Abstract
Enterococcus faecalis is a life-threatening bacterium that resists high levels of antibiotics or chemical preservatives. In this study, we aimed to investigate the inactivation of E. faecalis in fresh pineapple juice (FPJ) with two different cold atmospheric plasmas (CAP) reinforced by H2O2/H2O cold vapor: a plasma jet and a surface dielectric barrier discharge (SDBD). CAP treatments for 300 s with plasma jet and 420 s with SDBD caused an 8.2 log reduction of E. faecalis. The concentration of reactive oxygen and nitrogen species induced in FPJ by plasmas was also evaluated. In terms of quality attributes of FPJ, no noticeable color changes (ΔE) were observed. Furthermore, a trifle of loss of organic content such as sugars and organic acids was observed after treatments. These results suggest that our rapid CAP strategy effectively inactivated E. faecalis in FPJ with no change of color and negligible effects on other physicochemical properties.
Similar content being viewed by others
Introduction
Market demand for safe and high-quality fruit juices is increasing since their pleasant flavors and nutritional benefits are known to consumers. However, the health hazards of contaminated fruit juices are also well documented since the pathogenic microorganisms can grow and duplicate in fruit juices and cause infectious diseases in consumers1. The high nutritional richness of fruit juices can also be an attractive environment for microorganisms to proliferate2. Enterococcus faecalis (ATCC 29212) is a well-known and health-endangering bacteria in agricultural products. These bacteria are non-motile, gram-positive, with the capacity of causing a variety of life-threatening infections among which endocarditis and urinary tract infection are the most common ones3. E. faecalis strains can grow at a temperature of 60° and resist high levels of many antimicrobial agents4. E. faecalis strains are known to have irrefutable antibiotic resistance which can contribute to their pathogenicity5. Hence, effective methods are required to inactivate this pathogen in fruit juices. Since thermal treatments cannot be used for juices, the Addition of chemical preservative agents is currently a widely used method in food industries6. Various preservative agents such as sodium benzoate, calcium propionate, and potassium sorbate are used by commercial producers to prevent microbial spoilage in different food products. Among these chemical preservatives, sodium benzoate is usually added to fruit juices and beverages7. High levels of sodium benzoate might prevent microorganisms from spoiling the products but can have negative side effects on human cells as well7,8. Sodium benzoate can have cytotoxic effects on human cells and cause damage to different tissues. It has been proved that sodium benzoate can have genotoxic effects on human cells and cause mutation and damage to DNA, and consequently promoting normal cells into cancer cells9. On the other hand, E. faecalis strains are known to have remarkable tolerance to chemical agents and antibiotics10. Disadvantages of traditional preservation methods have created a desire of designing new non-thermal technologies for inactivating microorganisms to upgrade the levels of food safety and quality. These technologies, including intense cold atmospheric-pressure plasma (CAP), rely heavily on combining physical and chemical processes to disable or passivate a variety of microorganisms at low temperatures and short treatment times2,11,12,13,14. It is a well-known fact that plasma techniques generate reactive oxygen and nitrogen species (RONS) such as electrons, radicals, ions, ozone, atomic nitrogen/oxygen11,15. The antimicrobial capacity of cold plasma has been investigated by several scholars before. Most of the reported and suggested mechanisms for the antimicrobial activity of cold plasma are literally based on the generation of reactive oxygen species (ROS), reactive nitrogen species (RNS), and a minor contribution from ultraviolet light (UV)16. RNS and ROS seem to be responsible for severe damage to microbial DNA and cell wall17,18. There are various methods for cold plasma generation such as surface dielectric barrier discharge (SDBD), plasma jets, coronas, and microwave discharges2,19. It has been shown that the RONS generated in plasma are device-dependent. Thus it is rational to conclude that switching devices may not lead to the same outcome. Among the above-mentioned methods, SDBD and plasma jet can present a low-cost and effective antimicrobial cold plasma which causes minimum damage to the nutritional properties and physicochemical parameters in food products20,21. Many studies have shown the antimicrobial potential of cold plasma on various microorganisms19,22,23,24,25. So far, some studies have hired cold plasma for inactivating bacteria in fruit juices22,26,27. Liao et al., Wang et al., and Xiang et al. investigated the antimicrobial activity of cold plasma on apple juice contaminated with Escherichia coli and Zygosaccharomyces rouxii, respectively. Liao et al. microbiological assay showed 4.3 log CFU/ml of reduction and Wang et al. and Xiang et al. observed 5–6.8 log CFU/ml of reduction. The effect of plasma treatment on apple juice was investigated in the mentioned studies, a loss of some antioxidants and phenolic compounds such as vitamin C and no significant change in other nutrients was reported28,29,30. Hou et al.31 studied the antimicrobial influence of non-thermal plasma on blueberry juice against Bacillus sp. and reported 7.2 log CFU/ml of reduction in total with low cost and loss of quality. Mahnot et al.32 successfully inactivated Salmonella Typhimurium in coconut water by 5 log CFU/ml of reduction using high voltage atmospheric cold plasma with minimum changes in nutritional content. A commercialized and optimistic nonthermal food processing technique should achieve effective microbial inactivation and minimal loss of quality. Still little is known about the true nature of the RONS's role in inactivating microorganisms; In fact, today’s main concern in plasma food processing is to find the most effective RONS in microorganism inactivation since it can lead to more accurate and reliable inactivation at lower treatment times.
Our aims in this study were to (1) design and optimize CAP devices for food processing in industries to increase food safety and quality; (2) investigate the influence of CAP treatments on the conventional physicochemical properties of fresh pineapple juice (FPJ); (3) evaluate the concentration of the RONS in treated FPJ samples; also (4) establishing an innovative strategy of plasma treatments for reliable and rapid disinfection of fruit juices.
Materials and methods
CAP treatments
The new strategy implemented by us in this study consisted of combining atmospheric pressure cold plasma generators (the plasma jet and the SDBD) with cold vapors of hydrogen peroxide (H2O2) and water (H2O) to increase the production level of RONS species. In this study, we investigated the effects of plasma-induced species on the bactericidal properties of the liquid medium (pineapple juice) and its physicochemical properties. Due to the above considerations, a surface dielectric barrier discharge (SDBD) structure, as well as a jet in the after-glow region, have been used33. The following is a description of the plasma treatment and the details of the structures used in this study.
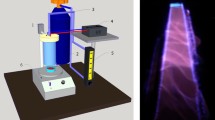
The SDBD and the plasma jet structures were shown schematically in Fig. 1. The SDBD structure was depicted in Fig. 1a. In this plasma source, an electrode arrangement consisting of aluminum adhesive tapes glued edge to edge on the top and back surfaces of a 4 mm thick glass dielectric. The dimensions of the aluminum strips were 2 cm by 18 cm and spaced 2 cm apart. The applied voltage on the electrodes was 12 kV AC with 6.2 kHz. On the upper surface of the SDBD structure, wide and thin strips of Kapton tape were glued as a strong dielectric to prevent corona and loss of electrical power. The power of the SDBD was 4.2 watts. Pure argon gas (99.999%) was used as the carrier gas to transfer H2O2/H2O vapors from the hydrogen peroxide-water mixture bottle into the plasma reactor for both sources (see Fig. 1c). The mole fraction of H2O2 in water was \( M_{{H_{2} O_{2} }} /M_{{H_{2} O}} = 0.3\) in the liquid phase and that of vapor mixture was evaluated to be \( M_{{H_{2} O_{2} }} /M_{{H_{2} O}} = 0.034\) in the mixture of argon, hydrogen peroxide, and water vapors. The difference in heat of vaporization of the hydrogen peroxide and water led to a significant decrease in the mole fraction of H2O2 concerning the liquid phase. Following Giguère and Maass34, the total vapor pressure of the hydrogen peroxide and water was assumed to be 20 Torr at 30 °C. The partial pressure of H2O2 and H2O was estimated to be 0.65 Torr and 19.35 Torr, respectively. Regarding these partial pressures at 30 °C, the concentrations for H2O2 and H2O vapors were obtained to be 856 and 25,492 ppm (part per million), respectively. These estimations determine the upper limits for mole fractions of hydrogen peroxide and water vapors based on Giguere and Maass's findings at 30 °C. The argon gas flowing over the mixture with 4 slm (standard liters per minute) carried the vapors from the liquid bottle into the gas discharge zone (see Fig. 1a, c). A mass flow controller (Seven stars D07-19B) and a flow readout box (Seven star D08-1F) were used to adjust and read the gas flow. The plasma jet also consisted of a copper tube as the powered electrode, a quartz glass tube as the dielectric barrier, and a ring electrode as the ground wrapped around the outer surface of the quartz (see Fig. 1b). Argon gas with H2O2/H2O vapors flows through the inner tube into the electric gas discharge gap. The whole structure was placed inside the epoxy resin as a high-aptitude electrical insulator to avoid the occurrence of an electric spark. The outer body of the jet structure was made of PTFE (Polytetrafluoroethylene). Frequency, peak voltage, and power for the plasma jet structure were 4.7 kHz, 14.4 kV, and 4.2 W, respectively. For both plasma sources, the plasma power was considered to be the same to compare the performance of the two devices under similar conditions. In all experiments, the ambient temperature and relative humidity were constant at 23 °C and 70%, respectively. The treatment time for the structures used in this study was 30, 90, 180, and 300 s, with the difference that for the SDBD structure, the sample was also exposed to plasma for 7 min. Juice samples were placed in a petri dish and each treatment was performed in triplicate. The surface of the samples (pineapple juice) is placed at a distance of 1 cm from the conical tip of the dielectric nozzle in the plasma jet structure and also at a distance of 2 cm from the lower electrode in the SDBD structure. The gas temperature of plasma was 50 °C for the SDBD device and 32.5 °C for the jet device. All treatments with both devices were also performed without plasma to ensure the contribution of plasma to the inactivation of bacteria and every other effect.
Schematic diagram of (a) SDBD-CP. 1: high voltage power supply, 2: dielectric barrier, 3: lower electrode, 4: upper electrode, 5: sample, 6: plasma discharge, 7: Kapton Tape, 8: gas inlet. (b) Plasma jet. 1: high voltage power supply, 2: gas inlet, 3: high-voltage electrode, 4: epoxy resin, 5: ring electrode, 6: quartz dielectric nozzle, 7: Teflon (PTFE) tube, 8: plasma plume, 9: sample. (c) Cold vapor section 1: argon gas inlet, 2: gas outlet.
Preparation of pineapple juice
To prepare fresh and uninfected pineapple juice, five pineapples were collected from the same plantation. After being washed up they were skinned and squeezed in a biological safety cabinet class III (LAMSYSTEM BMB-III-Laminar-S PROTECT 1E-C.001-120). Pulps and other unwanted particles were carefully removed by performing a simple filtration under the safety cabinet. The volume of each pineapple juice sample was 10 mL in all of our experiments. And the distance between the top of the sample and the surface of the SDBD was 2 cm. The distance between the top of the sample and the tip of the jet device was 1 cm, too.
Preparation of bacterial strain
The standard strain of E. faecalis (ATCC 29212) was cultured on Mueller–Hinton Agar (Ibresco i23118) and placed in an incubator at 37 °C for 24 h then transferred into the normal saline solution and made up to the concentration of 1.5 × 109 CFU/ml (5 McFarland). To prepare an infected fruit juice sample for CAP treatment, 1 ml of the above-mentioned solution was added to 9 ml of juice to achieve the final concentration of 1.5 × 108 CFU/ml (0.5 McFarland).
Microbiological analysis
To present an accurate Log chart, the untreated and CAP treated samples were serially diluted in 0.9% normal saline solution. Then, 0.1 ml of each diluted solution was plated on a Mueller–Hinton agar medium culture and incubated at 37 °C for 24 h.
Determination of organic content
Organic acids such as citric acid and malic acid, vitamin C, and sugars such as glucose, fructose, and sucrose were determined before and after the CAP treatment by reverse phase-high performance liquid chromatography (HPLC) procedure described by Tyagi, et al.35. All measurements were repeated three times.
Determination of vitamin C
The concentration of vitamin C in treated and untreated samples was determined using a Phenomenex Luna C18 column and a mobile phase of acetonitrile and 10 mM potassium dihydrogen orthophosphate buffer (40:60, pH = 2.1). The mobile phase was gently released with the flow rate of 1 ml/min and the column temperature was fixed at 50 °C. Detections were done with a UV detector set at a wavelength of 268 nm.
Determination of organic acids
In the case of citric acid and malic acid, the separation was performed using a Phenomenex Luna C18 column associated with the mobile phase of ammonium di-hydrogen orthophosphate (NH4H2PO4) buffer (2% w/v, pH = 2.18) and the flow rate of 1 ml/min. The temperature was stabilized at 32 °C. The detection of both organic acids was done by a UV–Vis detector set at a wavelength of 214 nm.
Determination of sugars
A binary gradient elution Phenomenex Luna NH2 column with the mobile phase of acetonitrile and water (75:25 v/v) and a flow rate of 1 ml/min was used for the separation of sugars. The column temperature was 40 °C. detections were done using a refractive index detector.
pH, temperature, and total dissolved solids (TDS) measurements
The pH and temperature of treated and untreated samples were measured immediately after the treatment times were finished. Both pH and temperature measurements were done at the same time using an AZ 86502 pH and thermometer. Total dissolved solids (TDS) measurements were also been taken care of by a NEWCON digital TDS meter.
Colorimetric measurements
Color is another important physicochemical factor in fruit juice. The color parameters of treated and untreated samples were measured by a hunter lab colorimeter (ColorFlex, Hunter Associates Laboratory, Inc., Reston, 232 Virginia, USA) in the CIE LAB space. Color conditions of samples were quantified as L* (whiteness/darkness), a* (redness/greenness), and b* (yellowness/blueness). Total color difference (ΔE) in samples after CAP treatment was shown as follows:
The letters with subscript 0 are the color values of the samples before and the letters with subscript i are the color values of treated samples.
RONS measurements
ROS
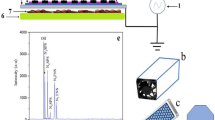
The concentration of H2O2 in the liquid sample was measured directly by UV/Vis spectroscopy using a reagent solution of potassium titanium(IV) oxalate dihydrate in H2O/H2SO436. Despite the H2O2, other ROS radicals cannot be measured directly in water solution due to their short-living nature. Other radicals do not exist long enough to be measured by reagents; hence a special group of compounds known as spin traps must be hired to scavenge these ROS and form a spin trap-radical complex which can keep long enough to be measured by an electron paramagnetic resonance (EPR)37. The concentration of hydroxyl radical (·OH), ozone (O3)/atomic oxygen (O), and hydroperoxyl (·OOH) were measured by the EPR method with thanks to spin traps. PBS solutions of spin traps 2,2,6,6-tetramethylpiperidine (TEMP, ≥ 99%), 5,5-dimethyl-1-pyrroline N-oxide (DMPO, ≥ 98%) and 5-(diethoxyphosphoryl)-5-methyl-1-pyrroline N-oxide (DEPMPO, ≥ 99%) were prepared at the concentration of 0.1 M, ready to be used. 0.1 M solution of singlet oxygen (O2(a1∆g)) scavenger sodium azide (NaN3, ≥ 99%) was added to TEMP to make difference between the amount of 2,2,6,6-tertramethylpiperidine 1-oxyl (TEMPO) formed from O3/O and O238,39. An aqueous solution of 5-(2, 2-dimethyl-1, 3-propoxycyclophosphoryl)-5-methyl-1-pyrroline N-oxide (CYPMPO) and phenyl-tert-butyl nitrone (PBN) were used to scavenge superoxide radical anions (O2·_)40,41. In a typical experiment, PBS and water solutions containing spin traps were suspended in fresh pineapple samples by shaking them roughly at − 16 °C for 21 h. After preparations, spin traps containing samples were exposed to each plasma device for 30–420 s.
EPR measurements and detections were done with a Magnettech MiniScope MS 200 spectrometer. Required parameters including frequency, modulation frequency, modulation amplitude, power, sweeping time, and sweeping width were precisely adjusted according to the research of Privat-Maldonado et al.39, and all scans were repeated three times. Analyzed samples were prepared in 50 µL glass capillaries for measurements. The results were procured by double integration (SpectrumViewer ver. 2.6.3) of the respective simulated spectra (NIH P.E.S.T. WinSIM software ver. 0.96) of the formed radical adducts.
RNS
Most RNS consists of nitrate and nitrite anions which are long-lived reactive species. Both nitrate and nitrite radicals were measured using ion chromatography columns. Cartridge Strata® C18-E was used to omit organic molecules from the samples to ensure accurate evaluation by the ion chromatography method. Column Metrosep A Supp 10—250/4.0 was hired with anion eluent composition of 5 mM Na2CO3 + 5 mM NaHCO3 and flow of 1 ml/min and pressure level of 14.94 MPa. Metrohm-model 881 compact IC pro 1 was hired with a recording time of 36 min and manual integration to detect the concentration of nitrate and nitrite in treated and untreated samples.
Statistical analysis
Each experimental condition, preparation, and requirement was performed in triplicate and each experiment was separately repeated three times. Data are presented as mean ± standard deviation (SD). Differences among groups are also reported using the statistical package SPSS 24.0 (IBM, Armonk, NY, USA). The chosen procedure for comparing the data was one-way ANOVA combined with Duncan’s multiple comparison test at a 95% confidence level and a significance level of p < 0.05.
Results and discussions
Plasma-induced H2O2 and generation of RONS in the pineapple juice
Evaluating the induced concentration of the ROS in the juice for both CAP devices is essential to clarify which one of them could be more responsible for the inhibition of the bacteria described above. As shown in Fig. 2, the levels of H2O2 in SDBD treated samples were significantly lower than the plasma jet and the increasing rate of the H2O2 concentration during the treatment in plasma jet was faster than SDBD. The H2O2 concentration after SDBD treatment for 30 s was 400 μM (13.60 ppm), which had risen to 800 μM (27.21 ppm) with treatment time to 300 s and remained 800 μM with the treatment time of 420 s. In the case of the plasma jet, the concentration of H2O2 was 1500 μM (51.02 ppm) after 30 s and increased rapidly to 8000 μM (272.10 ppm) after 300 s. Besides H2O2, ROS radicals such as superoxide, hydroxyl, ozone, atomic oxygen, and hydroperoxyl were also generated in plasma and induced in the FPJ during the treatment times from 30 to 420 s. Evaluating the concentration of ROS radicals with spin-trapping and EPR showed that plasma jet induces more ROS into the samples than SDBD, nevertheless, the increase of concentration was observed during the treatment times in both devices. As shown in Fig. 2 excluding the hydrogen peroxide, the concentration and increasing rates of ozone and atomic oxygen were higher than other ROS in both plasma devices.
Despite the combination of the gas feed used, RNS are generated in plasma from the nitrogen of the environment regarding the fact that both devices were open-air42.
During plasma treatments with the jet, the average concentration of the nitrate was 200 μM in control samples and after 30 s it significantly increased to 250 μM but after 90 s it had a significant decrease to 170 μM; after 180 s the concentration arose significantly to 190 μM and gently increased to 200 μM after 300 s. In the case of SDBD, the average concentration of nitrate in control samples did not arise after 30 s of treatment but after 90 s it significantly increased to 250 μM but after 180 s it slightly decreased to 240 μM. After 300 s it surprisingly decreased to 210 μM and then gently increased to 220 μM.
The concentration of nitrite anions was very low in comparison to nitrate anions and out of the scope of the ion chromatography method described elsewhere.
Previous investigations have demonstrated that RONS produced by plasma during treatments with jet or SDBD may play an important role in microbial inactivation43,44. RONS can induce critical damages to the cell wall, cell membrane, or cellular components. Mentioned changes can inactivate the spoiling microorganisms make it impossible for them to recover2,45.
Plasma device and treatment time-dependent inactivation of E. faecalis
Populations of E. faecalis in FPJ Before and after plasma jet and SDBD treatments are shown in Table 1, treatments with SDBD and jet disabled E. faecalis in FPJ samples in a time and device-dependent manner. No significant change was observed after 30 s of treatment with both devices but after reaching the time of 90 s the population started to reduce significantly. Finally, 8.2 log reduction E. faecalis was accomplished by treating samples with SDBD at 4.2 W for 420 s, while the same reduction was observed by exposure of jet at 4.2 W for 300 s. Treating samples without plasma but under the same conditions for the same times did not cause sufficient disinfection. These results strongly indicate that SDBD and jet can remarkably eliminate E. faecalis from FPJ but in different treatment times.
To show that the presence of plasma had a great contribution to the disinfection of the pineapple juice, all the microbiological tests were performed without the plasma but at the presence of the gas flow of Ar/H2O2/H2O. The absence of plasma was the only difference between gas flow treated samples and plasma-treated samples and all other experimental conditions were set the same as with the plasma treatment. The remaining bacterial population of each sample was counted after the end of its treatment time. The results of gas flow treated samples can be seen in Table 2. It can be concluded from Table 2 that total disinfection can’t be achieved by gas flow treatment at the mentioned times. The statistical analysis of the results showed that treating the samples for 90 s with both devices had no significant effect on the bacterial population in the samples.
To show that H2O2 is not the only responsible RONS for the bactericidal activity, we checked the bactericidal capability of H2O2 by adding pure H2O2 to the samples. As shown in Fig. 2 the highest concentration of H2O2 during our treatments with plasma jet was 8000 µM and that of the SDBD treatments was 800 µM. We prepared the mentioned concentrations with pure H2O2 and fresh pineapple juice. After preparing each solution the bacterial population of 0.5 McFarland (8.2 log) was immediately added to each solution and shook three times gently. The enumeration of the remaining bacterial population was performed 60 s after the addition of 0.5 McFarland. All of the mentioned procedure was replicated three times. The population of bacteria in the H2O2 added pineapple juice has been reduced to 1.33 ± 0.15 log after the addition of 8000 µM H2O2. And the addition of 800 µM of H2O2 reduced the bacterial population to 5.06 ± 0.49 log. This experiment showed that neither any of the 8000 µM and 800 µM concentrations of H2O2 can eradicate the bacterial population of infected samples, therefore the H2O2 is not lonely responsible for the bactericidal potential of our cold atmospheric plasma strategy.
Influences of CAP treatments on conventional physicochemical properties of FPJ
The main physicochemical characters of our FPJ samples, including pH, temperature, and total dissolved solids (TDS) were profiled and measured before and after plasma treatments. As shown in Table 3, the average pH of control samples was 3.90 by 300 s of exposure to plasma by jet, the average pH increased slowly to 3.96. The same increase of pH was observed after 420 s of treatment with SDBD. The presence of ROS and NOx anions may cause changes in pH as was reported in some studies30,32. The impact of CAP treatment on TDS was not significantly changed after 90 s of treatment with plasma jet and SDBD. But referring to Table 3, the TDS of samples increased to 2047 ppm and 2070 ppm after 300 s and 420 s of plasma jet and SDBD treatments, respectively. Among pH and TDS, the temperature might also be an important parameter in food processing since it has been reported that raise in temperature can lead to the degradation of oligosaccharides in fruit juices46,47. The temperature of all treated and untreated samples was measured immediately after treatment times. As shown in Table 3, the temperature of control samples was equal to the room temperature (22.33 °C) and it was not significantly changed after 90 s of treatment with plasma jet but after 180 s it increased to 22.67 °C and 23.67 °C after 300 s. Treatment of samples with SDBD had no significant effect on the temperature of the samples until 300 s but after that, it increased to 23.33 °C. The treatment of juice samples with our chosen methods might have induced changes in the temperature of them but it is unlikely to cause any degradation in nutrients47.
Influences of CAP treatments on the color of FPJ
The color of fruit juices and beverages is usually the result of the presence of a group of macromolecules called “Carotenoids”48. Carotenoids are also believed to contribute to the antioxidant potential of fruit juices49. Three color indexes L*, a*, and b*, and the color difference from control samples ΔE were measured before and after treatment times, and this procedure was repeated three times50,51. The color parameters of the samples are listed in the CIE LAB color coordinates in Table 4. During the treatment times with SDBD, the amount of L* and a* were not significantly changed after 180 s but the amount of b* suddenly decreased from 23.72 to 14.08 after 30 s of treatment and it was not significantly changed after 180 s, but after that, it started to decline to 10.04. During the treatment times with plasma jet, the amount of L* and a* were not significantly changed until 180 s of treatment but the amount of b* reduced from 23.72 to 15.10 after 30 s and it declined to 9.01 by 300 s. Significant changes of L* and a* during the treatment times might be in contact with the fact that carotenoids can get degraded by free radicals and reactive species52,53,54. Regarding the concentration of ROS and RNS in our treated samples, free radicals could be responsible for the loss of the carotenoids in our treated samples. In conflict with L*, a*, and b*, the amount of ΔE was not significantly changed at all. In summary, plasma treatments with both SDBD and plasma jet device mostly changed the amount of b*, but the other color indicators were slightly declined and ΔE was not significantly changed at all.
Influences of CAP treatments on the organic content of FPJ
The organic content of FPJ, particularly sugars, organic acids, and vitamin C are known to have health benefits for consumers of every age, and therefore their presence is an important nutrition quality of fruit juices and beverages55. On the other hand, sugars and organic acids cause the sweetness and sourness of the taste respectively, thus they play a key role in the flavor of fruit juices which is commercially important for processed juices and beverages35,56. The concentration of nutrients in pineapple juice before and after plasma treatments is detailed in Table 5. All values are in milligrams per 100 ml. Plasma treatments made no significant difference to the concentration of vitamin C until 180 s of treatment with SDBD and 90 s of treatment with plasma jet. Almost the same manner was observed in the case of citric acid and malic acid, there was no significant difference between the concentration of citric acid by the time of 90 s of SDBD and 30 s of plasma jet and the untreated samples but after the mentioned treatment times it was slowly reduced. The concentration of malic acid did was not significantly changed after 180 s of SDBD treatment and 90 s of plasma jet treatment. In the case of sugars, fructose and glucose showed similar behavior during treatment times but the concentration of the sucrose was an exception! The average concentration of fructose was not significantly changed until 90 s of treatment with the SDBD and plasma jet, after the mentioned treatment time the concentration of the fructose reduced slowly. The average concentration of glucose was not significantly changed until 90 s and 180 s of exposure to SDBD and plasma jet, respectively. The average concentration of glucose reduced slowly after the mentioned treatment times. The indifference with glucose and fructose, the average concentration of sucrose reduced after 30 s of treatment with the SDBD and plasma jet. On the other hand, the reduction rate of sucrose was somehow higher than glucose and fructose. The reason for this phenomenon might be related to the molecular structure of sucrose; sucrose is a disaccharide made of a glucose and fructose molecule which are attached by a special kind of covalent bond known as the glycosidic linkage. The glycosidic linkage can be disrupted by ROS and hydrogen peroxide57,58. In short, ROS and hydrogen peroxide can turn sucrose molecules into glucose and fructose molecules and that may illustrate the difference in reduction rates between monosaccharides and sucrose.
Conclusion
In this study, the efficiency of reinforced plasma jet and SDBD by H2O2 and H2O cold vapors on the inactivation of E. faecalis in FPJ was investigated. The results showed that exposure of plasma jet and SDBD at 4.2 W for 300 s and 420 s respectively, resulted in an 8.2 log reduction of E. faecalis cells in FPJ. Both plasma jet and SDBD induced a remarkable deal of ROS and RNS in treated samples resulting in a log reduction of E. faecalis. Some reactive species such as H2O2 had higher concentrations than others and are believed to have more contribution in inactivating bacteria cells and the following log reduction since a comparison between the log reductions and the concentration of RONS in both plasma devises shown in Fig. 2 indicates that the reduction of log and the increase of ROS especially H2O2 happened simultaneously.
Gentle decreasing rate on some of the organic content of the FPJ and minimal damage to them supports the fact that the plasma caused damage to the organic content of FPJ in both devices was negligible. Besides, plasma treatments caused adverse effects on color characteristics of FPJ samples, and no significant effect on the color difference (ΔE) between control and treated samples.
In summary, our results and findings explicitly suggest that our rapid innovative strategy of plasma treatments can effectively inactivate E. faecalis (8.2-log CFU/ml) in FPJ with minimal damage to its quality and no addition of harmful materials. In the future, studies and investigations should focus on proposing optimized, low-cost, and scale-up plasma procedures or devices for wide applications in food industries. It was also concluded that although the SDBD enhanced by H2O2/H2O has relatively less disinfectant power, due to its large scale, it can promise great potential for disinfection of fruit juices in line with commercial plans.
References
Cheng, R. M., Churey, J. J. & Worobo, R. W. Inactivation of Salmonella enterica and spoilage microorganisms in orange juice treated with dimethyl dicarbonate (DMDC). Int. J. Food Microbiol. 285, 152–157 (2018).
Misra, N. N., Tiwari, B. K., Raghavarao, K. S. M. S. & Cullen, P. J. Nonthermal plasma inactivation of food-borne pathogens. Food Eng. Rev. 3, 159–170 (2011).
Kau, A. L. et al. Enterococcus faecalis tropism for the kidneys in the urinary tract of C57BL/6J mice. Infect. Immun. 73, 2461 (2005).
Amyes, S. G. B. Enterococci and streptococci. Int. J. Antimicrob. Agents 29, S43–S52 (2007).
Hanchi, H., Mottawea, W., Sebei, K. & Hammami, R. The genus enterococcus: Between probiotic potential and safety concerns—An update. Front. Microbiol. 9, 1791 (2018).
Walker, M. & Phillips, C. A. The effect of preservatives on Alicyclobacillus acidoterrestris and Propionibacterium cyclohexanicum in fruit juice. Food Control 19, 974–981 (2008).
Zengin, N., Yüzbaşıoğlu, D., Ünal, F., Yılmaz, S. & Aksoy, H. The evaluation of the genotoxicity of two food preservatives: Sodium benzoate and potassium benzoate. Food Chem. Toxicol. 49, 763–769 (2011).
Mamur, S., Yüzbaşıoğlu, D., Ünal, F. & Yılmaz, S. Does potassium sorbate induce genotoxic or mutagenic effects in lymphocytes?. Toxicol. In Vitro 24, 790–794 (2010).
Ishidate, M. & Odashima, S. Chromosome tests with 134 compounds on Chinese hamster cells in vitro—A screening for chemical carcinogens. Mutat. Res. Fundam. Mol. Mech. Mutagen. 48, 337–353 (1977).
Franz, C. M. A. P. et al. Incidence of virulence factors and antibiotic resistance among enterococci isolated from food. Appl. Environ. Microbiol. 67, 4385 (2001).
Ambrico, P. F. et al. Surface dielectric barrier discharge plasma: A suitable measure against fungal plant pathogens. Sci. Rep. 10, 3673 (2020).
Bourke, P., Ziuzina, D., Boehm, D., Cullen, P. J. & Keener, K. The potential of cold plasma for safe and sustainable food production. Trends Biotechnol. 36, 615–626 (2018).
Korachi, M., Turan, Z., Şentürk, K., Şahin, F. & Aslan, N. An investigation into the biocidal effect of high voltage AC/DC atmospheric corona discharges on bacteria, yeasts, fungi and algae. J. Electrost. 67, 678–685 (2009).
Schlüter, O. et al. Opinion on the use of plasma processes for treatment of foods*. Mol. Nutr. Food Res. 57, 920–927 (2013).
Chen, D. et al. Evaluation of Cronobacter sakazakii inactivation and physicochemical property changes of non-fat dry milk powder by cold atmospheric plasma. Food Chem. 290, 270–276 (2019).
Laroussi, M. & Leipold, F. Evaluation of the roles of reactive species, heat, and UV radiation in the inactivation of bacterial cells by air plasmas at atmospheric pressure. Int. J. Mass Spectrom. 233, 81–86 (2004).
Korachi, M. & Aslan, N. The effect of atmospheric pressure plasma corona discharge on pH, lipid content and DNA of bacterial cells. Plasma Sci. Technol. 13, 99–105 (2011).
Yusupov, M. et al. Plasma-induced destruction of bacterial cell wall components: A reactive molecular dynamics simulation. J. Phys. Chem. C 117, 5993–5998 (2013).
Misra, N. N., Pankaj, S. K., Frias, J. M., Keener, K. M. & Cullen, P. J. The effects of nonthermal plasma on chemical quality of strawberries. Postharvest Biol. Technol. 110, 197–202 (2015).
Albertos, I. et al. Effects of dielectric barrier discharge (DBD) generated plasma on microbial reduction and quality parameters of fresh mackerel (Scomber scombrus) fillets. Innov. Food Sci. Emerg. Technol. 44, 117–122 (2017).
Cullen, P. J. et al. Translation of plasma technology from the lab to the food industry. Plasma Process. Polym. 15, 1700085 (2018).
Dasan, B. G. & Boyaci, I. H. Effect of cold atmospheric plasma on inactivation of Escherichia coli and physicochemical properties of apple, orange, tomato juices, and sour cherry nectar. Food Bioprocess Technol. 11, 334–343 (2018).
Fernández, A., Noriega, E. & Thompson, A. Inactivation of Salmonella enterica serovar Typhimurium on fresh produce by cold atmospheric gas plasma technology. Food Microbiol. 33, 24–29 (2013).
Georgescu, N., Apostol, L. & Gherendi, F. Inactivation of Salmonella enterica serovar Typhimurium on egg surface, by direct and indirect treatments with cold atmospheric plasma. Food Control 76, 52–61 (2017).
Sohbatzadeh, F., Hosseinzadeh Colagar, A., Mirzanejhad, S. & Mahmodi, S. E. coli, P. aeruginosa, and B. cereus bacteria sterilization using afterglow of non-thermal plasma at atmospheric pressure. Appl. Biochem. Biotechnol. 160, 1978–1984 (2010).
Almeida, F. D. L. et al. Effects of atmospheric cold plasma and ozone on prebiotic orange juice. Innov. Food Sci. Emerg. Technol. 32, 127–135 (2015).
Ozen, E. & Singh, R. K. Atmospheric cold plasma treatment of fruit juices: A review. Trends Food Sci. Technol. 103, 144–151 (2020).
Liao, X. et al. Application of a dielectric barrier discharge atmospheric cold plasma (Dbd-Acp) for Eshcerichia coli inactivation in apple juice. J. Food Sci. 83, 401–408 (2018).
Wang, Y. et al. Inactivation of yeast in apple juice using gas-phase surface discharge plasma treatment with a spray reactor. LWT Food Sci. Technol. 97, 530–536 (2018).
Xiang, Q. et al. Effects of dielectric barrier discharge plasma on the inactivation of Zygosaccharomyces rouxii and quality of apple juice. Food Chem. 254, 201–207 (2018).
Hou, Y. et al. Effect of cold plasma on blueberry juice quality. Food Chem. 290, 79–86 (2019).
Mahnot, N. K., Mahanta, C. L., Keener, K. M. & Misra, N. N. Strategy to achieve a 5-log Salmonella inactivation in tender coconut water using high voltage atmospheric cold plasma (HVACP). Food Chem. 284, 303–311 (2019).
Roth, J. R. Industrial plasma engineering: Volume 2: Applications to nonthermal plasma processing (CRC Press, Boca Raton, 2001).
Giguère, P. A. & Maass, O. Vapour pressures and boiling points of binary mixtures of hydrogen peroxide and water. Can. J. Res. 18, 181–193 (1940).
Tyagi, G. et al. Rapid determination of main constituents of packed juices by reverse phase-high performance liquid chromatography: An insight in to commercial fruit drinks. J. Food Sci. Technol. 51, 476–484 (2014).
Sellers, R. M. Spectrophotometric determination of hydrogen peroxide using potassium titanium(IV) oxalate. Analyst 105, 950–954 (1980).
Gorbanev, Y. & Bogaerts, A. in Atmospheric pressure plasma—From Diagnostics to Applications (eds Anton Nikiforov & Zhiqiang Chen) (IntechOpen, 2018).
Privat-Maldonado, A. et al. Nontarget biomolecules alter macromolecular changes induced by bactericidal low-temperature plasma. IEEE Trans. Radiat. Plasma Med. Sci. 2, 121–128 (2018).
Privat-Maldonado, A., Gorbanev, Y., Dewilde, S., Smits, E. & Bogaerts, A. Reduction of human glioblastoma spheroids using cold atmospheric plasma: The combined effect of short- and long-lived reactive species. Cancers 10, 394 (2018).
Gorbanev, Y., O’Connell, D. & Chechik, V. Non-thermal plasma in contact with water: The origin of species. Chem. Eur. J. 22, 3496–3505 (2016).
Tani, A., Ono, Y., Fukui, S., Ikawa, S. & Kitano, K. Free radicals induced in aqueous solution by non-contact atmospheric-pressure cold plasma. Appl. Phys. Lett. 100, 254103 (2012).
Graves, D. B. Reactive species from cold atmospheric plasma: Implications for cancer therapy. Plasma Process. Polym. 11, 1120–1127 (2014).
Kondeti, V. S. S. K. et al. Long-lived and short-lived reactive species produced by a cold atmospheric pressure plasma jet for the inactivation of Pseudomonas aeruginosa and Staphylococcus aureus. Free Radic. Biol. Med. 124, 275–287 (2018).
Valinataj Omran, A. et al. Single channel atmospheric pressure transporting plasma and plasma stream demultiplexing: physical characterization and application to E. coli bacteria inactivation. J. Phys. D Appl. Phys. 50, 315202 (2017).
Bourke, P., Ziuzina, D., Han, L., Cullen, P. J. & Gilmore, B. F. Microbiological interactions with cold plasma. J. Appl. Microbiol. 123, 308–324 (2017).
Polydera, A. C., Stoforos, N. G. & Taoukis, P. S. Quality degradation kinetics of pasteurised and high pressure processed fresh Navel orange juice: Nutritional parameters and shelf life. Innov. Food Sci. Emerg. Technol. 6, 1–9 (2005).
Vikram, V. B., Ramesh, M. N. & Prapulla, S. G. Thermal degradation kinetics of nutrients in orange juice heated by electromagnetic and conventional methods. J. Food Eng. 69, 31–40 (2005).
Zulueta, A., Esteve, M. J. & Frígola, A. Carotenoids and color of fruit juice and milk beverage mixtures. J. Food Sci. 72, C457–C463 (2007).
Gardner, P. T., White, T. A. C., McPhail, D. B. & Duthie, G. G. The relative contributions of vitamin C, carotenoids and phenolics to the antioxidant potential of fruit juices. Food Chem. 68, 471–474 (2000).
Amirabadi, S., Milani, J. M. & Sohbatzadeh, F. Application of dielectric barrier discharge plasma to hydrophobically modification of gum arabic with enhanced surface properties. Food Hydrocoll. 104, 105724 (2020).
Bursać Kovačević, D. et al. Effects of cold atmospheric gas phase plasma on anthocyanins and color in pomegranate juice. Food Chem. 190, 317–323 (2016).
Henry, L. K. et al. Effects of ozone and oxygen on the degradation of carotenoids in an aqueous model system. J. Agric. Food Chem. 48, 5008–5013 (2000).
Mordi, R. C., Ademosun, O. T., Ajanaku, C. O., Olanrewaju, I. O. & Walton, J. C. Free radical mediated oxidative degradation of carotenes and xanthophylls. Molecules 25, 1038 (2020).
Ribeiro, D., Freitas, M., Silva, A. M. S., Carvalho, F. & Fernandes, E. Antioxidant and pro-oxidant activities of carotenoids and their oxidation products. Food Chem. Toxicol. 120, 681–699 (2018).
Dhakal, S., Balasubramaniam, V. M., Ayvaz, H. & Rodriguez-Saona, L. E. Kinetic modeling of ascorbic acid degradation of pineapple juice subjected to combined pressure-thermal treatment. J. Food Eng. 224, 62–70 (2018).
Laorko, A., Tongchitpakdee, S. & Youravong, W. Storage quality of pineapple juice non-thermally pasteurized and clarified by microfiltration. J. Food Eng. 116, 554–561 (2013).
Duan, J. & Kasper, D. L. Oxidative depolymerization of polysaccharides by reactive oxygen/nitrogen species. Glycobiology 21, 401–409 (2010).
Qin, C. Q., Du, Y. M. & Xiao, L. Effect of hydrogen peroxide treatment on the molecular weight and structure of chitosan. Polym. Degrad. Stab. 76, 211–218 (2002).
Acknowledgements
The authors would like to extend thanks to Dr. Sepideh Amirabadi and Mr. Ehsan Shakerinasab for their charitable assistance in the statistical analysis of our data.
Author information
Authors and Affiliations
Contributions
F.S.: supervision, conceptualization, resources, writing—review and editing, design of CAP devices, investigation, validation. H.Y.: conceptualization, investigation, financial resources, writing—review and editing, preparation bacteria strain, microbiological assay, evaluation of organic content and physiochemical properties, formal analysis, validation. A.-H.S.: conceptualization, investigation, evaluation of colorimetric parameters, microbiological assay, investigation. A.S.: conceptualization, investigation, software, formal analysis, writing—review and editing, visualization, design of CAP devices, investigation, validation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sohbatzadeh, F., Yazdanshenas, H., Soltani, AH. et al. An innovative strategy to rapidly inactivate 8.2-log Enterococcus faecalis in fresh pineapple juice using cold atmospheric plasma. Sci Rep 11, 16010 (2021). https://doi.org/10.1038/s41598-021-95452-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-95452-2
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.