Abstract
It is hypothesized that vitamin D deficiency could be related to ovarian reserve. This systematic review and meta-analysis was undertaken to analyze the possible association between vitamin D and ovarian reserve among adolescent and adult women. All eligible studies identified through the ISI Web of Science, PubMed, and Scopus were included up to May 2021. A random-effects meta-analysis model was implemented and a weighted mean difference (WMD) and 95% confidence interval (CI) were calculated. A total of 38 papers covering 8608 individuals were enrolled in this systematic review and meta-analysis. Antral follicle count (AFC) was significantly lower among Asians (WMD − 0.65; 95% CI − 1.28 to − 0.01; P = 0.04; I2 = 0.0%) and luteinizing hormone (LH) levels were higher in non-Asians (WMD 2.16 IU/L; 95% CI 0.20 to 4.12; P = 0.031; I2 = 9.3%) with vitamin D insufficiency/deficiency. Also, there was a negative correlation between vitamin D and LH/FSH ratio in women with normal body mass index (BMI) (Fisher’s Z: − 0.18; 95% CI − 0.37 to − 0.008; P = 0.041; I2 = 51.5%). Although there were no significant associations between serum vitamin D levels and any of the intended ovarian reserve markers, subgroup analyses have found significant findings regarding AFC, LH, and LH/FSH ratio. In order to understand the underlying mechanisms of vitamin D in female reproduction, further attempts are needed.
Similar content being viewed by others
Introduction
Vitamin D is an essential nutrient with a hormone-like activity that was initially recognized for its importance in bone health and calcium–phosphate homeostasis1. Though, the recent vitamin D deficiency pandemic has emphasized other functions2. Growing documents suggest that vitamin D deficiency upsurges the risk of various chronic disorders including obesity, type 1 diabetes mellitus, cardiovascular, infectious, and autoimmune diseases; certain types of cancer; depression, and chronic pain2.
More recently, a regulatory role for vitamin D has been suggested in female fertility3,4. In this context, previous epidemiological investigations have proposed a seasonality in female reproductive capacity which might be explained partially by seasonal variation in serum levels of vitamin D5. Biological activities of vitamin D are applied through the vitamin D receptors (VDR) that have been detected in the ovary especially in granulosa cells and theca cells, endometrium and placenta6. This diverse VDR expression proposes a potential role of vitamin D in female reproduction7. Though the underlying mechanism by which vitamin D may involve in reproductive physiology is poorly known, a direct link between vitamin D and ovarian steroidogenesis has been proposed. This link is derived from several in-vitro and in-vivo studies indicating that vitamin D could stimulate steroidogenesis in ovarian cells by modulating the mRNA and protein expression levels of steroidogenic enzymes including Cyp11a1, StAR, Cyp19a1, and 3β-HSD8,9. Reproductive potential of an individual is mainly explained by the quality and the quantity of ovarian primordial follicles that was diminished as women get older. Therefore, several markers have suggested to illustrate the ovarian reserve status. Low anti-Mullerian hormone (AMH), low antral follicle count (AFC), low luteinizing hormone (LH), high follicle-stimulating hormone (FSH), and low LH/FSH ratio may represent a diminished ovarian reserve status10.
Nevertheless, the findings of experimental studies are consistent enough to suggest the association between vitamin D and ovarian reserve, the evidence of human studies are commonly inconsistent, with some documents supporting this relation11,12 and others failing to detect any significant association7,13,14. For example, Dennis et al.11 have suggested that vitamin D may pose a regulatory role in the production of AMH; however, the works of Drakopoulos et al.7, Pearce et al.13, and Shapiro et al.15 did not verify this association. With regard to the conflicting findings and the increasing trend of interest about the role of vitamin D in female reproduction, this study collects the available documents to clarify this issue. We aimed to perform a systematic review and meta-analysis to reach a firm conclusion about the possible link between serum vitamin D levels and ovarian reserve markers including AMH, AFC, LH, and FSH among adolescent and adult women using observational studies.
Methods
The present study was designed and conducted based on the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) Statements16 and also was registered (Prospero database: CRD42020191703).
Data source and search strategy
The electronic databases ISI Web of Science, PubMed, and Scopus were systematically searched from the earliest available date to May 2021 to identify relevant studies. Two investigators (A.A and E.K) independently searched the above-mentioned databases to find studies on the association between vitamin D and ovarian reserve, using the following keywords: (“ovarian reserve” OR “oocyte reserve” OR “Anti Mullerian hormone” OR “Mullerian inhibiting factor” OR “anti Mullerian factor” OR “Mullerian inhibitory substance” OR “Mullerian inhibiting hormone” OR “Mullerian inhibiting substance” OR “Mullerian regression factor” OR “AMH” OR “Follicle-stimulating hormone” OR “FSH” OR “Luteinizing hormone” OR “LH” OR “Antral follicle count” OR “AFC”) AND (“vitamin D” OR “25-Hydroxyvitamin D” OR “cholecalciferol” OR “ergocalciferol” OR “calciol” OR “vitamin D3” OR “25(OH)D3”).
The bibliographic lists of any of the eligible studies were also scanned to detect any additional qualified ones. We also contacted expert scientists in the field of ovarian reserve and vitamin D to lower the chance of missing any additional studies.
Study selection and eligibility criteria
The PICO (Population/intervention/comparison/outcome) components were as follows: P (adolescent and adult premenopausal women with vitamin D deficiency/insufficiency), I (serum levels of vitamin D), C (women with a normal level of serum vitamin D), O (ovarian reserve markers including AMH, AFC, LH, FSH, and LH/FSH ratio). The inclusion criteria were as follows: (1) original human observational studies either with case–control, cross-sectional, or longitudinal design; (2) published in the English language; (3) assessed serum levels of at least one of the ovarian reserve markers including AMH, AFC, LH, FSH, and LH/FSH ratio in association with 25(OH)D; and (4) those presented as (4.1) comparison of ovarian reserve markers (AMH, AFC, LH, FSH, and LH/FSH ratio) between women with vitamin D insufficiency/deficiency and vitamin D sufficient ones; or (4.2) correlation between 25(OH)D and ovarian reserve markers (AMH, AFC, LH, FSH, and LH/FSH ratio).
The exclusion criteria were as follows: (1) Experimental studies; (2) recruited pregnant, lactating, or postmenopausal women; and (3) poster abstracts, case reports, review articles, editorials, and non-original full-length articles, or those without original data or articles with no appropriate outcome measures. Two assessors independently (A.A and E.K) conducted the selection process. Any disagreement was resolved through discussion with a third reviewer (R.A).
Data extraction
The following data were extracted: first author's name, year of publication, geographical location, sample size, participant characteristics including health status, age and body mass index (BMI), 25(OH)D assay method, cut-off values of vitamin D status, the season of sample collection, study design, reported ovarian reserve markers, and statistical adjustment.
Quality assessment
The quality assessment of eligible studies was performed by two reviewers (A.A and E.K) individually using the Newcastle–Ottawa Scale (NOS) star system (ranged, 0–9 stars)17, which focuses on selection, comparability, and outcome. Studies scoring ≥ 7, 4–6, and ≤ 3 points were assumed as high, moderate, and low quality, respectively18.
Statistical analysis
The present study was performed to present the association between vitamin D and ovarian reserve quantitatively. Prior to the calculation of the effect size, the concentration of AMH was converted to ng/mL, LH to IU/l, and FSH to IU/l. In the current study, we calculated two types of effect sizes: (1) weighted mean difference (WMD) in AMH, AFC, LH, or FSH between vitamin D insufficiency/deficiency and sufficient vitamin D groups; and (2) Fisher’s Z of the correlation between 25(OH)D and AMH, AFC, LH, FSH or LH/FSH. If a document provided the results stratified by certain variables like age, BMI, and participants’ health status, it was divided into two different studies supposed to be independent of each other. In the absence of the mean and standard deviation (SD), values of median and range or median and interquartile range were converted into mean and SD based on related formulas19. Fisher’s Z and its SE using correlation coefficients (r) and sample size (N) were calculated by the relevant formula20. Heterogeneity between effect size of included studies was estimated by chi-squared (χ2) test and I2 statistic [I2 index < 40 (low heterogeneity), 40–75 (moderate heterogeneity) and > 75% (high heterogeneity)]21. When there was no significant heterogeneity, the effect size was calculated using a fixed-effects model. Otherwise, a random-effects model was used22. Subgroup analyses were done based on different characteristics of included studies, whenever possible, to check the sources of heterogeneity. Publication bias was assessed using Egger’s and Begg’s statistics23 and in the presence of significant publication bias, trim & fill analysis was performed to detect any possibly missed study. The sensitivity analyses were also conducted to evaluate the influence of every single study on the stability of the meta-analysis findings. The statistical analyses were done using STATA statistical program version 11.2 (Stata Corporation, College Station, TX, USA). A two-sided p-value < 0.05 was considered statistically significant.
Ethical approval
All analyses were based on previous published studies; thus, no ethical approval was required.
Results
Characteristics of included studies
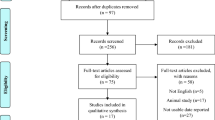
The primary search yielded 1648 articles. A total of 36 eligible articles involving 7882 individuals were included in this study with a sample size ranging from 26 to 851. Participants' mean age ranged from 17.8 to 42.5 years old and BMI from 20.7 to 35.7 kg/m2. The enrolled studies were published between 2009 and 2020 of which 9 were from Turkey24,25,26,27,28,29,30,31,32, 5 from United States12,15,33,34,35, 3 from Iran36,37,38, 3 from Poland39,40,41, 2 from South-Korea14,42, 2 from Saudi Arabia43,44, 2 from China45,46, 2 from India47,48. Others were from Spain49, Belgium7, Slovakia50, Egypt51, Bosnia and Herzegovina52, Australia53, Japan54, and Canada55. Moreover, 21 studies were cross-sectional in design7,14,24,25,26,27,28,29,33,36,37,39,40,42,43,46,47,49,51,52,54, 9 case–control30,31,32,38,41,44,45,48,50 and 6 cohorts12,15,34,35,53,55. Seventeen studies mentioned the season of sample collection7,14,15,27,32,33,34,35,37,38,40,41,42,52,53,54,55. Twenty-five studies selected serum vitamin D < 20 ng/ml as deficient, eight studies serum vitamin D < 10 ng/ml, and the others did not mention the cut-off values. Based on the NOS, 23 studies were ranked as high quality7,12,15,24,27,28,29,32,33,34,35,37,42,43,44,45,46,48,49,50,53,54,55 and 13 moderate14,25,26,30,31,36,38,39,40,41,47,51,52, respectively. Table 1 provides the primary information of enrolled studies and Fig. 1 provides the study selection process applied for this systematic review and meta-analysis.
Findings from the systematic review
Four studies have examined the association between 25(OH)D and ovarian reserve markers, however, due to insufficient data, they were described qualitatively.
In the first study, Ghadimi et al.38, have assessed the association between vitamin D and metabolic parameters of PCOS in high-school girls (mean age: 17.85 years old) through a case–control design. The study included 104 PCOS individuals and 88 non-PCOS controls. Based on Pearson’s test, no significant correlation was found between 25(OH)D levels and LH and FSH levels.
The other investigation has been conducted by Jukic et al.33 in 2015 to explore the relationship between FSH and serum vitamin D among 527 premenopausal women (mean age: 17.85 years old). In this cross-sectional study, 25(OH)D and urinary FSH levels were inversely correlated (P = 0.003).
The other study in 2018 was conducted by Arefi et al.36 to explore the correlation between vitamin D deficiency and ovarian reserve through a cross-sectional study of 189 Iranian infertile women (mean age: 32.21, mean BMI: 26.7 kg/m2). The result of this study proposed a highly significant correlation between vitamin D and AFC (p < 0.001).
The last evidence was conducted among infertile and fertile females (18–40 years old) to investigate the correlation of vitamin D deficiency with serum AMH. The result of this cross-sectional study failed to show any significant correlation between vitamin D and AMH in either fertile or infertile women47.
Findings from meta-analysis
Comparison of ovarian reserve markers between women with vitamin D insufficiency/deficiency and sufficient ones
Serum 25(OH)D levels and AFC
The analysis of six studies7,29,49,54,55 with 2242 participants revealed that AFC is lower in patients with vitamin D insufficiency/deficiency compared to their controls (WMD − 0.56; 95% CI − 1.12 to − 0.004; P = 0.052) without significant heterogeneity (I2 = 0.0%, P = 0.555) (Fig. 2). Subgroup analysis revealed a significant result only among Asian population (WMD − 0.65; 95% CI − 1.28 to − 0.01; P = 0.04) (Table 2). Findings from sensitivity analysis revealed that the exclusion of Drakopoulos et al.7 (WMD = − 0.61; 95% CI − 1.19 to − 0.04) and Fabris et al.49 (WMD = − 0.63; 95% CI − 1.23 to − 0.02) studies from the analysis changed the overall result.
Serum 25(OH)D levels and AMH
Serum levels of AMH were compared between 1561 women with vitamin D insufficiency/deficiency and 924 women with sufficient vitamin D status using 8 studies7,15,28,40,49,54 yielded non-significant difference (WMD 0.02 ng/mL; 95% CI − 0.40 to 0.43; P = 0.929) with evidence of heterogeneity (I2 = 63.2%, P = 0.008) (Fig. 3). Furthermore, subgroup analysis did not change the results (Table 2). Meta-analysis findings were not sensitive to individual studies.
Serum 25(OH)D levels and FSH
The analysis of seven datasets15,28,29,48,52,55 including 1164 participants showed that serum FSH was not significantly associated with vitamin D status (WMD − 0.04 IU/l; 95% CI − 0.47 to 0.40; P = 0.870) with significant heterogeneity (I2 = 55.9%, P = 0.034) (Fig. 4). In addition, subgroup analysis did not change the findings (Table 2). The overall meta-analysis result for FSH was not sensitive to individual studies.
Serum 25(OH)D levels and LH
The difference of LH according to the vitamin D status was examined in six studies28,29,48,50,52 which was not significant (WMD 0.05 IU/l; 95% CI − 0.67 to 0.76; P = 0.900) (Fig. 5). Substantial heterogeneity was not observed (I2 = 39.7%, P = 0.141) among the included studies. Subgroup analysis of geographical areas revealed that serum LH was significantly higher among the non-Asian population with vitamin D insufficiency/deficiency compared to the control group (WMD 2.16 IU/l; 95% CI 0.20 to 4.12; P = 0.031) with no evidence of heterogeneity (I2 = 9.3%, P = 0.294) (Table 2). Excluding individual studies did not change the overall meta-analysis results.
Publication bias
No evidence of publication bias was observed for AFC (Begg’s test: P = 0.573, Egger’s test: P = 0.655), AMH (Begg’s test: P = 0.621, Egger’s test: P = 0.836), and FSH (Begg’s test: P = 0.293, Egger’s test: P = 0.401). There was evidence of publication bias for LH (Begg’s test: P = 0.039, Egger’s test: P = 0.251) and trim & fill analysis was applied. Two studies were filled and meta-analysis was done with new dataset but the results did not change (WMD − 0.23 ng/mL; 95% CI − 1.08 to 0.60; P = 0.577; I2 = 55.7%).
The correlation between ovarian reserve markers and serum 25(OH)D levels
The correlation between AFC and 25(OH)D levels
There was no significant correlation between AFC and 25(OH)D using 5 studies7,14,53,54 with 1391 participants (Fisher’s Z: 0.03; 95% CI − 0.03 to 0.08; P = 0.343) with no evidence of heterogeneity (I2 = 0.0%, P = 0.845) (Fig. 6). Findings were not sensitive to any individual studies.
The correlation between AMH and 25(OH)D levels
Twenty studies7,14,28,32,34,37,40,41,42,45,46,51,53,54 with 3406 subjects evaluated the correlation between AMH and 25(OH)D. There was no significant correlation between AMH and 25(OH)D (Fisher’s Z: − 0.03; 95% CI − 0.11 to 0.04; P = 0.355) with considerable heterogeneity (I2 = 73.3%, P < 0.001) (Fig. 7). Subgroup analysis was performed based on geographical areas, participants’ health status, and study design, however, the overall results did not change (Table 3). The overall meta-analysis result for AMH was not sensitive to individual studies.
The correlation between FSH and 25(OH)D levels
Correlation between FSH and 25(OH)D was observed in 13 studies14,25,26,27,28,30,32,34,43,44,45,48 with 1908 participants. Overall, there was no significant association between FSH and 25(OH)D (Fisher’s Z: − 0.06; 95% CI − 0.18 to 0.06; P = 0.357) (Fig. 8). There was evidence of substantial heterogeneity among the effect size of included studies (I2 = 83.7%, P < 0.001). Subgroup analysis based on participants’ health status and study design did not change the findings (Table 3). Excluding individual studies did not change the overall meta-analysis results.
The correlation between LH and 25(OH)D levels
The correlation between LH and vitamin D was not significant in the meta-analysis of seven studies26,28,30,32,44,48 with 919 participants (Fisher’s Z: − 0.09; 95% CI − 0.29 to 0.11; P = 0.372). Furthermore, evidence of significant heterogeneity was observed (I2 = 87.2%, P < 0.001) (Fig. 9). Subgroup analysis based on participants’ health status and study design did not change the overall findings (Table 3). Meta-analysis findings were not sensitive to individual studies.
The correlation between LH/FSH ratio and 25(OH)D levels
The correlation between LH/FSH ratio and 25(OH)D was examined in 8 studies24,31,32,39,43,50 with 786 participants. There was no significant association between LH/FSH ratio and 25(OH)D (Fisher’s Z: 0.004; 95% CI − 0.22 to 0.21; P = 0.971) with evidence of considerable heterogeneity (I2 = 84.4%, P < 0.001) (Fig. 10). Subgroup analysis revealed a negative correlation between LH/FSH ratio and 25(OH)D among women with normal BMI (Fisher’s Z: − 0.18; 95% CI − 0.37 to − 0.008; P = 0.041). The findings were not sensitive to any individual studies.
Publication bias
No evidence of publication bias was observed for AFC (Begg’s test: P = 0.624, Egger’s test: P = 0.911), AMH (Begg’s test: P = 0.381, Egger’s test: P = 0.990), FSH (Begg’s test: P = 0.951, Egger’s test: P = 0.651), LH (Begg’s test: P = 0.362, Egger’s test: P = 0.082), and LH/FSH ratio (Begg’s test: P = 0.216, Egger’s test: P = 0.751).
Discussion
In order to identify new nutritional factors associated with women’s fertility, various attempts have been conducted. However, interpreting the literature to wrap up a conclusion is a difficult process for clinicians. Therefore, a comprehensive systematic review and meta-analysis of available literature can represent the most reliable evidence. Although previous systematic review and meta-analysis examined the relationship between concentrations of vitamin D and ovarian reserve56, that study focused only on AMH and included only 5 articles. Therefore, it was necessary to conduct a more comprehensive systematic review and meta-analysis on this relationship.
The present systematic review and meta-analysis of 36 observational studies examined the association between serum vitamin D levels and ovarian reserve markers including AMH, AFC, FSH, LH, and LH/FSH ratio in the adolescent and adult population of premenopausal women. Although, there was no significant association between serum vitamin D levels and any of the intended ovarian reserve markers, some of the subgroup analyses have found significant findings. AFC was significantly lower among Asians and LH was higher in the non-Asian population with vitamin D insufficiency/deficiency. Moreover, there was a negative correlation between vitamin D and LH/FSH ratio in women with normal BMI.
There are some points that should be taken into account when interpreting the results. First of all, there are substantial inter-assay differences in the performance of commercially available kits for serum vitamin D assay57. This notion may affect the results and play a considerable role as a heterogeneity factor. Additionally, seasonal variation in serum vitamin D should be considered when interpreting the results58. This notable fact has been excused in some papers59,60 and such inconsistency among the season of sample collection could also influence our final results. Lastly, there are several factors including race, skin color, use of skin protection (sunscreen), latitude, environmental pollution, aging, cultural and lifestyle issues that all can affect the synthesis and availability of vitamin D worthy to consider when interpreting the results4,61,62.
AMH is a glycoprotein hormone related to inhibin and activin and belongs to the family of transforming growth factor β (TGF-β), which has substantial functions in ovarian folliculogenesis63. AMH decreases follicle sensitivity to FSH64. Thus, there is absolute evidence that AMH is involved in the initiation of growth in follicles and FSH sensitivity65. The mechanism by which vitamin D may affect AMH and FSH is unclear. Similar to human studies, the findings of experimental studies regarding this association are also inconclusive66,67,68. Vitamin D may influence ovarian steroidogenesis, development of the follicles, and ovarian reserve7. AFC is a main prognosticator of the ovarian reserve and response to hormonal and follicle stimulation69. Furthermore, the related mechanism of vitamin D was regulated through VDR70,71, Thus, the ovarian reserve markers levels might be affected by the VDR polymorphism72. Interestingly, Szafarowska et al. reported that there is an association between polymorphisms of the VDR gene and AMH; however, they have not found any correlation between AMH levels and vitamin D concentrations in PCOS women73. On the other hand, some studies have represented that vitamin D deficiency and also single nucleotide polymorphism (SNP) of VDR did not affect dysmenorrhea, pelvic pain, or infertility74. Several studies have suggested that reduced vitamin D concentrations in PCOS and obese women may be associated with infertility75,76. A possible mechanism regarding the recent association might be decreased insulin sensitivity due to vitamin D deficiency77. Considering this hypothesis, insulin could elevate androgen biosynthesis and reduce sex hormone-binding globulin (SHBG) which resulted in hyperandrogenism. An excess amount of androgens is converted to estrogen. High estrogen concentration promotes the secretion of LH and represses FSH of the anterior pituitary78. Based on our findings, a negative correlation was observed regarding LH/FSH ratio with vitamin D suggesting that vitamin D status may contribute to hormonal dysregulation, even in women with normal BMI. According to the current meta-analysis, the issue that vitamin D levels are associated with ovarian reserve markers is still a controversial subject. Evidence is still unreliable as the randomized controlled trials are scarce, and the findings of available evidence are extremely heterogeneous.
On the other hand, the overall result of vitamin D and AFC showed a marginally significant association, whereas, the exclusion of Drakopoulos et al. and Fabris et al. studies from the analysis revealed a significant association between vitamin D and AFC. Based on the results of these studies, the change in results can be interpreted by Drakopoulos et al.'s study that was the only study to show that AFC was higher in women with vitamin D deficiency compared to those without vitamin D deficiency. In addition, Fabris et al.'s study demonstrated the least difference between the two groups with and without vitamin D deficiency in relation to AFC. As a result, the exclusion of these studies was able to make a significant result overall.
One of the substantial limitations in our study is the lack of evaluation of ethnicity in relation to vitamin D status, given that the vast majority of patients included in studies were Caucasian. Also, different methods of measuring vitamin D and the health status of the participants can be considered as other factors. Nevertheless, other parameters in the present meta-analysis were not sensitive to individual studies. The present meta-analysis had other limitations. There was significant heterogeneity in our study that may have affected the results and diminished the generalizability of the findings. The probable sources of heterogeneity might be differences in age, BMI, study design, vitamin D and ovarian reserves markers assay methods and kits, the season of sample collection, geographical variation, and the quality of the studies. Furthermore, the observational design of the included studies precludes us to examine the causality. Another limitation that may influence the findings is regarding vitamin D binding protein concentrations and VDR's polymorphisms that were not measured by the included studies. Moreover, seasonal variation of vitamin D was not taken into account in some of the included studies. In addition, the selected subgroup for the current study was not pre-specified that might be a source of bias and a limitation of the present systematic review and meta-analysis. Furthermore, the ovarian reserve mostly refers to the number and quality of dormant primordial follicles that cannot be explained completely by serum levels of AMH, AFC, LH, FSH, and LH/FSH ratio. On the other hand, these biochemical markers were selected as surrogate variables to illustrate the ovarian reserve. Another point to consider is the included studies did not use the same cut-off values for determining the patients’ vitamin D status. Moreover, there are potential confounders in the association between vitamin D and ovarian reserve including age, BMI, dietary intake, smoking, physical activity, and etc. Since most of the included studies did not comprehensively adjust for these confounders, this issue can influence our findings and should be considered as possible limitation.
Conclusion
Based on what was discussed, although, there was no significant association between serum vitamin D levels and any of the intended ovarian reserve markers, some subgroup analyses have found significant findings. AFC was significantly lower among Asians and LH was higher in non-Asian population with vitamin D insufficiency/deficiency. Moreover, there was a negative correlation between vitamin D and LH/FSH ratio in women with normal BMI. In order to understand the underlying mechanisms of vitamin D in female reproduction, further attempts are needed.
References
Holick, M. F. Vitamin D deficiency. N. Engl. J. Med. 357(3), 266–281 (2007).
Hossein-nezhad, A. & Holick, M. F. (eds) Vitamin D for Health: A Global Perspective. Mayo Clinic Proceedings (Elsevier, 2013).
Luk, J., Torrealday, S., Neal Perry, G. & Pal, L. Relevance of vitamin D in reproduction. Hum. Reprod. 27(10), 3015–3027 (2012).
Arab, A., Hadi, A., Moosavian, S. P., Askari, G. & Nasirian, M. The association between serum vitamin D, fertility and semen quality: A systematic review and meta-analysis. Int. J. Surg. 71, 101–109 (2019).
Voulgaris, N. et al. Vitamin D and aspects of female fertility. Hormones 16(1), 5–21 (2017).
Kinuta, K. et al. Vitamin D is an important factor in estrogen biosynthesis of both female and male gonads. Endocrinology 141(4), 1317–1324 (2000).
Drakopoulos, P. et al. The effect of serum vitamin D levels on ovarian reserve markers: A prospective cross-sectional study. Hum. Reprod. 32(1), 208–214 (2017).
Yoshizawa, T. et al. Mice lacking the vitamin D receptor exhibit impaired bone formation, uterine hypoplasia and growth retardation after weaning. Nat. Genet. 16(4), 391–396 (1997).
Parikh, G. et al. Vitamin D regulates steroidogenesis and insulin-like growth factor binding protein-1 (IGFBP-1) production in human ovarian cells. Horm. Metab. Res. 42(10), 754–757 (2010).
Levi, A. J. et al. Reproductive outcome in patients with diminished ovarian reserve. Fertil. Steril. 76(4), 666–669 (2001).
Dennis, N. A. et al. The level of serum anti-Müllerian hormone correlates with vitamin D status in men and women but not in boys. J. Clin. Endocrinol. Metab. 97(7), 2450–2455 (2012).
Merhi, Z. O. et al. Circulating vitamin D correlates with serum antimüllerian hormone levels in late-reproductive-aged women: Women’s Interagency HIV Study. Fertil. Steril. 98(1), 228–234 (2012).
Pearce, K., Gleeson, K. & Tremellen, K. Serum anti-Mullerian hormone production is not correlated with seasonal fluctuations of vitamin D status in ovulatory or PCOS women. Hum. Reprod. 30(9), 2171–2177 (2015).
Chang, E. M., Kim, Y. S., Won, H. J., Yoon, T. K. & Lee, W. S. Association between sex steroids, ovarian reserve, and vitamin D levels in healthy nonobese women. J. Clin. Endocrinol. Metab. 99(7), 2526–2532 (2014).
Shapiro, A. J., Darmon, S. K., Barad, D. H., Gleicher, N. & Kushnir, V. A. Vitamin D levels are not associated with ovarian reserve in a group of infertile women with a high prevalance of diminished ovarian reserve. Fertil. Steril. 110(4), 761–766 (2018).
Moher, D., Liberati, A., Tetzlaff, J. & Altman, D. G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 8(5), 336–341 (2010).
Wells, G. et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-analyses (2000).
Uhland, A. M., Kwiecinski, G. G. & DeLuca, H. F. Normalization of serum calcium restores fertility in vitamin D-deficient male rats. J. Nutr. 122(6), 1338–1344 (1992).
Hozo, S. P., Djulbegovic, B. & Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 5(1), 13 (2005).
Lenhard, W. & Lenhard, A. Hypothesis Tests for Comparing Correlations. Psychometrica. https://www.psychometricade/correlation.html. (2014).
Green, S. & Higgins, J. Cochrane Handbook for Systematic Reviews of Interventions. Version (2005).
DerSimonian, R. & Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 7(3), 177–188 (1986).
Egger, M., Smith, G. D., Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109), 629–634 (1997).
Yildizhan, R. et al. Serum 25-hydroxyvitamin D concentrations in obese and non-obese women with polycystic ovary syndrome. Arch. Gynecol. Obstet. 280(4), 559–563 (2009).
Kulaksizoglu, M. et al. Risk factors for diabetes mellitus in women with primary ovarian insufficiency. Biol. Trace Elem. Res. 154(3), 313–320 (2013).
Kebapcilar, A. G. et al. Is there a link between premature ovarian failure and serum concentrations of vitamin D, zinc, and copper?. Menopause 20(1), 94–99 (2013).
Ersoy, E. et al. Vitamin D levels in patients with premature ovarian failure. Ginekol. Pol. 87(1), 32–36 (2016).
Arslan, E., Gorkem, U. & Togrul, C. Is there a relationship between vitamin D deficiency status and PCOS in infertile women?. Geburtshilfe Frauenheilkd. 79(7), 723–730 (2019).
Inal, Z. O., Inal, H. A. & Gorkem, U. Sexual function and depressive symptoms in primary infertile women with vitamin D deficiency undergoing IVF treatment. Taiwan. J. Obstet. Gynecol. 59(1), 91–98 (2020).
Yilmaz, S. A. et al. The relationship between Polycystic ovary syndrome and vitamin D levels. Turk. J. Obstet. Gynecol. 12(1), 18–24 (2015).
Bostanci, E. I., Ozler, S., Yilmaz, N. K. & Yesilyurt, H. Serum 25-hydroxy vitamin D levels in turkish adolescent girls with polycystic ovary syndrome and the correlation with clinical/biochemical parameters. J. Pediatr. Adolesc. Gynecol. 31(3), 270–273 (2018).
Kokanali, D., Karaca, M., Ozaksit, G., Elmas, B. & Ustun, Y. E. Serum vitamin D levels in fertile and infertile women with polycystic ovary syndrome. Geburtshilfe Frauenheilkd. 79(5), 510–516 (2019).
Jukic, A. M. Z., Steiner, A. Z. & Baird, D. D. Association between serum 25-hydroxyvitamin D and ovarian reserve in premenopausal women. Menopause 22(3), 312–316 (2015).
Jukic, A. M. Z., Baird, D. D., Wilcox, A. J., Weinberg, C. R. & Steiner, A. Z. 25-Hydroxyvitamin D (25(OH)D) and biomarkers of ovarian reserve. Menopause 25(7), 811–816 (2018).
Harmon, Q. E. et al. Vitamin D and reproductive hormones across the menstrual cycle. Hum. Reprod. 35(2), 413–423 (2020).
Arefi, S. et al. Is the ovarian reserve influenced by Vitamin D deficiency and the dress code in an infertile Iranian population?. J. Ovarian Res. 11(1), 1–6 (2018).
Alavi, N., Ebrahimi, M. & Akbari-Asbagh, F. The effect of vitamin D status on ovarian reserve markers in infertile women: A prospective cross-sectional study. Int. J. Reprod. BioMed. 18(2), 85–92 (2020).
Ghadimi, R., Esmaeilzadeh, S., Firoozpour, M. & Ahmadi, A. Does vitamin D status correlate with clinical and biochemical features of polycystic ovary syndrome in high school girls?. Caspian J. Intern. Med. 5(4), 202–208 (2014).
Kozakowski, J., Kapuscinska, R. & Zgliczynski, W. Associations of vitamin D concentration with metabolic and hormonal indices in women with polycystic ovary syndrome presenting abdominal and gynoidal type of obesity. Ginekol. Pol. 85(10), 765–770 (2014).
Bednarska-Czerwinska, A., Olszak-Wasik, K., Olejek, A., Czerwinski, M. & Tukiendorf, A. Vitamin D and anti-Mullerian hormone levels in infertility treatment: The change-point problem. Nutrients 11(5), 1053 (2019).
Szafarowska, M. et al. Anti-Mullerian hormone level is associated with vitamin D receptor polymorphisms in women with polycystic ovary syndrome. J. Assist. Reprod. Genet. 36(6), 1281–1289 (2019).
Kim, S., Lee, J. R., Suh, C. S., Choi, Y. M. & Kim, S. H. Relationship between serum anti-mullerian hormone with vitamin D and metabolic syndrome risk factors in late reproductive-age women. Menopause 24(12), 1440 (2017).
Daghestani, M. H. Evaluation of biochemical, endocrine, and metabolic biomarkers for the early diagnosis of polycystic ovary syndrome among non-obese Saudi women. Int. J. Gynecol. Obstet. 142(2), 162–169 (2018).
Kensara, O. A. Prevalence of hypovitaminosis D, and its association with hypoadiponectinemia and hyperfollistatinemia, in Saudi women with naïve polycystic ovary syndrome. J. Clin. Transl. Endocrinol. 12, 20–25 (2018).
Xu, Z. F. et al. Correlation of serum vitamin D levels with ovarian reserve markers in patients with primary ovarian insufficiency. Int. J. Clin. Exp. Med. 12(4), 4147–4153 (2019).
Zhu, J. P., Wang, J., Chen, Y., Ji, Y. Q. & Xiong, M. Anti-Mullerian hormone levels in Chinese women younger than 30 years and 30 years or older and correlated biochemical indices. Obstet. Gynecol. Surv. 72(1), 33–38 (2017).
Lata, I., Tiwari, S., Gupta, A., Yadav, S. & Yadav, S. To Study The Vitamin D levels in infertile females and correlation of vitamin D deficiency with AMH levels in comparison to fertile females. J. Hum. Reprod. Sci. 10(2), 86–90 (2017).
Ganie, M. A. et al. Impact of hypovitaminosis D on clinical, hormonal and insulin sensitivity parameters in normal body mass index polycystic ovary syndrome women. J. Obstet. Gynaecol. 36(4), 508–512 (2016).
Fabris, A. M. et al. Impact of vitamin D levels on ovarian reserve and ovarian response to ovarian stimulation in oocyte donors. Reprod. Biomed. Online. 35(2), 139–144 (2017).
Figurová, J., Dravecká, I., Javorský, M., Petríková, J. & Lazúrová, I. Prevalence of vitamin D deficiency in Slovak women with polycystic ovary syndrome and its relation to metabolic and reproductive abnormalities. Wien. Klin. Wochenschr. 128(17–18), 641–648 (2016).
Bakeer, E. et al. Anti-Müllerian hormone as a diagnostic marker in Egyptian infertile polycystic ovary syndrome females: Correlations with vitamin D, total testosterone, dyslipidemia and anthropometric parameters. J. Med. Biochem. 37(4), 448–455 (2018).
Velija-Ašimi, Z. Evaluation of the association of vitamin D deficiency with gonadotropins and sex hormone in obese and non-obese women with polycystic ovary syndrome. Med. Glas. 11(1), 170–176 (2014).
Pearce, K., Gleeson, K. & Tremellen, K. Serum anti-Mullerian hormone production is not correlated with seasonal fluctuations of vitamin D status in ovulatory or PCOS women. Hum Reprod. 30(9), 2171–2177 (2015).
Wong, H. Y. Q. et al. Independent association of serum vitamin D with anti-Mullerian hormone levels in women with polycystic ovary syndrome. Clin. Endocrinol. (Oxf.) 89(5), 634–641 (2018).
Garbedian, K., Boggild, M., Moody, J. & Liu, K. E. Effect of vitamin D status on clinical pregnancy rates following in vitro fertilization. CMAJ Open 1(2), E77 (2013).
Moridi, I., Chen, A., Tal, O. & Tal, R. The association between vitamin D and anti-Müllerian hormone: A systematic review and meta-analysis. Nutrients 12(6), 1567 (2020).
Snellman, G. et al. Determining vitamin D status: A comparison between commercially available assays. PLoS ONE 5(7), e11555 (2010).
Diffey, B. Modelling the seasonal variation of vitamin D due to sun exposure. Br. J. Dermatol. 162(6), 1342–1348 (2010).
Holick, M. F. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am. J. Clin. Nutr. 80(6), 1678S-S1688 (2004).
Glew, R. H., Crossey, M. J., Polanams, J., Okolie, H. I. & VanderJagt, D. J. Vitamin D status of seminomadic Fulani men and women. J. Natl Med. Assoc. 102(6), 485–490 (2010).
Askari, G., Rafie, N., Miraghajani, M., Heidari, Z. & Arab, A. Association between vitamin D and dry eye disease: A systematic review and meta-analysis of observational studies. Contact Lens Anterior Eye 43(418), 425 (2020).
Arab, A., Golpour-Hamedani, S. & Rafie, N. The association between vitamin D and premenstrual syndrome: A systematic review and meta-analysis of current literature. J. Am. Coll. Nutr. 38(7), 648–656 (2019).
Deffieux, X. & Antoine, J. Inhibins, activins and anti-Müllerian hormone: Structure, signalling pathways, roles and predictive value in reproductive medicine. Gynecol. Obstet. Fertil. 31(11), 900 (2003).
Dewailly, D. et al. Interactions between androgens, FSH, anti-Müllerian hormone and estradiol during folliculogenesis in the human normal and polycystic ovary. Hum. Reprod. Update 22(6), 709–724 (2016).
Oh, S. R., Choe, S. Y. & Cho, Y. J. Clinical application of serum anti-Müllerian hormone in women. Clin. Exp. Reprod. Med. 46(2), 50 (2019).
Görkem, Ü., Küçükler, F. K., Toğrul, C. & Gülen, Ş. Vitamin D does not have any impact on ovarian reserve markers in infertile women. Gynecol. Obstet. Reprod. Med. 23(2), 79–83 (2017).
Bakhshalizadeh, S. et al. Modulation of steroidogenesis by vitamin D3 in granulosa cells of the mouse model of polycystic ovarian syndrome. Syst. Biol. Reprod. Med. 63(3), 150–161 (2017).
Dicken, C. L. et al. Peripubertal vitamin D3 deficiency delays puberty and disrupts the estrous cycle in adult female mice. Biol. Reprod. 87(2), 51 (2012).
Muttukrishna, S. et al. Antral follicle count, anti-mullerian hormone and inhibin B: Predictors of ovarian response in assisted reproductive technology?. BJOG 112(10), 1384–90 (2005).
Krishnan, A. V. et al. Novel pathways that contribute to the anti-proliferative and chemopreventive activities of calcitriol in prostate cancer. J. Steroid Biochem. Mol. Biol. 103(3–5), 694–702 (2007).
Björkhem-Bergman, L., Lehtihet, M., Rane, A. & Ekström, L. Vitamin D receptor rs2228570 polymorphism is associated with LH levels in men exposed to anabolic androgenic steroids. BMC. Res. Notes 11(1), 51 (2018).
Malloy, P. J., Peng, L., Wang, J. & Feldman, D. Interaction of the vitamin D receptor with a vitamin D response element in the Mullerian-inhibiting substance (MIS) promoter: Regulation of MIS expression by calcitriol in prostate cancer cells. Endocrinology 150(4), 1580–1587 (2009).
Szafarowska, M. et al. Anti-Müllerian hormone level is associated with vitamin D receptor polymorphisms in women with polycystic ovary syndrome. J. Assist. Reprod. Genet. 36(6), 1281–1289 (2019).
Vilarino, F. L. et al. Analysis of vitamin D receptor gene polymorphisms in women with and without endometriosis. Hum. Immunol. 72(4), 359–363 (2011).
Kokanalı, D., Karaca, M., Ozakşit, G., Elmas, B. & Üstün, Y. E. Serum vitamin D levels in fertile and infertile women with polycystic ovary syndrome. Geburtshilfe Frauenheilkd. 79(05), 510–516 (2019).
Fang, F. et al. Effect of vitamin D supplementation on polycystic ovary syndrome: A systematic review and meta-analysis of randomized controlled trials. Complement. Ther. Clin. Pract. 26, 53–60 (2017).
Durmaz, Z. H. et al. Does vitamin D deficiency lead to insulin resistance in obese individuals?. Biomed. Res. 28(17), 7419 (2017).
Johansson, J. & Stener-Victorin, E. Polycystic ovary syndrome: Effect and mechanisms of acupuncture for ovulation induction. Evid.-Based Complement. Altern. Med. 2013, 762615 (2013).
Author information
Authors and Affiliations
Contributions
A.A, E.K and R.A contributed to the conception of research. A.A, E.K and M.R searched databases, screened articles and extracted data. A.A performed statistical analysis; and all authors contributed to the writing and revision of the manuscript. R.A revised the final edition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Karimi, E., Arab, A., Rafiee, M. et al. A systematic review and meta-analysis of the association between vitamin D and ovarian reserve. Sci Rep 11, 16005 (2021). https://doi.org/10.1038/s41598-021-95481-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-95481-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.