Abstract
Elevated fibroblast growth factor 23 (FGF23) levels are associated with adverse outcome in populations with cardiovascular disease and chronic kidney failure. It is unclear if FGF23 has significance in prognosis estimation in patients with acute heart failure (HF) when compared to traditional risk estimation tools. Serum levels of intact FGF23 were assessed in 139 patients admitted to the Intermediate Care Unit of a tertiary hospital for acute HF. Patients were followed-up for one year. After exclusion of patients who were lost to follow-up, data outliers, and patients with sampling errors, the final study cohort comprised 133 patients. The Seattle Heart Failure (SHF) Model was used to estimate one-year survival. FGF23 levels correlated with HF severity and were strongly associated with one-year mortality. Associations between one-year outcome and FGF23, assessed on day 1 after admission, were still evident after multivariable adjustment (OR 15.07; 95%CI 1.75–129.79; p = 0.014). FGF23 levels predicted the one-year outcome with similar accuracy as the SHF Model, both if assessed on day 1 and on day 2 after admission (FGF23d1: AUC 0.784; 95%CI 0.669–0.899; FGF23d2: AUC 0.766; 95%CI 0.631–0.901; SHF: AUC 0.771; 95%CI 0.651–0.891). The assessment of FGF23 in patients with acute HF might help identify high-risk patients that are more prone to complications, need a closer follow-up and more aggressive treatment.
Similar content being viewed by others
Introduction
Despite major advances in diagnostics and therapy options, cardiovascular diseases (CVD) are still associated with a high morbidity and mortality worldwide, especially if the onset is acute. Multiple tools for risk estimation are available and usually consist of a set of clinical parameters, often combined with imaging results, summarized in a risk score. In acute situations, however, the assessment of complex risk scores is often time-consuming and therefore not feasible in daily routine. The identification of singular biomarkers that are capable of accurately predicting an individual’s risk might help identify patients who need a more aggressive treatment strategy and a closer follow-up.
Fibroblast growth factor 23 (FGF23) is a phosphaturic hormone mainly produced in the bone that controls mineral homeostasis by regulating serum phosphate, parathyroid hormone, and 1,25-(OH)2-vitamin D3. FGF23 has been identified as an early biomarker of impaired kidney function, as FGF23 levels rise prior to the development of hyperphosphatemia, 1,25-(OH)2D3 deficiency, hypocalcemia, or secondary hyperparathyroidism1,2. Chronic kidney disease (CKD) is associated with an increased risk of CVD, and cardiovascular event rate increases as eGFR declines3. FGF23 has been proposed as sensitive biomarker for adverse renal and extrarenal outcomes4,5. Heart failure (HF) is the second most common cause of secondary FGF23 excess. FGF23 rises during HF exacerbations, associates with disease severity, and predicts risk of HF-related death and hospitalization, as well as likelihood of therapeutic response to angiotensin-converting enzyme inhibitor (ACE inhibitor) therapy irrespective of kidney function6,7,8,9. However, while recent data indicate that the prognostic impact of FGF23 in chronic HF is limited10, less is known about the prognostic value of FGF23 levels obtained shortly after hospitalization for acute HF in critically ill patients.
FGF23 has been shown to be produced and released by cardiac fibroblasts in response to acute myocardial damage11. Considering that FGF23 can be rapidly obtained from a single blood draw, we hypothesized that this biomarker might be valuable for prognosis estimation especially in patients with acute HF where timely decision-making is mandatory. In this single-center study, we assessed serum levels of intact FGF23 in 139 patients who had been admitted to the Intermediate Care Unit of University Hospital Aachen for acute HF. We sought to correlate FGF23 to clinical and echocardiographic parameters indicating HF severity. Furthermore, we aimed to assess the capability of FGF23 to predict the one-year mortality in comparison with the well-established Seattle Heart Failure (SHF) Model.
Results
Study population and patient characteristics
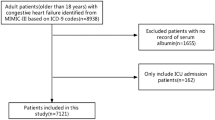
A total of 139 patients admitted to the Intermediate Care Unit of University Hospital Aachen for acute HF met the initial inclusion criteria of the study. After exclusion of patients lost to follow-up, patients with incomplete assessments of FGF23 levels, and outliers with extremely high FGF23 levels, the final study cohort encompassed a total of 133 patients (Supplementary Fig. S1).
Patient characteristics are shown in Supplementary Table S1. Mean age was 66.98 ± 12.22 years (range 32–94), and 96 patients (72.2%) were male. From all patients with acute HF on admission, 37 patients (27.8%) had previously known HF (chronic decompensated HF), whereas de novo HF was diagnosed in 96 patients (72.2%). 9 patients (6.8%) were diagnosed with HFpEF, 58 patients (43.6%) were classified as HFmrEF, and 66 patients (49.6%) had HFrEF. 50 patients had no limitations of physical activity before admission (NYHA I, 37.6%), 38 patients had slight limitations (NYHA II, 27.8%), 29 patients reported a marked limitation of physical activity (NYHA III, 21.8%), and 17 patients were unable to perform any physical activity without discomfort (NYHA IV, 12.8%).
110 patients (82.7%) survived, and 23 patients (17.3%) died within one year after admission.
Associations between FGF23 levels and LVEF
Serum levels of intact FGF23 were assessed at admission (day 1) and on the following day (day 2). There was a modest inverse correlation between logFGF23 levels and LVEF, both on day 1 (r = − 0.31, p = < 0.001) and on day 2 (r = − 0.27, p = 0.005) (Fig. 1a,b). However, after controlling for age, NT-proBNP, and eGFR, the correlation between logFGF23 levels and LVEF lost significance (day 1: r = − 0.09; p = 0.338; day 2: r = 0.007; p = 0.942, Table 1). Table 2 shows mean logFGF23 levels in patients with HFpEF, HFmrEF, and HFrEF with and without adjustment for age, NT-proBNP, and eGFR in a one-way between-groups analysis of covariance. Patients with HFpEF and HFrEF had significantly higher logFGF23 levels compared to patients with HFmrEF, both if assessed on day 1 (Fig. 1c) and on day 2 (Fig. 1d). After adjustment for age, NT-proBNP, and eGFR, logFGF23 levels on day 1 were still significantly higher in patients with HFpEF compared to patients with HFmrEF, while significance was lost for the comparison between HFmrEF and HFrEF. There were no significant differences in logFGF23 levels on day 2 among HFpEF, HFmrEF, and HFrEF patients when age, NT-proBNP, and eGFR were controlled for.
Relationship between LVEF and FGF23 levels. (a, b) In an unadjusted analysis, FGF23 levels, assessed on day 1 (a) and on day 2 (b) showed a modest inverse correlation with LVEF in patients with acute HF. (c, d) Patients who were diagnosed with HFpEF or HFrEF had significantly higher FGF23 levels compared with HFmrEF patients, both when FGF23 was assessed on day 1 (c) and on day 2 (d).
FGF23 levels are associated with clinical severity of heart failure
Increased NT-proBNP levels can serve as a marker for the clinical severity of the disease12. Thus, given the significantly increased logFGF23 levels in patients with preserved LVEF, we investigated whether logFGF23 showed a robust correlation with NT-proBNP rather than LVEF when adjusted for confounding covariates. On day 1 and day 2 after admission for acute HF, logFGF23 levels were positively correlated with NT-proBNP levels, both in an unadjusted analysis (day 1: r = 0.49, p = < 0.001; day 2: r = 0.59, p = < 0.001; Fig. 2a,b) and after controlling for age, eGFR, and LVEF (day 1: r = 0.25; p = 0.005; day 2: (r = 0.42; p = < 0.001) (Table 1). Furthermore, logFGF23 levels on day 1 and on day 2 increased with worsening NYHA and Killip classification status (Fig. 2c–f, Table 1), suggesting associations between FGF23 levels and clinical severity of HF. LogFGF23 levels on day 1 also showed a robust correlation with echocardiographic functional parameters of HF, including LVEDD, LVESD, and LA area, both in an unadjusted analysis and when adjusted for age, NT-proBNP, eGFR, and LVEF, while we did not observe correlations between logFGF23 levels and septum thickness, deceleration time (DT), E/A, E/E’, RVSP, and TAPSE (Table 1).
Association between FGF23 levels and severity of heart failure. (a, b) FGF23 levels were positively correlated with NT-proBNP levels, both when FGF23 was assessed on day 1 (a) and on day 2 (b). (c–f) Indicating associations between FGF23 levels and clinical severity of HF, FGF23 levels increased with worsening NYHA (c, d) and Killip classification status (e, f).
Elevated FGF23 levels are associated with a higher mortality in patients with acute heart failure
At one-year follow-up, 23 patients had died (17.3%) and 110 (82.7%) out of 133 HF patients were still alive. Patients who died within one year after admission to the Intermediate Care Unit had significantly higher logFGF23 levels than patients who survived this period, both on day 1 and on day 2 in the unadjusted analysis (Supplementary Table S1).
Elevated logFGF23 levels assessed on day 1 still correlated significantly with one-year mortality in a logistic regression analysis, after adjustment for LVEF, eGFR, and NT-proBNP (OR 15.07; 95%CI 1.75–129.79; p = 0.014; Table 3). No association with mortality was observed when FGF23 was assessed on day 2 after admission (OR 6.57; 95%CI 0.69–62.69; p = 0.102; Table 3). We observed a higher percentage of non-survivors among patients with chronic decompensated HF as compared to patients with de novo HF (Table 1), and patients with chronic decompensated HF were in worse clinical condition as compared to patients with de novo HF (Supplementary Table S2). Importantly, we noticed higher logFGF23 levels in chronic decompensated HF patients. Therefore, we included the condition of having “chronic decompensated HF” as a covariate in the model. Nevertheless, higher logGF23 levels on day 1 were still predictive of mortality (OR 10.33, 95%CI 1.25–85.79; p = 0.030; Supplementary Table S3).
FGF23 levels predict one-year mortality as accurately as the seattle heart failure model
The SHF model correctly classified one-year survival in 82.0% of HF patients with a sensitivity of 95.5% and a specificity of 17.4%. Higher SHF scores were associated with a higher likelihood of survival (OR 1.04; 95%CI 1.02–1.06; p = < 0.0001), and the AUC value of 0.771 (95%CI 0.651–0.891) showed a fair discrimination between patients who survived and patients who died within the first year after hospital admission (Fig. 3a). LogFGF23 levels on day 1 and on day 2 showed similar accuracy for the prediction of the one-year outcome in HF patients (logFGF23 day 1, AUC 0.784; 95%CI 0.669–0.899; sensitivity 97.3%, specificity 26.1%; logFGF23 day 2, AUC 0.766; 95%CI 0.631–0.901; sensitivity 96.7%, specificity 31.6%, Fig. 3b,c). We evaluated by logistic regression whether the addition of logFGF23 to the SHF score increased the percentage accuracy in classification. Addition of day 1-logFGF23 led to slightly increased sensitivity (96.4%) and specificity of the model (30.4%). Overall, the model including SHF scores and logFGF23 on day 1 correctly classified 85.0% of cases. Addition of logFGF23 levels on day 2 to the SHF model further increased specificity (42.1%) and sensitivity (97.8%), resulting in 88.3% accuracy in classification.
Prediction of the one-year endpoint in patients with acute heart failure. (a) The SHF Model provided a fair discrimination between patients who survived and patients who died within the first year after hospital admission. (b, c) LogFGF23 levels on day 1 (b) and on day 2 (c) predicted survival with similar accuracy as the SHF Model.
Discussion
In the present study we showed that elevated serum concentrations of FGF23 were associated with one-year mortality in critically ill patients with acute HF admitted to an intermediate care unit of a tertiary referral hospital. The ability of FGF23 levels to discriminate between survivors and non-survivors was similar to the well-established SHF Model. Moreover, addition of FGF23 levels to SHF led to increased accuracy in classification.
Risk stratification is indispensable for the clinical management of HF patients. Several models have been developed to standardize prognosis estimation. Such models usually comprise a set of demographics, laboratory, and imaging data, eventually producing a risk estimation score. However, the assessment of all variables contributing to a prognostic model can be challenging and time-consuming, especially in emergency situations. Therefore, in daily practice, the estimate of a patient’s prognosis in acute situations is often mainly based on the clinician’s experience.
Here, we showed for the first time that the assessment of FGF23 levels offers similar accuracy in prognosis estimation as the SHF model in patients hospitalized for acute HF. Since FGF23 levels can be easily assessed from the patients’ serum, FGF23 might serve as a risk estimation tool that is especially valuable in emergency situations.
While FGF23 is a strong predictor of mortality in dialysis patients13, elevated FGF23 levels have also been associated with an increased mortality in non-CKD populations, mainly driven by a higher risk for cardiovascular events5,9. It is well-known that FGF23 concentrations are elevated in patients with chronic HF14,15. Patients with cardiogenic shock have a tremendous increase in FGF23, which has been associated with poor outcome16,17.
While FGF23 has been highly praised for predicting HF-related outcome and prognosis in previous studies6,18,19,20, more recent work has challenged its independent predictive value10,21. FGF23 has been shown to be a powerful biomarker for onset and progression of CKD22,23, and levels rise even before serum creatinine24. Thus, it is unclear whether FGF23 elevation might simply represent an early manifestation of impaired kidney function, even if eGFR is seemingly normal.
It is unclear whether FGF23 excess is solely a biomarker or contributes mechanistically to adverse outcomes as only few studies have investigated causality. Although Faul et al. showed in animal models and in isolated rat cardiomyocytes that treatment with FGF23 may directly cause pathological cardiac remodeling25, the administration of FGF23 has also been suggested to increase blood pressure26, which itself might contribute to left-ventricular hypertrophy. Data from the EVOLVE trial (Evaluation of Cinacalcet HCl Therapy to Lower Cardiovascular Events) suggest lowering FGF23 levels by Cinacalcet is associated with lower rates of cardiovascular death and major cardiovascular events27. On the other hand, data on primary elevations of FGF23, as seen in x-linked hypophosphatemia28 and cardiovascular morbidity are conflicting29,30, and high circulating FGF23 levels did not alter cardiac function in mice31. Furthermore, it is unclear if FGF23 elevation in HF represents an effect of HF itself independent of CKD or is a consequence of subclinical reductions in renal perfusion that stimulate FGF23 production through the same pathways as in early CKD32.
Nevertheless, recent studies suggest that FGF23 may be produced and secreted by cardiac tissues themselves especially in response to ischemia, pressure overload, and acute decompensated HF, and that locally produced FGF23 stimulates pro-fibrotic factors that promote pathogenic cardiac remodeling11,33. Interestingly, FGF23 levels were higher in patients with HFpEF compared with HFmrEF patients in our study, and FGF23 levels were not associated with LVEF after adjustment for age, NT-proBNP, and eGFR. Instead, FGF23 levels correlated with more functional echocardiographic parameters of HF, such as LVEDD, LVESD, and LA area, even when LVEF was controlled for. Of note, this correlation was observed even though echocardiography was not performed at the worst state of decompensation, but within the first 72 h after admission when some patients in part may have recompensated. FGF23 has recently been shown to be higher in patients with HFpEF compared to subjects with normal LVEF, but without signs or symptoms of HF34. In line with previous studies20,34, FGF23 also correlated with NYHA and Killip classification status as clinical indicators of HF severity, albeit with the important caveat that most patients in our study had no or mild limitations of physical activity before admission (i.e., 65.4% of patients were NYHA I or II), and the vast majority (88.7% of patients) had no overt pulmonary edema (Killip class III) or signs of cardiogenic shock (Killip class IV) at admission.
However, while high FGF23 levels were consistently associated with a modestly increased risk of cardiovascular death, they were also associated with an increased risk of non-cardiovascular causes of death in a recent meta-analysis, suggesting that the relationship between FGF23 and CVD risk may be non-causal35. We did not distinguish between cardiovascular and non-cardiovascular causes of death, which is certainly a limitation in our study. It is also noteworthy that the odds ratio for non-survival for FGF23 was an order of magnitude higher than other metrics, suggesting FGF23 might indeed be a powerful marker of worse outcome in acute heart failure. However, at the same time, the wide 95% confidence interval suggests a great variance and therefore some uncertainty. Importantly, our sample size was rather small, and the single-center design of our study limits the generalizability of our findings. Therefore, larger studies, ideally in a multicentric setting, are necessary to validate FGF23 as a prognostic biomarker in acute HF. In addition, our study cohort was largely male, which, however, might be explained by the higher incidence and prevalence of HF in men that has been reported at all ages36.
Although it is possible that elevated FGF23 levels simply reflect the loss of filtration capacity rather than being an independent biomarker in patients with acute HF, we showed that FGF23 predicted one-year survival as accurately as the SHF Model in critically ill HF patients, with even higher accuracy by combining FGF23 and SHF. Because FGF23 is relatively easy to assess, it might be especially valuable in emergency situations (Fig. 4).
FGF23 levels predict the one-year outcome in patients with acute HF with similar accuracy as the SHF Model. This might be of interest especially in emergency situations, where the assessment of all variables contributing to a prognostic model can be challenging and time-consuming. The figure was created in Adobe Illustrator (Version 25.2.3; https://www.adobe.com/products/illustrator.html).
Conclusion
FGF23 levels were similarly accurate as the SHF Model in predicting survival at one year after hospitalization for acute HF in a critically ill patient cohort. Incremental accuracy for mortality prediction can be achieved by combining FGF23 levels with the SHF Model. The assessment of FGF23 levels might help identify patients who are more prone to complications, need a closer follow-up, and a more aggressive treatment.
Methods
Study population
A total of 139 patients admitted to the Intermediate Care Unit of University Hospital RWTH Aachen for acute HF between August 2016 and September 2018 were included in the study. Exclusion criteria were age < 18 years, pregnancy or breastfeeding, CKD requiring dialysis, heart or kidney transplantation, and primary admission to the Intensive Care Unit or transfer to the Intensive Care Unit within 12 h after admission to the Intermediate Care Unit. Furthermore, patients who died within 12 h after admission were excluded from the study. In accordance with the working hours of our clinical study center, patients were screened from Mondays–Thursdays between 7 am and 1 pm. The Independent Ethics Committee at the RWTH Aachen Faculty of Medicine approved the study protocol (EK 099/16), and all methods were in accordance with the Declaration of Helsinki and current guidelines. Written informed consent was obtained before study enrollment from all patients.
Clinical definition of acute heart failure
HF was defined in accordance with the current ESC guidelines37. Acute HF included patients with acute de novo HF and decompensated chronic HF. HF with preserved left-ventricular ejection fraction (LVEF) (HFpEF) was defined as LVEF ≥ 50%, HF with midrange LVEF (HFmrEF) was defined as an LVEF 40–49%, and HF with reduced EF (HFrEF) was defined as an LVEF < 40%. We used the NYHA and Killip functional classifications to describe the severity of symptoms.
Laboratory analysis
Blood samples were collected at the time of admission (“day 1”) and on the following day (“day 2”). Routine laboratory analyses were performed at our core laboratory facility. In addition, serum levels of intact FGF23 were measured using a fully automated chemiluminescent assay (Liaison XL, DiaSorin S.p.A., Saluggia, Italy).
Echocardiography
Routine echocardiographic examinations were performed at our echocardiography core facility within the first 72 h after hospitalization. The LVEF was calculated in the apical four- and two-chamber views using the modified biplane Simpson’s rule. Diastolic dysfunction was defined as E/e’ ≥ 13 and a mean e’ (septal and lateral wall) < 9 cm/s37. Further measurements included left ventricular end-diastolic diameter (LVEDD), left ventricular end-systolic diameter (LVESD), septum thickness, left atrial area (LA area), right-ventricular systolic pressure (RVSP), and tricuspid annular plane systolic excursion (TAPSE).
Study endpoints and follow-up
The primary endpoint was to identify any association between FGF23 levels and survival at one year in patients with acute HF. Secondary endpoints aimed to examine FGF23 as a biomarker for prognosis estimation in comparison to the SHF Model. Furthermore, we investigated associations between FGF23 and clinical and echocardiographic parameters of HF severity. All patients were followed up by phone calls to assess their survival at one year after admission.
Risk calculation
We used the SHF Model38 to calculate the anticipated 1-year survival (https://qxmd.com/calculate/calculator_203/seattle-heart-failure-model). Variables included in the model were age, LVEF, systolic blood pressure, weight, sex, NYHA class, etiology (ischemic vs. non-ischemic), dosages of furosemide, torsemide, bumetidine, metolazone, and HCTZ, usage of allopurinol, statins, ACE inhibitors, beta-blockers, angiotensin-receptor blockers, K-sparing diuretics, the presence of biventricular pacemakers, implantable cardioverter defibrillators, or combined devices, and blood levels of sodium, cholesterol, hemoglobin, lymphocytes, and uric acid.
Statistical analysis
Baseline characteristics were assessed by standard descriptive statistics. Continuous data are expressed as mean ± SD. Categorical data are presented as numbers and percentages. Shapiro–Wilk test was used to assess normality. Serum FGF23 levels were not normally distributed and therefore log transformed (logFGF23). Potential outliers were identified using the ROUT method, as described elsewhere39, based on a false discovery rate of 1%. Statistical differences between continuous variables were determined with student’s t-test or one-way ANOVA, followed by Tukey’s Multiple Comparisons testing, and between categorical variables with Chi Square Test. We used Pearson’s correlation to investigate the relationship between logFGF23 and LVEF, and between logFGF23 and NT-proBNP, and partial correlation was used to control for age, eGFR, NT-proBNP and/or LVEF. Direct logistic regression analyses were performed to assess associations between logFGF23 and survival at one year. Each model was adjusted for LVEF, eGFR, and NT-proBNP. Furthermore, we calculated areas under the ROC curve (AUCs) and the corresponding 95%-confidence intervals (CI) to compare the performances of the SHF Model and FGF23 for mortality prediction. All statistical analyses were performed using IBM SPSS statistics, version 22.0. Graphs were created in GraphPad Prism (GraphPad Software, Version 8.4.1). Figure 4 was created in Adobe Illustrator (Version 25.2.3).
Data availability
All data is available from the corresponding author upon reasonable request.
Abbreviations
- CVD:
-
Cardiovascular diseases
- FGF23:
-
Fibroblast growth factor 23
- CKD:
-
Chronic kidney disease
- HF:
-
Heart failure
- ACE inhibitor:
-
Angiotensin-converting enzyme inhibitor
- SHF:
-
Seattle Heart Failure Model
- LVEF:
-
Left-ventricular ejection fraction
- HFpEF:
-
Heart failure with preserved ejection fraction
- HFmrEF:
-
Heart failure with midrange ejection fraction
- HFrEF:
-
Heart failure with reduced ejection fraction
- LVEDD:
-
Left ventricular end-diastolic diameter
- LVESD:
-
Left ventricular end-systolic diameter
- LA area:
-
Left atrial area
- RVSP:
-
Right-ventricular systolic pressure
- TAPSE:
-
Tricuspid annular plane systolic excursion
- DT:
-
Deceleration time
- AUC:
-
Area under the ROC curve
References
Gutierrez, O. et al. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J. Am. Soc. Nephrol. 16, 2205–2215 (2005).
Hasegawa, H. et al. Direct evidence for a causative role of FGF23 in the abnormal renal phosphate handling and vitamin D metabolism in rats with early-stage chronic kidney disease. Kidney Int. 78, 975–980 (2010).
Go, A. S., Chertow, G. M., Fan, D., McCulloch, C. E. & Hsu, C. Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 351, 1296–1305 (2004).
Ix, J. H. et al. Fibroblast growth factor-23 and death, heart failure, and cardiovascular events in community-living individuals: CHS (Cardiovascular Health Study). J. Am. Coll. Cardiol. 60, 200–207 (2012).
Parker, B. D. et al. The associations of fibroblast growth factor 23 and uncarboxylated matrix Gla protein with mortality in coronary artery disease: The Heart and Soul Study. Ann. Intern. Med. 152, 640–648 (2010).
Plischke, M. et al. Inorganic phosphate and FGF-23 predict outcome in stable systolic heart failure. Eur. J. Clin. Invest. 42, 649–656 (2012).
Gruson, D., Ferracin, B., Ahn, S. A. & Rousseau, M. F. Comparison of fibroblast growth factor 23, soluble ST2 and Galectin-3 for prognostication of cardiovascular death in heart failure patients. Int. J. Cardiol. 189, 185–187. https://doi.org/10.1016/j.ijcard.2015.04.074 (2015).
Poelzl, G. et al. FGF23 is associated with disease severity and prognosis in chronic heart failure. Eur. J. Clin. Invest. 44, 1150–1158 (2014).
Udell, J. A. et al. Fibroblast growth factor-23, cardiovascular prognosis, and benefit of angiotensin-converting enzyme inhibition in stable ischemic heart disease. J. Am. Coll. Cardiol. 63, 2421–2428 (2014).
Stöhr, R. et al. Limited role for fibroblast growth factor 23 in assessing prognosis in heart failure patients: Data from the TIME-CHF trial. Eur. J. Heart Fail. 22, 701–709 (2020).
Schumacher, D. et al. Cardiac FGF23: New insights into the role and function of FGF23 after acute myocardial infarction. Cardiovasc. Pathol. 40, 47–54 (2019).
Pudil, R., Tichý, M., Praus, R., Bláha, V. & Vojácek, J. NT-proBNP and echocardiographic parameters in patients with acute heart failure. Acta Med. (Hradec Kralove) 50, 51–56 (2007).
Jean, G. et al. High levels of serum fibroblast growth factor (FGF)-23 are associated with increased mortality in long haemodialysis patients. Nephrol. Dial. Transplant. 24, 2792–2796 (2009).
Imazu, M. et al. Pathophysiological impact of serum fibroblast growth factor 23 in patients with nonischemic cardiac disease and early chronic kidney disease. Am. J. Physiol. Heart Circ. Physiol. 307, H1504-1511 (2014).
Wohlfahrt, P. et al. Association of fibroblast growth factor-23 levels and angiotensin-converting enzyme inhibition in chronic systolic heart failure. JACC Heart Fail. 3, 829–839 (2015).
Pöss, J. et al. FGF-23 is associated with increased disease severity and early mortality in cardiogenic shock. Eur. Heart J. Acute Cardiovasc. Care 2, 211–218 (2013).
Fuernau, G. et al. Fibroblast growth factor 23 in acute myocardial infarction complicated by cardiogenic shock: A biomarker substudy of the Intraaortic Balloon Pump in Cardiogenic Shock II (IABP-SHOCK II) trial. Crit. Care 18, 713 (2014).
Koller, L. et al. Fibroblast growth factor 23 is an independent and specific predictor of mortality in patients with heart failure and reduced ejection fraction. Circ. Heart Fail. 8, 1059–1067 (2015).
Scialla, J. J. et al. Fibroblast growth factor-23 and cardiovascular events in CKD. J. Am. Soc. Nephrol. 25, 349–360 (2014).
Ter Maaten, J. M. et al. Fibroblast growth factor 23 is related to profiles indicating volume overload, poor therapy optimization and prognosis in patients with new-onset and worsening heart failure. Int. J. Cardiol. 253, 84–90 (2018).
Emrich, I. E. et al. Strength of fibroblast growth factor 23 as a cardiovascular risk predictor in chronic kidney disease weaken by ProBNP adjustment. Am. J. Nephrol. 49, 203–211 (2019).
Chudek, J. et al. Fibroblast growth factor 23 (FGF23) and early chronic kidney disease in the elderly. Nephrol Dial Transplant 29, 1757–1763 (2014).
Fliser, D. et al. Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: The Mild to Moderate Kidney Disease (MMKD) Study. J. Am. Soc. Nephrol. 18, 2600–2608 (2007).
Lu, X. & Hu, M. C. Klotho/FGF23 axis in chronic kidney disease and cardiovascular disease. Kidney Dis. 3, 15–23 (2017).
Faul, C. et al. FGF23 induces left ventricular hypertrophy. J. Clin. Invest. 121, 4393–4408 (2011).
Andrukhova, O. et al. FGF23 regulates renal sodium handling and blood pressure. EMBO Mol. Med. 6, 744–759 (2014).
Moe, S. M. et al. Cinacalcet, fibroblast growth factor-23, and cardiovascular disease in hemodialysis: The evaluation of cinacalcet HCl therapy to lower cardiovascular events (EVOLVE) trial. Circulation 132, 27–39 (2015).
Jonsson, K. B. et al. Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N. Engl. J. Med. 348, 1656–1663 (2003).
Takashi, Y. et al. Patients with FGF23-related hypophosphatemic rickets/osteomalacia do not present with left ventricular hypertrophy. Endocr. Res. 42, 132–137 (2017).
Nehgme, R., Fahey, J. T., Smith, C. & Carpenter, T. O. Cardiovascular abnormalities in patients with X-linked hypophosphatemia. J. Clin. Endocrinol. Metab. 82, 2450–2454 (1997).
Pastor-Arroyo, E. M. et al. The elevation of circulating fibroblast growth factor 23 without kidney disease does not increase cardiovascular disease risk. Kidney Int. 94, 49–59 (2018).
Musgrove, J. & Wolf, M. Regulation and effects of FGF23 in chronic kidney disease. Annu. Rev. Physiol. 19, hysiol-021119 (2019).
Hao, H. et al. FGF23 promotes myocardial fibrosis in mice through activation of β-catenin. Oncotarget 7, 64649–64664 (2016).
Kanagala, P. et al. Fibroblast-growth-factor-23 in heart failure with preserved ejection fraction: Relation to exercise capacity and outcomes. ESC Heart Fail. 7, 4089–4099 (2020).
Marthi, A. et al. Fibroblast growth factor-23 and risks of cardiovascular and noncardiovascular diseases: A meta-analysis. J. Am. Soc. Nephrol. 29, 2015–2027 (2018).
Mehta, P. A. & Cowie, M. R. Gender and heart failure: A population perspective. Heart 92(Suppl 3), iii14–iii18 (2006).
Ponikowski, P. et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 18, 891–975 (2016).
Levy, W. C. et al. The Seattle Heart Failure Model: Prediction of survival in heart failure. Circulation 113, 1424–1433 (2006).
Motulsky, H. J. & Brown, R. E. Detecting outliers when fitting data with nonlinear regression—A new method based on robust nonlinear regression and the false discovery rate. BMC Bioinform. 7, 123 (2006).
Acknowledgements
We would like to thank Mrs. Roya Soltan (Institute for Molecular Cardiovascular Research (IMCAR), RWTH Aachen University) for her assistance with processing of the blood samples. We also would like to thank Prof. Dr. Ralf-Dieter Hilgers (Department of Medical Statistics, RWTH Aachen University) for overseeing the statistical analyses.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was funded by DiaSorin GmbH.
Author information
Authors and Affiliations
Contributions
A.C., C.C., V.B., and A.S. concepted and designed the study; A.C., R.F., C.C., E.L., and A.S. analyzed and interpreted the data; A.C., E.L., and A.S. drafted the manuscript; R.F., K.K., C.C., and V.B. revised it critically for important intellectual content. A.C. prepared Figs. 1–3, E.L. prepared Fig. 4. All authors agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
This study was funded by DiaSorin GmbH. AS and VB have received institutional research support from DiaSorin GmbH for the assessment of FGF23 in the serum samples. DiaSorin GmbH was not involved in the study design, sample collection, interpretation of data or writing of the report. The other authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cornelissen, A., Florescu, R., Kneizeh, K. et al. Intact fibroblast growth factor 23 levels and outcome prediction in patients with acute heart failure. Sci Rep 11, 15507 (2021). https://doi.org/10.1038/s41598-021-94780-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-94780-7
This article is cited by
-
FGF23 and klotho at the intersection of kidney and cardiovascular disease
Nature Reviews Cardiology (2024)
-
Oncostatin M is a regulator of fibroblast growth factor 23 (FGF23) in UMR106 osteoblast-like cells
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.