Abstract
General population-based cohort studies provide solid evidence on mass Helicobacter pylori (HP) eradication effects. Self-reported questionnaires are occasionally used in such studies to ascertain the HP eradication history. However, reports on the reliability of these questionnaires are lacking. This general population-based cohort study included 899 individuals with HP infection at the baseline survey who were reported to have eradicated it at the 5-year follow-up survey. Of these, the medical records of 280 patients were available for investigation, and the HP eradication status of 93 individuals was ascertained. Their medical records were reviewed, and the reliability of the self-reported questionnaire responses was assessed. Of the 91 individuals who successfully eradicated HP based on the medical records, 90 (98.9%) answered the self-reported questionnaire correctly, with an unweighted kappa value of 0.661 (p < 0.001). The difference between the self-reported and medical records age at eradication was within a 1-year range in most participants (86.8%). Similarly, the HP eradication procedure and the outcomes were reasonably matched. In conclusion, the responses to the self-reported HP eradication questionnaire were almost consistent with the medical records. Thus, HP eradication history assessment by a self-reported questionnaire is reliable for an epidemiological study in the general population.
Similar content being viewed by others
Introduction
Helicobacter pylori (HP) is a gram-negative, microaerophilic bacterium that infects the gastric epithelium. It is estimated that more than half of the world’s population is infected with HP1, making it the most widespread infection worldwide. Overall, HP infection shows a progressive decline in prevalence; however, in some Middle East countries, the prevalence has remained relatively stable2. The prevalence of HP in Japan has been rapidly decreasing partly due to the increase in the number of patients cured of HP infection since 2013, when the health insurance policy started covering HP gastritis3.
It is well known that HP infection contributes to the development of chronic gastritis, peptic ulcer disease, and gastric cancer4,5. The International Agency for Research on Cancer (IARC), a subsidiary of the World Health Organization, categorized HP in 1994 as a group 1 carcinogen for gastric cancer6, the fifth most frequently diagnosed cancer, and the third most common cause of cancer-related death worldwide7. The IARC reported that 2.2 million new cancer cases were attributable to infections in 2018, and HP was responsible for 810,000 of these cases8. The age-standardized HP infection incidence rate was estimated at 8.7 cases per 100,000 person-years, making HP the most important infectious cause of cancer worldwide8.
There is significant interest in mass HP eradication for gastric cancer prevention. The Kyoto global consensus report recommended that all individuals with HP infection should receive eradication therapy to prevent gastric cancer9. However, eradication benefits for baseline gastric cancer incidence vary across regions and populations10. A recent Cochrane Database of Systematic Reviews study reported moderate evidence that investigating and eradicating HP reduced the incidence of and mortality from gastric cancer in asymptomatically infected Asian individuals11. Similarly, there was minimal evidence of benefits in terms of all-cause mortality and adverse events.
Therefore, several epidemiological studies have evaluated the effect of HP eradication on gastric cancer; however, its beneficial or adverse effects remain uncertain, especially in the general population12. Therefore, large-scale cohort studies are necessary to clarify the effects of HP eradication. Such analysis of the outcomes is extremely important in Japan, the only country in the world that promotes HP eradication therapy to all patients with HP infection13. Indeed, self-reported questionnaires should be used to obtain the HP eradication history in a large-scale general population-based cohort study. It is necessary to collate the self-reported results with the medical records to ensure their accuracy; however, this becomes less easy as the survey scale increases. Furthermore, there are no reports clarifying the reliability of such questionnaires on HP eradication history. Therefore, this study aimed to examine the reliability of HP eradication history and its therapeutic outcomes, obtained through a self-reported questionnaire in a general population cohort.
Results
Consistency of HP eradication status between the medical records and self-reported responses
HP eradication status was confirmed in the medical records of 92 individuals, including 32.8% of the 280 individuals whose medical records were investigated (Table 1). Of 91 patients with successful HP eradication based on the medical records, 90 (97.8%) reported successful eradication in the self-reported questionnaire. Only one individual was confirmed on both the medical records and the self-reported questionnaire to have failed to eradicate HP. The unweighted kappa value for the HP eradication results between the self-reported response and the medical records was 0.661 (95% confidence interval [CI], 0.041–1.000, p < 0.001). The HP was eradicated by the second-line treatment in the individual who provided an incorrect response.
Consistency of age at HP eradication between the medical records and self-reported responses
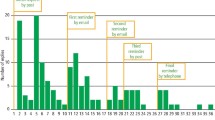
The self-reported age at HP eradication exactly matched the medical records in 27 individuals (29.7%), comprising 22 (30.6%) and five (26.3%) individuals with HP eradicated by the first- and second-line treatments, respectively (Fig. 1). The unweighted kappa values for the age at eradication between the self-reported response and the medical records were 0.177 (95% CI, 0.080–0.274, p < 0.001) for all, 0.184 (95% CI, 0.075–0.292, p < 0.001) in those with HP eradicated by the first-line treatment, and 0.130 (95% CI, − 0.065 to 0.326, p = 0.129) in those with HP eradicated by the second-line treatment. Only a few individuals had a complete match between the self-reported age at HP eradication and the medical records; however, the difference in the age of eradication for most individuals was within 1 year [79 individuals (86.8%) overall, 63 (87.5%) with HP eradicated by the first-line treatment, and 16 (84.2%) with HP eradicated by the second-line treatment; Fig. 1].
Differences in Helicobacter pylori (HP) eradication age between the medical records and self-reported responses. The eradication age differences between the medical records and self-reported responses are indicated by the following colors: blue, minus 3 years; orange, minus 2 years; gray, minus 1 year; yellow, no difference; sky blue, plus 1 year; green, plus 2 years; navy blue, plus 3 years.
Impact of individual characteristics on the differences in reported eradication age between the medical records and self-reported responses
No differences were observed in sex, birth age, smoking or alcohol drinking habit, exercise habit, educational background, and median height, weight, and body mass index between individuals whose self-reported age of eradication differed within 1 year from the medical records and those whose age of eradication differed by 2 years or more (Table 2). Individuals with a history of health checkups for gastric cancer were significantly more likely to have a difference of 2 years or more between the self-reported and medical records eradication age (Table 2).
HP eradication therapy
HP was eradicated in almost all individuals by a proton pump inhibitor (PPI)- or potassium-competitive acid blocker (p-CAB)-based triple therapy administered for 7 days (Table 3). The first-line and second-line eradication rates in this study were 77.4 and 95.0%, respectively. HP eradication rate was significantly higher with p-CAB-based therapy than with PPI-based therapy (p = 0.04). HP eradication was determined by 13C-urea breath tests using a cut-off value of 2.5‰ and/or a negative rapid urease test. The incidence of adverse events was 4.3% and 5.0% during the first-line and second-line eradication, respectively.
Discussion
This is the first study demonstrating an optimal internal consistency in HP eradication history results between self-reported questionnaires and the medical records. Mostly, differences in the reported age of eradication remained within 1 year. These results suggest that the studied self-reported questionnaire for HP eradication history is valid and a suitable tool for HP eradication history assessment in the general population.
Recent large-scale cohort studies evaluated the effect of HP eradication treatment on the prevention of gastric cancer by assessing the HP eradication history with various survey methods12,14,15. The HP eradication history was collected from the unified electronic medical records of all Veterans Health Administration (VHA) facilities for a retrospective cohort study with data of 371,813 patients in the VHA15. Another hospital-based study12 investigated the HP eradication history based on a self-reported questionnaire but did not report its reliability. In hospital-based cohort studies, it is generally considered that the HP eradication history should rely on a medical interview or self-reported questionnaire. Furthermore, the Swedish drug registry was used to obtain information on HP eradication treatment in a population-based, nationwide (n = 95,176) cohort study in Sweden14; however, eradication success was not confirmed. A survey method that strikes a balance between the accuracy and feasibility of the eradication history acquisition is required to more precisely estimate HP eradication effectiveness in large-scale cohort studies. A self-reported questionnaire might be a proper tool for examining the effect of HP eradication with valid confidence in general population cohort studies.
A history of health checkups for gastric cancer was more observed among the individuals whose self-reported age of eradication differed by more than 2 years from the medical records than among those with a difference of within 1 year. It is assumed that individuals with a history of such health checkups are more health-conscious and participate in various health-related activities. This could have contributed to the confusion in recalling the age at which HP was eradicated. Therefore, the extent of differences in the self-reported age at HP eradication might vary between hospital- and general population-based cohort studies. In addition, since individuals had to report the age by a number, the reported age depended on how it was rounded. Similarly, this could have contributed to the age difference from the medical records.
The medical records confirmed the actual eradication regimen and the method for efficacy evaluation in our study. Almost all individuals received PPIs- or p-CAB-based triple therapy for HP eradication following the guidelines for the management of HP infection in Japan16. Eradication efficacy was primarily determined by 13C-urea breath tests. The overall eradication rate was 77% for first-line eradication and 95% for second-line eradication, similar to those observed in previous reports17. Supposedly, the current decline in the first-line eradication rate in Japan is due to the impact of the high clarithromycin (CM) resistance rate of approximately 30%. In fact, metronidazole-based eradication therapy has been proven to be more effective than CM-based eradication therapy in a randomized trial conducted in Japan18. Thus, the low eradication rate observed for first-line eradication compared to second-line eradication in this study may be due to the influence of CM resistance. As previously reported19,20, the first-line HP eradication rate was higher with p-CAB-based triple therapy than with PPI-based triple therapy. p-CAB allows rapid, profound, and sustained suppression of gastric acid secretion21, which is an important factor for increasing the antibiotics sensitivity of HP22. The cytochrome P450 2C19 (CYP2C19) plays a role in the effect of PPIs on acid secretion, and a decrease in eradication rate has been reported in CYP2C19 extensive metabolizers23. In contrast, the effects of p-CAB and the eradication rate on p-CAB-based triple therapy are not influenced by CYP2C19 polymorphism19. Furthermore, a recent meta-analysis demonstrated that p-CAB-based therapy is superior to PPI-based therapy to eradicate CM-resistant HP strains24. These factors may explain the high eradication rate of p-CAB-based therapy compared to PPI-based therapy observed in this study.
This study had several limitations. It was feasible to collate the self-reported questionnaire results with the medical records for only a few individuals who visited two hospitals and two clinics in a single prefecture. This could affect the generalizability of the results to other populations. Therefore, further validation studies with a larger number of participants are required. The self-reported questionnaire reliability might be affected by differences in ethnicity, education, background, HP infection rate, and gastric cancer incidence rate. Therefore, the questionnaire should be validated in various other settings, and the content of the questionnaire and the questionnaire design should be clarified. To verify the effects of HP eradication interventions, it is important to confirm the success or failure of HP eradication and whether persistently infected patients truly do not have a history of HP eradication. However, a medical record survey is required at all hospitals or clinics visited by the individuals to verify no history of HP eradication, which could not be conducted in this study. The reliability of the self-reported questionnaire in these individuals may differ from the individuals in this study. Hence, additional validation, including individuals who reported no HP eradication, is required. The adverse effects of HP eradication therapy were reviewed retrospectively from a medical record survey. Therefore, the actual incidence of adverse effects may have been underestimated.
In conclusion, the HP eradication results on the self-reported questionnaire in this study were almost consistent with the information in the medical records. Although a complete agreement on the HP eradication age was not achieved, the differences were mostly within 1 year. This difference may have been influenced by the history of health check-ups for gastric cancer. Therefore, HP eradication history assessment by a self-reported questionnaire could be considered reliable and valid in epidemiological studies in the general population.
Methods
Study population
Participants in this study were enrolled from a general population-based cohort study, named the Yamagata study, that aims to identify novel therapeutic targets through the elucidation of risk factors, such as environmental factors and genetic background and their underlying pathogeneses in the development of lung, stomach, colorectal, liver, and breast cancers, as well as lifestyle-related diseases, such as stroke, acute myocardial infarction, hypertension, renal failure, and diabetes25. Participants in the Yamagata study underwent community-based annual health check-ups in seven cities (Yamagata-city, Sakata-city, Sagae-city, Yonezawa-city, Kaminoyama-city, Tendo-city, and Higashine-city) in Yamagata Prefecture, Japan, from 2009 to 2015. All participants agreed to be included in this cohort study (n = 21,300). The participants were investigated at baseline by a self-reported questionnaire and data collected from their health check-up results. All participants were scheduled to undergo a 5-year follow-up, and their self-reported questionnaires and health check-up results were collected at the follow-up survey. The selection flowchart for inclusion in this study is shown in Fig. 2. The HP infection status based on serum anti-HP immunoglobulin G (IgG) antibody titer, self-reported HP examination history, and the eradication results at the baseline survey (Fig. 3) were available for 14,870 individuals. Serum anti-HP IgG antibody titers were ≥ 10 U/mL in 5769 of 11,357 individuals with no history of HP eradication at the baseline survey. Of these, 899 individuals were reported to have eradicated HP at the 5-year follow-up survey. The health insurance database was searched for participant hospital visits, and the medical records were examined to evaluate the validity of the self-reported questionnaire regarding HP eradication history. For practical reasons, four medical institutions, two major hospitals, and two gastroenterology clinics in Yamagata City were available to validate the questionnaire information. These included the Yamagata University Hospital (n = 87), Yamagata Prefectural Central Hospital (n = 96), Oizumi Medical Clinic (n = 94), and Yaoita Clinic (n = 3). Among the 280 investigated medical records, 93 individuals could be evaluated for their HP eradication history. The other 187 individuals had visited the four institutions for reasons unrelated to HP eradication, such as visiting a doctor other than a gastroenterologist (n = 120), endoscopic screening for gastric (n = 21) or colorectal (n = 18) cancer, endoscopic treatment (n = 7), regular doctor visit (n = 7), and due to other unrelated symptoms (n = 14).
Of the 5769 HP-positive individuals, 4870 included those whose self-reported questionnaires were yet to be confirmed at the time of this study (n = 3587), those who reported having no HP eradication (n = 710), those for whom no clear age of eradication was mentioned (n = 250), and those who did not provide any description of their HP eradication history (n = 323).
This study was approved by the Ethics Review Committees of Yamagata University Faculty of Medicine (#2019-175), Yamagata Prefectural Central Hospital (October 2019, #103), Oizumi Medical Clinic, and Yaoita Clinic, and was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participants at enrollment in this cohort study.
Medical records investigation
We (YS, YA, MS, NM, HT, HO, and TY), all board-certified gastroenterologists of the Japanese Society of Gastroenterology, together with some board-certified HP infection physicians of the Japanese Society for Helicobacter Research (YS, YA, and HO), visited the medical institutions and reviewed the medical records regarding HP eradication treatment, including treatment regimens, outcomes, testing for eradication confirmation, adverse effects, the date of eradication, and explanation of the results.
Evaluation of serum anti-HP IgG antibody titer
Serum anti-HP IgG antibody titers were measured by enzyme-linked immunosorbent assay (ELISA) using the E-Plate Eiken or E-Plate II Eiken (Eiken Chemical Co., Ltd., Tokyo, Japan), following the manufacturer's instructions. Values greater than 10 U/mL were considered to indicate HP infection.
Statistical analysis
Data are presented as numbers (percentages) or medians (interquartile ranges). Continuous or categorical variables were compared by the two-tailed Wilcoxon rank-sum test or chi-squared test, respectively. The concordance rate between the self-reported questionnaire and the medical records was assessed by the unweighted or weighted kappa coefficient. All statistical analyses were performed using JMP, Version 14.3.0 (SAS Institute Inc., Cary, NC, USA). Values of p < 0.05 were considered statistically significant.
Data availability
All relevant original data are available from the corresponding author upon reasonable request.
References
Hooi, J. K. Y. et al. Global prevalence of Helicobacter pylori infection: Systematic review and meta-analysis. Gastroenterology 153, 420–429. https://doi.org/10.1053/j.gastro.2017.04.022 (2017).
Sjomina, O., Pavlova, J., Niv, Y. & Leja, M. Epidemiology of Helicobacter pylori infection. Helicobacter 23, e12514. https://doi.org/10.1111/hel.12514 (2018).
Hiroi, S., Sugano, K., Tanaka, S. & Kawakami, K. Impact of health insurance coverage for Helicobacter pylori gastritis on the trends in eradication therapy in Japan: Retrospective observational study and simulation study based on real-world data. BMJ Open 7, e015855. https://doi.org/10.1136/bmjopen-2017-015855 (2017).
Uemura, N. et al. Helicobacter pylori infection and the development of gastric cancer. N. Engl. J. Med. 345, 784–789. https://doi.org/10.1056/nejmoa001999 (2001).
Graham, D. Y. Helicobacter pylori update: Gastric cancer, reliable therapy, and possible benefits. Gastroenterology 148, 719.e3-731.e3. https://doi.org/10.1053/j.gastro.2015.01.040 (2015).
Schistosomes, liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon. IARC Monogr. Eval. Carcinog. Risks Hum. 61, 1–241 (1994).
Arnold, M. et al. Global burden of 5 major types of gastrointestinal cancer. Gastroenterology 159, 335.e15-349.e15. https://doi.org/10.1053/j.gastro.2020.02.068 (2020).
de Martel, C., Georges, D., Bray, F., Ferlay, J. & Clifford, G. M. Global burden of cancer attributable to infections in 2018: A worldwide incidence analysis. Lancet Glob. Health 8, e180–e190. https://doi.org/10.1016/s2214-109x(19)30488-7 (2020).
Sugano, K. et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut 64, 1353–1367. https://doi.org/10.1136/gutjnl-2015-309252 (2015).
Lee, Y. C. et al. Association between Helicobacter pylori eradication and gastric cancer incidence: A systematic review and meta-analysis. Gastroenterology 150, 1113.e5-1124.e5. https://doi.org/10.1053/j.gastro.2016.01.028 (2016).
Ford, A. C., Yuan, Y., Forman, D., Hunt, R. & Moayyedi, P. Helicobacter pylori eradication for the prevention of gastric neoplasia. Cochrane Database Syst. Rev. 7, CD005583. https://doi.org/10.1002/14651858.CD005583.pub3 (2020).
Bae, S. E. et al. The effect of eradication of Helicobacter pylori on gastric cancer prevention in healthy asymptomatic populations. Helicobacter 23, e12464. https://doi.org/10.1111/hel.12464 (2018).
Asaka, M., Kato, M. & Sakamoto, N. Road map to eliminate gastric cancer with Helicobacter pylori eradication and consecutive surveillance in Japan. J. Gastroenterol. 49, 1–8. https://doi.org/10.1007/s00535-013-0897-8 (2014).
Doorakkers, E., Lagergren, J., Engstrand, L. & Brusselaers, N. Helicobacter pylori eradication treatment and the risk of gastric adenocarcinoma in a Western population. Gut 67, 2092–2096. https://doi.org/10.1136/gutjnl-2017-315363 (2018).
Kumar, S., Metz, D. C., Ellenberg, S., Kaplan, D. E. & Goldberg, D. S. Risk factors and incidence of gastric cancer after detection of Helicobacter pylori infection: A large cohort study. Gastroenterology 158, 527.e7-536.e7. https://doi.org/10.1053/j.gastro.2019.10.019 (2020).
Kato, M. et al. Guidelines for the management of Helicobacter pylori infection in Japan: 2016 revised edition. Helicobacter 24, e12597. https://doi.org/10.1111/hel.12597 (2019).
Suzuki, S., Esaki, M., Kusano, C., Ikehara, H. & Gotoda, T. Development of Helicobacter pylori treatment: How do we manage antimicrobial resistance?. World J. Gastroenterol. 25, 1907–1912. https://doi.org/10.3748/wjg.v25.i16.1907 (2019).
Nishizawa, T. et al. Clarithromycin versus metronidazole as first-line Helicobacter pylori eradication: A multicenter, prospective, randomized controlled study in Japan. J. Clin. Gastroenterol. 49, 468–471. https://doi.org/10.1097/MCG.0000000000000165 (2015).
Murakami, K. et al. Vonoprazan, a novel potassium-competitive acid blocker, as a component of first-line and second-line triple therapy for Helicobacter pylori eradication: A phase III, randomised, double-blind study. Gut 65, 1439–1446. https://doi.org/10.1136/gutjnl-2015-311304 (2016).
Kiyotoki, S., Nishikawa, J. & Sakaida, I. Efficacy of vonoprazan for Helicobacter pylori eradication. Intern. Med. 59, 153–161. https://doi.org/10.2169/internalmedicine.2521-18 (2020).
Jenkins, H. et al. Randomized clinical trial: Safety, tolerability, pharmacokinetics and pharmacodynamics of repeated doses of TAK-438 (vonoprazan), a novel potassium-competitive acid blocker, in healthy male subjects. Aliment. Pharmacol Ther. 41, 636–648. https://doi.org/10.1111/apt.13121 (2015).
Graham, D. & Fischbach, L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut 59, 1143–1153. https://doi.org/10.1136/gut.2009.192757 (2010).
Furuta, T. & Graham, D. Pharmacologic aspects of eradication therapy for Helicobacter pylori infection. Gastroenterol. Clin N. Am. 39, 465–480. https://doi.org/10.1016/j.gtc.2010.08.007 (2010).
Li, M. et al. Systematic review with meta-analysis: Vonoprazan, a potent acid blocker, is superior to proton-pump inhibitors for eradication of clarithromycin-resistant strains of Helicobacter pylori. Helicobacter 23, e12495. https://doi.org/10.1111/hel.12495 (2018).
Yamagata University Genomic Cohort Consortium & Narimatsu, H. Constructing a contemporary gene-environmental cohort: Study design of the Yamagata molecular epidemiological cohort study. J. Hum. Genet. 58, 54–56. https://doi.org/10.1038/jhg.2012.128 (2013).
Acknowledgements
We wish to thank all the participants in this study. This work was supported by AMED under Grant Numbers JP18ck0106277 and JP20ck0106561h0001, and in part by the Japan Society for the Promotion of Science KAKENHI Grant Number JP20K08350.
Author information
Authors and Affiliations
Contributions
Y.S., Y.A., N.S., K.Y., E.S., and M.I. contributed to the study concept and design. Y.S., Y.A., M.S., N.M., H.T., H.O., and T.Y. contributed to data acquisition. Y.S., Y.A., N.S., K.Y., E.S., and M.I. contributed to the analysis and interpretation of data. Y.S., Y.A., N.S., and M.I. drafted the manuscript. M.W., K.I., T.K., T.K., S.T., Y.U., and M.I. critically revised the manuscript for important intellectual content. T.K., S.T., Y.U., and M.I. provided the final approval of the version to be published. All authors have read and approved the submitted version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sasaki, Y., Abe, Y., Shoji, M. et al. Reliability of self-reported questionnaire for epidemiological investigation of Helicobacter pylori eradication in a population-based cohort study. Sci Rep 11, 15605 (2021). https://doi.org/10.1038/s41598-021-95124-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-95124-1
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.