang, Z. et al. Involvement of CD226+ NK cells in immunopathogenesis of systemic lupus erythematosus. J. Immunol. 186, 3421–3431 (2011).
Kanaya, M. et al. Soluble DNAM-1, as a predictive biomarker for acute Graft-Versus-Host disease. PLoS One 11, 1–12 (2016).
Goshima, Y. et al. A mathematical model for dynamics of soluble form of DNAM-1 as a biomarker for graft-versus-host disease. PLoS One 15, 1–12 (2020).
Xu, Z. et al. Increased levels of soluble CD226 in sera accompanied by decreased membrane CD226 expression on peripheral blood mononuclear cells from cancer patients. BMC Immunol. 10, 1–8 (2009).
Hou, S., Zheng, X., Wei, H., Tian, Z. & Sun, R. Recombinant soluble CD226 protein directly inhibits cancer cell proliferation in vitro. Int. Immunopharmacol. 19, 119–126 (2014).
Takahashi, N. et al. Increased soluble CD226 in sera of patients with cutaneous T-cell lymphoma mediates cytotoxic activity against tumor cells via CD155. J. Investig. Dermatol. 137, 1766–1773 (2017).
Mosaad, Y. M. et al. Association between CD226 polymorphism and soluble levels in rheumatoid arthritis: Relationship with clinical activity. Immunol. Investig. 47, 264–278 (2018).
Yu, X. et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat. Immunol. 10, 48–57 (2009).
Stanietsky, N. et al. The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. Proc. Natl. Acad. Sci. USA 106, 17858–17863 (2009).
Hafler, J. P. et al. CD226 Gly307Ser association with multiple autoimmune diseases. Genes. Immun. 10, 5–10 (2009).
Suzuki, T. et al. Non-synonymous variant (Gly307Ser) in CD226 is associated with susceptibility in Japanese rheumatoid arthritis patients. Mod. Rheumatol. 23, 200–202 (2013).
Lee, Y. H., Bae, S. C. & Song, G. G. Association between the CTLA-4, CD226, FAS polymorphisms and rheumatoid arthritis susceptibility: A meta-analysis. Hum. Immunol. 76, 83–89 (2015).
Fasth, A. E. R., Björkström, N. K., Anthoni, M., Malmberg, K. J. & Malmström, V. Activating NK-cell receptors co-stimulate CD4+CD28− T cells in patients with rheumatoid arthritis. Eur. J. Immunol. 40, 378–387 (2010).
Dieudé, P. et al. Association of the CD226 Ser307 variant with systemic sclerosis. Arthritis Rheum. 63, 1097–1105 (2011).
Jin, J., Chou, C., Lima, M., Zhou, D. & Zhou, X. Systemic sclerosis is a complex disease associated mainly with immune regulatory and inflammatory genes. Open Rheumatol. J. 8, 29–42 (2014).
Ayano, M. et al. Increased CD226 expression on CD8+ T cells is associated with upregulated cytokine production and endothelial cell injury in patients with systemic sclerosis. J. Immunol. 195, 892–900 (2015).
Arriens, C., Wren, J. D., Munroe, M. E. & Mohan, C. Systemic lupus erythematosus biomarkers: The challenging quest. Rheumatology (Oxford) 56, i32-45 (2017).
Capecchi, R., Puxeddu, I., Pratesi, F. & Migliorini, P. New biomarkers in SLE: From bench to bedside. Rheumatology (Oxford) https://doi.org/10.1093/rheumatology/keaa484 (2020).
Jeltsch-David, H. & Muller, S. Neuropsychiatric systemic lupus erythematosus: Pathogenesis and biomarkers. Nat. Rev. Neurol. 10, 579–596 (2014).
Hanly, J. G., Kozora, E., Beyea, S. D. & Birnbaum, J. Review: Nervous system disease in systemic lupus erythematosus: Current status and future directions. Arthritis Rheumatol. 71, 33–42 (2019).
Liu, S. et al. Treatment of murine lupus with TIGIT-Ig. Clin. Immunol. 203, 72–80 (2019).
Hochberg, M. C. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 40, 1725 (1997).
Gladman, D. D., Ibañez, D. & Urowitz, M. B. Systemic lupus erythematosus disease activity index 2000. J. Rheumatol. 29, 288–291 (2002).
Cook, R. J., Gladman, D. D., Pericak, D. & Urowitz, M. B. Prediction of short term mortality in systemic lupus erythematosus with time dependent measures of disease activity. J. Rheumatol. 27, 1892–1895 (2000).
Isenberg, D. A. et al. BILAG 2004. Development and initial validation of an updated version of the British Isles Lupus Assessment Group’s disease activity index for patients with systemic lupus erythematosus. Rheumatology (Oxford) 44, 902–906 (2005).
Ruperto, N. et al. International consensus for a definition of disease flare in lupus. Lupus 20, 453–462 (2011).
Acknowledgements
This work was supported by Japan Society for the Promotion of Science [Grant number JSPS KAKENHI 19K17887]. The authors thank Enago for the English language review.
Author information
Authors and Affiliations
Contributions
M.N. and M.Ay. participated in study conception and design. M.N., M.Ay., K.K., S.K., K.H., and S.I. participated in data acquisition and analysis. M.N., M.Ay., K.K., H.M., Y.K., M.Ak., N.O., Y.A., K.A., T.H., and H.N. contributed to the interpretation of results. M.N. was a major contributor in writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nakano, M., Ayano, M., Kushimoto, K. et al. Association of elevated serum soluble CD226 levels with the disease activity and flares of systemic lupus erythematosus. Sci Rep 11, 16162 (2021). https://doi.org/10.1038/s41598-021-95711-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-95711-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.