Abstract
This study assesses how circadian rhythms of heart rate (HR), HR variability (HRV) and activity change during long-term missions in space and how they relate to sleep quality. Ambulatory 48-h ECG and 96-h actigraphy were performed four times on ten healthy astronauts (44.7 ± 6.9 years; 9 men): 120.4 ± 43.7 days (Before) launch; 21.1 ± 2.5 days (ISS01) and 143.0 ± 27.1 days (ISS02) after launch; and 86.6 ± 40.6 days (After) return to Earth. Sleep quality was determined by sleep-related changes in activity, RR-intervals, HRV HF- and VLF-components and LF-band. The circadian amplitude of HR (HR-A) was larger in space (ISS01: 12.54, P = 0.0099; ISS02: 12.77, P = 0.0364) than on Earth (Before: 10.90; After: 10.55 bpm). Sleep duration in space (ISS01/ISS02) increased in 3 (Group A, from 370.7 to 388.0/413.0 min) and decreased in 7 (Group B, from 454.0 to 408.9/381.6 min) astronauts. Sleep quality improved in Group B from 7.07 to 8.36 (ISS01) and 9.36 (ISS02, P = 0.0001). Sleep-related parasympathetic activity increased from 55.2% to 74.8% (pNN50, P = 0.0010) (ISS02). HR-A correlated with the 24-h (r = 0.8110, P = 0.0044), 12-h (r = 0.6963, P = 0.0253), and 48-h (r = 0.6921, P = 0.0266) amplitudes of the magnetic declination index. These findings suggest associations of mission duration with increased well-being and anti-aging benefitting from magnetic fluctuations.
Similar content being viewed by others
Introduction
Previous investigations reported that circadian rhythm disruption and sleep problems, with impact on human aging, are pervasive among astronauts1,2,3,4,5,6,7. Nevertheless, several recent investigations provide evidence of anti-aging effects of long-duration space travel. In the National Aeronautics and Space Administration (NASA) Twin Study, the identical twin astronaut monitored before, during, and after a 1-year mission onboard the ISS had lengthened telomeres as compared to his twin serving as a genetically matched ground control8. Because telomere length is considered a marker of cellular aging, aging being usually associated with decreased telomere length9,10, the NASA twin study suggests a possible anti-aging effect of long-duration space travel. Another study on blood DNA methylation of six participants of the Mars-500 mission, a high-fidelity 520-day ground simulation experiment, showed that mission duration was associated with significant decreases in epigenetic aging at the 168- and 300-day time points11. DNA methylation, including DNAmPhenoAge, is a robust predictor of mortality risk that is also highly correlated with chronological age11,12.
Other investigations also showed anti-aging or even longevity effects of space travel, such as the significantly extended lifespan of Caenorhabditis elegans after a 9-day spaceflight13, and of Drosophila melanogaster after a 13-day spaceflight14. Our previous study also showed anti-aging effects of 6-month space flights, which were associated with improved HRV reflecting good health and anti-aging in seven astronauts15. Surprisingly, we found that magnetic fluctuations affect the brain’s default mode network (DMN) activity by stimulating the VLF component of HRV in a light-dependent manner and/or with help from the circadian clock.
Human aging on Earth is also generally associated with a weakening of the circadian system and a decrease in sleep efficiency. Earlier, based on analysis of ECG records of 7 astronauts during long-duration missions on the ISS, we observed an improvement in SDANN, Triangular Index, and TF after 4.5 months in space15. We then hypothesized that these results could indicate an anti-aging effect. Herein, we revisit our earlier finding based on data from 10 astronauts, and investigate possible mechanisms underlying anti-aging effects of long-duration space travel, focusing on the circadian system. We assess the circadian variation of heart rate (HR) and endpoints of heart rate variability (HRV) and estimate sleep efficiency based on 48-h ambulatory ECG records complemented by 96-h actigraphy. We examine (1) how circadian rhythms change in space, a larger 24-h amplitude assumed to reflect a stronger circadian system; and (2) how spaceflight affects sleep duration, symptoms of insomnia, and sleep quality. In order to understand how the observed changes may have occurred, we study (3) how spaceflight affects parasympathetic nerve activity, and (4) how magnetic fluctuations in space affect circadian behavior.
Subjects and methods
Subjects
Ten healthy astronauts (9 men, 1 woman) participated in this ISS (International Space Station) JAXA (Japan Aerospace Exploration Agency) investigation, named “Biological Rhythms 48 Hrs”. Their mean (± SD) age was 44.7 ± 6.9 years. Their mean stay in space was 155.7 ± 26.0 days. Astronauts had passed class III physical examinations from the National Aeronautics and Space Administration (NASA). The study was approved by the Institutional Review Boards of NASA, ESA (European Space Agency) and JAXA. Informed consent was obtained from all participants. A detailed explanation of the study protocol was given to the astronauts before they gave written, informed consent, according to the Declaration of Helsinki Principles. All methods were performed in accordance with the JAXA/ESA/NASA guidelines and regulations.
Universal Time Coordinated (UTC) is used aboard the ISS. The windows are covered during night hours to give the impression of darkness because the station experiences 16 sunrises and sunsets per day. Astronauts follow a strict 24-h routine, waking up at 06:00 and retiring for sleep at 21:3016. At the time of data collection of this study, artificial lighting from both incandescent and fluorescent light sources was used on the ISS; maximal light intensity was 700 lx17.
Experimental protocols
Around-the-clock ambulatory 48-h ECG records were obtained by using a two-channel Holter recorder (FM-180; Fukuda Denshi). Measurements were made four times. First (Before), recording occurred 120.4 ± 43.7 (mean ± SD; range: 50–183) days before launch, except for one astronaut who, due to technical problems, was recorded on day 469 after return to Earth. Two recordings were obtained on the ISS, early (ISS01, 21.1 ± 2.5, 18–26 days after launch), and late (ISS02, 143.0 ± 27.1, 103–183 days after launch) during their mission. The last recording (After) was obtained 86.6 ± 40.6 (17–156) days after return to Earth. In one astronaut, actigraphy could not be performed during ISS02, and in two astronauts, ambulatory 48-h ECG records were not obtained after return to Earth, because of a recording failure due to electrodes’ poor contact to the skin.
The 48-h ECG records were subdivided into two 24-h spans to assess sleep duration and quality of sleep, and to compare HRV endpoints between days of relatively higher or lower magnetic activity.
Astronauts were classified by their sleep duration in space: in Group A (n = 3), sleep duration lengthened in space during both ISS01 and ISS02 compared to Before, and in Group B (n = 7), sleep duration was shorter during both ISS01 and IDD02 than Before.
Analysis of heart rate variability
Data collection and measurement procedures were conducted as previously reported15,18,19,20,21. Briefly, for HRV measurements, RR intervals between normal QRS waveforms were extracted as normal-to-normal (NN) intervals. Time-domain HRV indices, including r-MSSD and pNN50, and frequency-domain measures, including conventional VLF- (0.003–0.04 Hz), LF- (0.04–0.15 Hz), and HF- (0.15–0.40 Hz) components22, as well as LF-band (0.01–0.05 Hz), MF1-band (0.05–0.10 Hz), MF2-band (0.10–0.15 Hz), and HF-band (0.15–0.20 Hz), reflecting an activation of the default mode network (DMN)21 were obtained with the MemCalc/CHIRAM (Suwa Trust GMS, Tokyo, Japan) software23.
Actigraphy
A 96-h actigraphy record started 48 h before the start of 48-h ECG monitoring. Actigraphy was shown to be highly correlated with polysomnographically-defined sleep timing, even under spaceflight conditions5,6,24. Astronauts wore wrist-borne actiwatches (Actiwatch Spectrum; Phillips/Respironics, Murrysville, PA, USA) on the nondominant wrist, providing sleep/wake activity in 1-min epochs.
Cosine curve fitting for estimating period, amplitude and acrophase by cosinor
The circadian period of activity and ECG-derived measures was determined by the Maximum Entropy Method (MEM)23. Single 24-h, 12-h or 48-h cosine curves were fitted independently to the data by cosinor25,26,27 to estimate their respective amplitude and acrophase in addition to the MESOR (Midline Estimating Statistic Of Rhythm, a rhythm-adjusted mean). Changes in circadian amplitude assessed the response in circadian rhythmicity to the space environment.
Determining sleep duration, and scoring sleep quality and symptoms of insomnia
Sleep at night was estimated by actigraphy, RR-intervals, heart rate and changes in the HF-component of HRV, as shown in the left panel of Fig. 1. Sleep duration was determined as the average between two consecutive sleep spans. An “insomnia score” between 0 and 10 was determined based on four items. A score of 3, 2, or 1 was assigned if the average sleep duration was below 4.5 h, between 4.5 and 6 h, or between 6 and 7.5 h, respectively. A score of 3, 2, or 1 was assigned if the average sleep latency was more than 30 min, between 20 and 30 min, or between 10 and 20 min, respectively. A score of 1 was assigned if sleep was interrupted. A score of 3, 2, or 1 was assigned if the time of awakening was earlier than usual on Earth by more than 60 min, 40 to 60 min, or 20 to 40 min, respectively. The insomnia score was obtained by summing the scores from the four items.
Estimation of sleep span (left) and assessment of sleep quality (right). Left: Sleep span is estimated by wrist activity data with postural change (row 1), RR-intervals (row 2), heart rate (row 3), and changes in the HF-component (row 4). This example shows day-to-day differences in the sleep-related increase in the HF-component of HRV. The spectral power of the HF-component is higher on the first day (row 4). In addition, the nocturnal HR dip is blunted on the second day as compared to the first day (row 3). Right: Representative example of a 48-h concomitant record of wrist activity, RR-intervals, and HF-component, LF-band and VLF-component of HRV. Right and left results are from different astronauts. Sleep quality is assessed by determining whether there is a sleep-related (1) decrease in activity (row 1); and increase during the sleep span in (2) RR-intervals (row 2); (3) power in the HF-component (row 3); (4) power of the LF-band (row 4); and (5) power of the VLF-component (row 5). A sleep quality score is obtained by summing the 5 items for each night, on a scale of 0 to 10 points (Yes/No is 1/0).
Sleep quality was estimated by using not only the actigraphy data and RR-intervals, but also the spectral power of the HF-component, LF-band and VLF-component of HRV, as shown in the right panel of Fig. 1, because these indices reflect the quality of rest, activity of the DMN, and fluctuations that are important for health, respectively. Five items contributed to determining the sleep quality score. Each item had a 2-point scale (0 to 1 for each of two consecutive nights, corresponding to an answer of “No” or “Yes” to each question item). Summing the scores from the five items yielded a score on a scale of 0 to 10, a higher score meaning a better sleep quality. The five items consist of whether there is a sleep-related (1) decrease in activity; and increase during sleep in (2) RR-intervals; (3) power in the HF-component; (4) power of the LF-band; and (5) power of the VLF-component. In the example shown in the right panel of Fig. 1, a sleep-related increase in RR-intervals was prominent during sleep on both nights (counted as 1 point each). Similarly, sleep-related increases in the power of the HF-component, LF-band, and VLF-component were also observed on both nights, as was a sleep-related decrease in activity. Accordingly, the sleep quality score in this case is 10.
Assessment of parasympathetic activity by nocturnal HR dip and sleep-related HRV rise in space
A blunted HR dip during sleep has been associated with all-cause mortality28. HR is expected to decrease by at least 10% during sleep as compared to the awake span. Non-dipping was defined as a decrease of less than 10%. HRV is also known to increase during sleep. The nocturnal rise was calculated by comparison with the awake span. Changes in sleep-related HR-dipping and HRV-rising during spaceflight are assessed.
Assessment of magnetic fluctuations
The ISS is protected from the space environment by Earth's magnetic field. The ISS orbits the Earth every 90 min at an altitude of 330 to 480 km. As we reported previously15, we used geomagnetic measurements at 1-min intervals available from the Auroral Observatory of the University of Tromsø, Norway (69°39′ N, 18°56′ E). The indices considered herein are total intensity (F, in nT), declination (D, angle between geographic and magnetic north, in degrees), inclination (I, angle between horizontal plane and magnetic direction, in degrees), horizontal intensity (H, in nT), and vertical intensity (Z, in nT).
Historically, the estimation of the ionospheric electric field used ionospheric currents and field-aligned currents from ground magnetic records, along with incoherent scatter radars, satellite measurements of X-ray and UV aurorae29. This type of study over the past two hundred years about the structure and temporal changes of the Sun–Earth space, now called space weather, has become of great interest to space science30,31,32.
Effects of magnetic fluctuations on the circadian amplitude of HR in space
Correlations between the circadian amplitude of HR and the 12-, 24-, or 48-h amplitudes of geomagnetic indices were estimated using Pearson’s correlation coefficient.
Statistical analyses
Data were expressed as mean ± standard deviation (SD). Space-related changes in HRV endpoints were assessed by computing differences between their values assumed during ISS01 and/or ISS02 and those obtained before launch (Before), analyzed by the paired t-test. Effects of magnetic activity on HRV endpoints were also compared to before launch (Before) by the paired t-test, using the Stat Flex (Ver. 6) software (Artec Co., Ltd., Osaka, Japan). P-values less than 0.05 were considered to indicate statistical significance.
Results
Circadian rhythm of sleep–wake cycle and heart rate during spaceflight
Actigraphy records spanned on average 5453.5 ± 828.5 min (Before), 6565.6 ± 1270.1 min (ISS01), 6761.0 ± 529.0 min (ISS02) and 5273.7 ± 1290.0 min (After). The corresponding estimates of the circadian period were 23.55-h (Before), 24.32-h (ISS01), 23.66-h (ISS02) and 23.91-h (After). Estimates of the acrophase at a 24-h trial period were 14:32 (Before), 14:15 (ISS01), 14:12 (ISS02) and 14:13 (After). Circadian periods and 24-h acrophases did not change statistically significantly in space. The circadian amplitude of activity did not change with statistical significance either. The circadian sleep–wake cycle was resilient to the mission environment in space (Table 1).
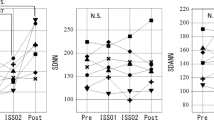
The circadian amplitude of HR increased during spaceflight from 10.90 bpm (Before) to 12.54 (ISS01) and 12.77 (ISS02), returning to 10.55 (After), Fig. 2. There were no statistically significant changes in the circadian period or acrophase of HR in space. Such an amplification of the circadian variation in HR differs from results of previous investigations2,3,4,5,6,7.
The 48-h and 3.5-day amplitudes of activity tended to be reduced in space. The 48-h amplitude dropped from 39.85 ± 30.14 (Before) to 22.12 ± 16.05 (ISS01) (P = 0.1041) and 27.63 ± 17.93 (ISS02), and the 84-h amplitude dropped from 44.51 ± 37.22 (Before) to 18.57 ± 16.36 (ISS01) (P = 0.0588) and 22.64 ± 14.46 (ISS02). Similarly, the 48-h amplitude of HR was reduced in space from 3.78 ± 1.55 (Before) to 2.23 ± 9.90 (ISS01, P = 0.0426) and 2.91 ± 1.30 (ISS02). These results reinforce the higher prominence of the circadian rhythm of these variables in space.
Sleep duration and scoring of sleep quality and symptoms of insomnia
In group A, insomnia and quality of sleep scores did not statistically significantly change in space, as compared to pre-launch, Table 2. By definition, astronauts in group B shortened their sleep duration in space, which changed from 454.0 min (Before) to 408.9 min (ISS01, P = 0.0201) and 381.6 min (ISS02, P = 0.0011), returning to 426.7 min (After), Fig. 3 (left). The quality of sleep score improved from 7.07 (Before) to 8.36 (ISS01), and 9.36 (ISS02, P = 0.0001), returning to 6.42 (After), Fig. 3 (right). No statistically significant change was noted for the insomnia score. The change in sleep duration may reflect a regression toward the mean as sleep duration before launch was shorter in Group A than in Group B (370.7 vs. 454.0 min, P = 0.0984). Sleep duration no longer differed in space between the two groups (ISS01: 388.0 vs. 408.9 min, P = 0.5549; ISS02: 413.0 vs. 381.6 min, P = 0.3720) or after return to Earth (474.5 vs. 426.7 min, P = 0.3791). Sleep quality did not differ either between the two groups, before launch (7.83 vs. 7.07, P = 0.2836), in space (ISS01: 9.00 vs. 8.36, P = 0.4257; ISS02: 7.83 vs. 9.36, P = 0.2680) or after return to Earth (6.25 vs. 6.42, P = 0.9120).
Changes in sleep duration (left) and quality of sleep score (right) in Group B astronauts. Sleep duration shortened from 454.0 ± 64.7 min (Before) to 408.9 ± 43.8 min (ISS01, P = 0.0201) and 381.6 ± 49.2 min (ISS02, P = 0.0011) during spaceflight, returning to 426.7 ± 67.5 min after the mission (left). Quality of sleep improved from 7.07 ± 1.10 to 8.36 ± 1.25 (ISS01, P = 0.1148) and 9.36 ± 0.95 (ISS02, P = 0.0001), returning to 6.42 ± 1.93 after return to Earth (right).
Nocturnal HR dip and HRV rise during long-duration spaceflight
As shown in Table 3, HR dropped by more than 10% during sleep in both groups on the ISS. In Group B, the HR dip became deeper during ISS01 (P = 0.0375) and ISS02 (P = 0.0375) as compared to pre-launch. The nocturnal rise increased during ISS02 in r-MSSD (P = 0.0492) and pNN50 (P = 0.0010), HRV indices reflecting parasympathetic activity. The nocturnal dip also decreased reciprocally in LF/HF (P = 0.0161), which reflects sympathetic activity. During ISS02, the nocturnal rise also increased in the MF1-band reflecting DMN activity (P = 0.0070), and in the VLF-component showing well-being (P = 0.0303), Table 3.
Effects of magnetic fluctuations on circadian HR amplitude in space
MEM spectra of geomagnetic indices during spans matching the 48-h ECG records showed not only a peak around one cycle in 24 h, but also peaks around one cycle in 48 h and around one cycle in 12 h, corresponding to the circaduodian and circasemidian components, respectively. The circadian amplitude of HR correlated statistically significantly with the circadian (r = 0.8110, P = 0.0044), circasemidian (r = 0.6963, P = 0.0253) and circaduodian (r = 0.6921, P = 0.0266) amplitudes of the geomagnetic declination index, Fig. 4. Among geomagnetic indices, the geomagnetic declination index showed the strongest association, as shown in Table 4. The circasemidian amplitude of the geomagnetic declination index also correlated statistically significantly with the circadian amplitude of the VLF-component (r = 0.6963, P = 0.0253), MF1-band (r = 0.7398, P = 0.0144), and HF-band (r = 0.7702, P = 0.0091), Table 4. Its circadian amplitude also correlated with the circadian amplitude of the VLF-component (r = 0.7383, P = 0.0148). An effect of magnetic fluctuations was not found to affect the circadian acrophase of HR or any of the HRV endpoints.
Representative example of correlation between the circadian amplitude of HR and the amplitude of the 12-, 24- and 48-h components of the geomagnetic declination (D) of the geomagnetic field. Effect of magnetic fluctuations on the circadian amplitude of human HR is suggested by its strong correlation with the 12-h (circasemidian; r = 0.6963, P = 0.0253, left panel), circadian (r = 0.8110, P = 0.0044, middle panel), and 48-h (circaduodian; r = 0.6921, P = 0.0266, right panel) amplitudes of D, the declination of the geomagnetic field.
Discussion
The most striking result of this investigation is the larger prominence of the circadian HR rhythm in space in ten astronauts living on the ISS for about 6 months, Fig. 2, an indication that long-duration spaceflight may have slowed down aging. These results contrast with earlier reports by others2,3,4,5,6,7. Most of these studies, however, were conducted during shorter missions on the MIR station or on shuttle missions. Daily schedules may not have been as regimented as in current ISS conditions, and no information on the lighting conditions were provided2,3,4,5,6. Results from the only study that was conducted on the ISS during long-duration missions similar to ours showed that sleep duration was shorter and of poorer quality when crewmembers’ sleep episodes were misaligned relative to the estimated endogenous circadian temperature minimum7. The large circadian amplitude of HR and of some of the HRV endpoints may be an indication that in our study circadian misalignment may have minimal, although we did not measure temperature. Another aspect of our results, which was not examined in these earlier studies and may deserve further investigation is our finding that both the 3.5-day component of activity and the 48-h component of HR weakened in space, supporting an enhancement of the circadian rhythm.
Misalignment of the circadian clock could impair the health of astronauts. Evidence from genetic animal models and from humans under circadian misalignment (such as shift work) shows that disrupting the coordination between the endogenous clock and physiological processes can seriously affect health. Circadian disruption can lead to cardiovascular, metabolic and psychiatric disorders, cancer and cognitive decline in the aged. The International Agency for Research on Cancer (IARC), part of the World Health Organization (WHO), enlisted circadian disruption as a probable human carcinogenic (Group 2A) in 200733,34,35,36,37,38,39,40,41,42,43,44. Conversely, enforcement of the circadian rhythm impairs cancer progression and increases quality of life41. In patients with lung and breast cancer, the presence of a circadian rhythm is a survival predictor45,46; patients with colorectal cancer who have a robust circadian rhythm survive longer and have a better quality of life than patients who have a misaligned circadian system and poor sleep47,48. Thus, enhancement of the circadian HR rhythm in space, observed herein, may be associated with well-being and reflect a heightened health status. Whether the amplified circadian HR rhythm stems from the regular daily routine on the ISS or from exposure to the space environment itself remains to be determined. Below, we review how long-duration space travel might promote anti-aging.
Among the several reasons why the circadian rhythm of HR strengthened in space, the astronauts’ strictly regulated scheduled day on the ISS comes to mind first. Because the body has peripheral clocks located in each organ (heart, lung, liver, muscles, kidneys, retina, etc.) that optimize the function of each organ according to the environmental context, allowing adaptation of the organism to environmental changes, the circadian clock network has an adaptive function49,50. The astronauts’ day starts by awakening at 06:00, followed by post-sleep activities and a morning inspection of the station. The crew then eats breakfast and takes part in a daily planning conference with Mission Control before starting work at around 08:10. The first scheduled exercise of the day follows, after which the crew continues work until 13:05. Following a one-hour lunch break, the afternoon consists of more exercise and work before astronauts carry out their pre-sleep activities beginning at 19:30, including dinner and a crew conference. The scheduled sleep span begins at 21:30. Such a regular life schedule, in particular, a regular light/dark cycle, regular arousal stimuli, regular food intake, and in principle regularly programmed exercise can thus synchronize the circadian system of astronauts51,52.
In addition, space-environmental stimuli, including magnetic fluctuations and microgravity can be involved in the amplification of the circadian rhythm of HR. The role of magnetic fluctuations in circadian clock function, how sleep enhances parasympathetic tone, and how the DMN is activated, gauged by HRV behavior in the VLF, MF1- and HF-bands, are additional insights obtained from the present investigation (Table 4). A recent study53 found that both the amplitude and period of the endogenous circadian oscillation are affected by magnetic stimulation, and that the effects are strongly circadian stage-dependent. Applied at the appropriate phase, magnetic stimulation enhances the circadian amplitude of bioluminescence of cultured suprachiasmatic nucleus slices.
Microgravity can also induce an over 3-fold increase in the circadian amplitude of clock genes54,55,56,57. In addition to light, gravity is another synchronizer affecting circadian patterns with significant changes of general behavior, hormone synthesis, body temperature and metabolism54.
Sleep in space
Sleep duration was shorter in space than on Earth in 7 of the 10 astronauts (Group B). On average, they slept 6 h and 49 min during ISS01 and 6 h 22 min during ISS02, which represents 90.1% and 84.1% of their sleep duration before launch, respectively; after return to Earth, they slept 7 h 7 min (94.0% of pre-launch sleep duration), Fig. 3 (left) and Table 2. In the other 3 astronauts (Group A), sleep duration during ISS01 and ISS02 was 6 h and 28 min and 6 h and 53 min, respectively (Table 2).
Sleep duration is associated with several health and functional consequences, with both short (≤ 6 h) and long (≥ 9 h) sleep duration increasing the risk of negative outcomes58,59,60,61,62,63,64. Several studies have shown that short sleep duration, as well as long sleep duration, impacts inflammation65. In the population-based InCHIANTI study (n = 751), concentrations of TNF and CRP were increased at extremes of both short and long sleep. Sleep duration between 6 and 7 h observed during space travel suggests that it is sufficient for attenuating inflammatory disease risk at the systemic, cellular, and genomic levels, with implications for inflammaging and molecular processes of aging, both in Group A and B astronauts, as Irwin and Opp proposed65.
Insomnia independently contributes to the risk of inflammaging processes of aging66,67. The insomnia score of astronauts of both Groups A and B was kept well between 1.83 and 2.71 during space travel (Table 2).
The quality of sleep score of astronauts improved in space, another sign that aging may have slowed down on the ISS. In astronauts of Group A, the increase was mostly noted during ISS01 (from 7.83 to 9.00, P = 0.0198), whereas in astronauts of Group B, the sleep score increased from 7.07 before launch to 8.36 during ISS01 and to 9.36 during ISS02 (P = 0.0001), Table 2 and Fig. 3 (right). Sleep quality is a complex construct that is quite difficult to evaluate with sufficient precision68,69,70. We estimated quality of sleep based on actigraphy and on the circadian profiles of several HRV endpoints (Fig. 1). Among them, the HF-component is known to express quality of sleep22,71; the LF-band is thought to reflect an activation of the temporoparietal junction (TPJ), a central member of hubs of the DMN, serving a broader adaptive purpose21,72,73; the VLF-component reflects fluctuations that are fundamental to health and well-being, because the heart itself intrinsically generates the VLF-component of HRV15,74,75,76. Since these endpoints were used to derive the quality of sleep score, and since they are associated with health preservation, we can consider that a high quality of sleep score is indicative of health.
We found that sleep quality was improved in space, contrary to previous investigations. Numerous studies have linked sleep to the circadian rhythm, also in humans42,77,78. Magnetic stimulation in space may be another contributory factor. Several previous investigations indeed demonstrated that magnetic stimulation at night induced deep sleep slow waves79,80.
Parasympathetic activity assessed by sleep-related HR dip and HRV rise in space
Sleep-related parasympathetic activity improved in astronauts of Group B, reaching values similar to those of astronauts in Group A. The nocturnal rise in HRV, reflecting parasympathetic activity, increased in space during ISS02 as compared to pre-flight: r-MSSD increased from 25.1 to 37.3% (P = 0.0492) and pNN50 from 55.2% to 74.8% (P = 0.0010). These responses in space agree with work by Gundel et al., who suggested that the parasympathetic drive to the heart during sleep may be stronger in space than on Earth81. Reciprocally, LF/HF, reflecting sympathetic activity, decreased in space compared to pre-flight (from 42.1% to 27.2% during ISS02, P = 0.0161). Hence, the nocturnal HR dip was more pronounced in space than before launch: it increased from 20.9% to 24.1% (ISS01, P = 0.0375) and 24.8% (ISS02, P = 0.0384), returning to pre-launch values after return to Earth (20.4%), Table 3. In addition, we found that the MF1-band, reflecting DMN activity, and the VLF-component, fundamental to health and well-being, increased during ISS02 as compared to pre-flight (MF1: from 6.9% to 31.1.%, P = 0.0070; VLF: from 34.1% to 49.8%, P = 0.0303), Table 3.
Since a blunted nocturnal decrease in HR (by less than 10%) has been associated with all-cause mortality82,83,84, responses observed in HR and HRV indices may suggest a positive physiological adaptation to microgravity in space.
Effects of magnetic fluctuations on the circadian amplitude of HR
Earlier, we reported a possible anti-aging effect of magnetic fluctuations in space, as gauged by HRV endpoints of astronauts during long-duration missions on the ISS15. Herein, we confirm this result by our comparison of several HRV endpoints between the day of higher versus the day of lower magnetic activity. In astronauts of Group B (n = 7) during ISS01, days of higher magnetic activity are associated with a higher MESOR of the HF-component (164.2 ± 84.5 vs. 145.3 ± 85.6, P = 0.0381), of pNN50 (7.2 ± 6.0 vs. 5.9 ± 5.9, P = 0.0361), of the VLF-component (2644.7 ± 1151.3 vs. 2292.7 ± 1016.3, P = 0.0040), and of the LF-band (1663.1 ± 786.8 vs. 1482.5 ± 724.3, P = 0.0450). During ISS02, the power of the HF-component is larger and the nocturnal HR dip is more pronounced on the day of higher magnetic activity, as illustrated for one case in Fig. 1 (left).
The circadian amplitude of HR correlated positively with magnetic disturbances in space along the scales of 24 h (r = 0.8110, P = 0.0044), 12 h (r = 0.6963, P = 0.0253) and 48 h (r = 0.6921, P = 0.0266), Table 4 and Fig. 4. As life depends on processes fluctuating at many different frequencies, many showing co-periodisms with the broad environment, it is not surprising that several components of space weather are associated with an amplification of the circadian HR rhythm in space. Invisible fluctuating cosmic factors (such as solar activity and magnetic disturbances) can affect virtually every cell and electrical circuits in biological systems85,86,87. Although the underlying mechanisms are far from being fully understood, our previous studies based on 7-day/24-h ECG found a significant graded decrease in HRV endpoints in response to geomagnetic storms. Based on these results, we postulated the existence of a light-dependent human magnetoreception system88,89 and proposed that human cryptochrome-2 can act as a magnetic sensor90.
As humans respond to space weather, Halberg et al.87 proposed that broad time structures (chronomes) in humans resonate with cycles found in the environment. The study of these effects is part of chronomics medicine, including chronoastrobiology27,87, the science studying effects of heliogeomagnetics on biota. On Earth, circaseptans in human HR were amplified during spans when they were detected in solar activity86. Herein, we showed that magnetic fluctuations also had a distinct impact on the circadian rhythm of important cardiovascular functions in space.
Limitations
This is the first report showing that magnetic activity can reinforce the circadian system in space. The study is limited, however, by the relatively short ECG records of 48 h, monitored only twice during a 6-month space mission. Longer ECG records are desirable as shown by our previous 7-day/24-h investigations on Earth27,85,88,89.
Another limitation is the lack of brain oscillatory activity data, as discussed in our previous investigations15,21, although a number of studies showed that HRV is like a mirror reflecting functions of the neural network72,91,92. However, these associations are extremely complex93,94,95, and we need future investigations to directly depict the brain oscillatory activity in space.
Future studies would benefit from including measures of telomere length and DNA methylation, which are generally accepted indicators of anti-aging, as a complement to the increased prominence of circadian rhythms and the increase in sleep quality documented herein.
Fully understanding physiology in space is challenging in view of the multiple factors involved beyond weightlessness. Fluid redistribution, isolation, and the changed work environment are elements that all contribute to the astronauts' well-being and physiological adaptation to space. Results herein have shed light on the merit of distinguishing between different stages of adaptation to the space environment, realizing that some mechanisms involved may take several months. Much work remains to be done, however, before arriving at unambiguous and reliable answers to the multi-faceted questions that long-duration spaceflights entail.
Conclusion
Herein, we provided evidence that in space the circadian rhythm of HR was strengthened, sleep quality was improved, and parasympathetic tone was enhanced at night, by taking advantage of magnetic fluctuations and by activating DMN activities. Our previous investigation also showed that magnetic fluctuations in the magnetosphere can affect and enhance HRV endpoints in space, thereby perhaps conveying an anti-aging effect, probably in association with the brain DMN, in a light-dependent manner and/or with help from the circadian clock15. These findings suggest associations of long-duration space travel with increased well-being and anti-aging properties.
Such information could enable the formulation of novel hypotheses, or account for unanticipated results, for instance from data collected during a magnetic storm that we now know can affect the heart and brain. Thus, magnetic disturbances have become a concern in programs of space exploration, even though further studies on the molecular mechanisms underlying the action of the electromagnetic fields, in combination with microgravity, are warranted.
Data availability
Restrictions from Japan’s Aerospace Exploration Agency apply to the availability of the data supporting the findings of this study. which were used under license for the current study. As such, they are not publicly available.
References
Mohler, S. R. Age and space flight. Aviat. Space Environ. Med. 56, 714–717 (1985).
Santy, P. A., Kapanka, H., Davis, J. R. & Stewart, D. F. Analysis of sleep on Shuttle missions. Aviat Space Environ Med. 59, 1094–1097 (1988).
Gundel, A., Polyakov, V. V. & Zulley, J. The alteration of human sleep and circadian rhythms during spaceflight. J. Sleep Res. 6, 1–8 (1997).
Monk, T. H., Buysse, D. J., Billy, B. D., Kennedy, K. S. & Willrich, L. M. Sleep and circadian rhythms in four orbiting astronauts. J. Biol. Rhythms. 13, 188–201 (1998).
Dijk, D. J. et al. Sleep, performance, circadian rhythms, and light-dark cycles during two space shuttle flights. Am. J. Physiol. Regul. Integr. Comp. Physiol. 281, R1647–R1664 (2001).
Barger, L. K. et al. Prevalence of sleep deficiency and use of hypnotic drugs in astronauts before, during, and after spaceflight: an observational study. Lancet Neurol. 13, 904–912 (2014).
Flynn-Evans, E. E., Barger, L. K., Kubey, A. A., Sullivan, J. P. & Czeisler, C. A. Circadian misalignment affects sleep and medication use before and during spaceflight. NPJ Microgravity. 2, 15019 (2016).
Garrett-Bakelman, F. E. et al. The NASA Twins Study: A multidimensional analysis of a year-long human spaceflight. Science 364, eaau8650 (2019).
Turner, K. J., Vasu, V. & Griffin, D. K. Telomere biology and human phenotype. Cells 8, 73 (2019).
Lulkiewicz, M., Bajsert, J., Kopczynski, P., Barczak, W. & Rubis, B. Telomere length: how the length makes a difference. Mol. Biol. Rep. 47, 7181–7188 (2020).
Nwanaji-Enwerem, J. C. et al. A longitudinal epigenetic aging and leukocyte analysis of simulated space travel: The Mars-500 mission. Cell Rep. 33, 108406 (2020).
Lu, A. T. et al. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging (Albany NY) 11, 303–327 (2019).
Honda, Y. et al. Genes down-regulated in spaceflight are involved in the control of longevity in Caenorhabditis elegans. Sci. Rep. 2, 487 (2012).
Ma, L., Ma, J. & Xu, K. Effects of spaceflight on the circadian rhythm, lifespan and gene expression of Drosophila melanogaster. PLoS ONE 10, e0121600 (2015).
Otsuka, K. et al. Anti-aging effects of long-term space missions, estimated by heart rate variability. Sci. Rep. 9, 8995 (2019).
Ohshima, H. Healthy life learned from space medicine. Auton. Nerv. Syst. (Tokyo) 54, 73–76 (2017).
Whitmire, A.M. et al. Risk of performance errors due to sleep loss, circadian desynchronization, fatigue, and work overload. In Human Health and Performance Risks of Space Exploration Missions: Evidence Reviewed by the NASA Human Research Program, (ed. McPhee, J.C. & Charles, J.B.) 85–116 (NASA SP-2009-3405, 2009).
Yamamoto, N. et al. Effects of long-term microgravity exposure in space on circadian rhythms of heart rate variability. Chronobiol. Int. 32, 327–340 (2015).
Otsuka, K. et al. Intrinsic cardiovascular autonomic regulatory system of astronauts exposed long-term to microgravity in space: observational study. NPJ Microgravity. 1, 15018 (2015).
Otsuka, K. et al. Long-term exposure to space’s microgravity alters the time structure of heart rate variability of astronauts. Heliyon. 2, e00211 (2016).
Otsuka, K. et al. Circadian challenge of astronauts’ unconscious mind adapting to microgravity in space, estimated by heart rate variability. Sci. Rep. 8, 10381 (2018).
Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation 93, 1043–1065 (1996).
Saito, K. et al. (eds) A recent advances in time series analysis by maximum entropy method (Hokkaido University Press, Sapporo, 1994).
Monk, T. H., Buysse, D. J. & Rose, L. R. Wrist actigraphic measures of sleep in space. Sleep 22, 948–954 (1999).
Bingham, C., Arbogast, B., Cornelissen, G. G., Lee, J. K. & Halberg, F. Inferential statistical methods for estimating and comparing cosinor parameters. Chronobiologia 9, 397–439 (1982).
Cornelissen, G. Cosinor-based rhythmometry. Theor. Biol. Med. Model. 11, 16 (2014).
Otsuka, K., Cornelissen, G. & Halberg, F. Chronomics and Continuous Ambulatory Blood Pressure Monitoring—Vascular Chronomics: From 7-Day/24-Hour to Lifelong Monitoring 870 + lxxv (Springer, Tokyo, 2016).
Ben-Dov, I. Z. et al. Blunted heart rate dip during sleep and all-cause mortality. Arch. Intern. Med. 167, 2116–2121 (2007).
Kamide, Y., Richmond, A. D. & Matsushita, S. Estimation of ionospheric electric field, ionospheric currents and field-aligned currents from ground magnetic records. J. Geophys. Res. 86, 801–813 (1981).
Kamide, Y. Estimate of electromagnetic quantities in space from ground magnetic records. Science 241, 328–330 (1988).
Kamide, Y. et al. Combining electric field and aurora observations from DE 1 and 2 with ground magnetometer records to estimate ionospheric electromagnetic quantities. J Geophys Res. 94, 6723–6738 (1989).
Kamide, Y. & Balan, N. The importance of ground magnetic data in specifying the state of magnetosphere–ionosphere coupling: a personal view. Geosci. Lett. 3, 10 (2016).
Hastings, M. H., Reddy, A. B. & Maywood, E. S. A clockwork web: circadian timing in brain and periphery, in health and disease. Nat. Rev. Neurosci. 4, 649–661 (2003).
Davis, S. & Mirick, D. K. Circadian disruption, shift work and the risk of cancer: A summary of the evidence and studies in Seattle. Cancer Causes Control. 17, 539–545 (2006).
Masri, S., Kinouchi, K. & Sassone-Corsi, P. Circadian clocks, epigenetics, and cancer. Curr. Opin. Oncol. 27, 50–56 (2015).
Morris, C. J., Purvis, T. E., Hu, K. & Scheer, F. A. Circadian misalignment increases cardiovascular disease risk factors in humans. Proc. Natl. Acad. Sci. U.S.A. 113, E1402–E1411 (2016).
Cornelissen, G. & Otsuka, K. Chronobiology of aging: A mini-review. Gerontology 63, 118–128 (2017).
Cornelissen, G. Metabolic syndrome, adiponectin, sleep, and the circadian system. EBioMedicine 33, 20–21 (2018).
Chellappa, S. L., Vujovic, N., Williams, J. S. & Scheer, F. A. J. L. Impact of circadian disruption on cardiovascular function and disease. Trends Endocrinol. Metab. 30, 767–779 (2019).
Stenvers, D. J., Scheer, F. A. J. L., Schrauwen, P., la Fleur, S. E. & Kalsbeek, A. Circadian clocks and insulin resistance. Nat. Rev. Endocrinol. 15, 75–89 (2019).
Sulli, G., Lam, M. T. Y. & Panda, S. Interplay between circadian clock and cancer: New frontiers for cancer treatment. Trends Cancer 5, 475–494 (2019).
Xie, Y. et al. New insights into the circadian rhythm and its related diseases. Front. Physiol. 10, 682 (2019).
Hadadi, E. et al. Chronic circadian disruption modulates breast cancer stemness and immune microenvironment to drive metastasis in mice. Nat. Commun. 11, 3193 (2020).
Kervezee, L., Kosmadopoulos, A. & Boivin, D. B. Metabolic and cardiovascular consequences of shift work: The role of circadian disruption and sleep disturbances. Eur. J. Neurosci. 51, 396–412 (2020).
Sephton, S. E., Sapolsky, R. M., Kraemer, H. C. & Spiegel, D. Diurnal cortisol rhythm as a predictor of breast cancer survival. J. Natl. Cancer Inst. 92, 994–1000 (2000).
Sephton, S. E. et al. Diurnal cortisol rhythm as a predictor of lung cancer survival. Brain Behav. Immun. 30 Suppl, S163–S170 (2013).
Mormont, M. C. et al. Marked 24-h rest/activity rhythms are associated with better quality of life, better response, and longer survival in patients with metastatic colorectal cancer and good performance status. Clin. Cancer Res. 6, 3038–3045 (2000).
Innominato, P. F. et al. Circadian rest-activity rhythm as an objective biomarker of patient-reported outcomes in patients with advanced cancer. Cancer Med. 7, 4396–4405 (2018).
Johnson, C. H. Testing the adaptive value of circadian systems. Methods Enzymol. 393, 818–837 (2005).
Charrier, A., Olliac, B., Roubertoux, P. & Tordjman, S. Clock genes and altered sleep-wake Rhythms: Their role in the development of psychiatric disorders. Int. J. Mol. Sci. 18, 938 (2017).
Sanchis-Gomar, F. et al. Physical exercise as an epigenetic modulator: Eustress, the “positive stress” as an effector of gene expression. J. Strength Cond. Res. 26, 3469–3472 (2012).
Hamaguchi, Y., Tahara, Y., Hitosugi, M. & Shibata, S. Impairment of circadian rhythms in peripheral clocks by constant light is partially reversed by scheduled feeding or exercise. J. Biol. Rhythms. 30, 533–542 (2015).
Kassahun, B. T., Bier, M. & Ding, J. Perturbing circadian oscillations in an in vitro suprachiasmatic nucleus with magnetic stimulation. Bioelectromagnetics 41, 63–72 (2020).
Ranieri, D., Cucina, A., Bizzarri, M., Alimandi, M. & Torrisi, M. R. Microgravity influences circadian clock oscillation in human keratinocytes. FEBS Open Bio 5, 717–723 (2015).
Yang, S. et al. Simulated microgravity influences circadian rhythm of NIH3T3 cells. Biol. Rhythm. Res. 47, 897–907 (2016).
Ranieri, D. et al. Simulated microgravity triggers epithelial mesenchymal transition in human keratinocytes. Sci. Rep. 7, 538 (2017).
Chen, L. et al. BMAL1 Disrupted intrinsic diurnal oscillation in rat cerebrovascular contractility of simulated microgravity rats by altering circadian regulation of miR-103/CaV1.2 signal pathway. Int. J. Mol. Sci. 20, 3947 (2019).
Youngstedt, S. D. & Kripke, D. F. Long sleep and mortality: rationale for sleep restriction. Sleep Med. Rev. 8, 159–174 (2004).
Grandner, M. A. & Drummond, S. P. Who are the long sleepers? Towards an understanding of the mortality relationship. Sleep Med. Rev. 11, 341–360 (2007).
Gallicchio, L. & Kalesan, B. Sleep duration and mortality: a systematic review and meta-analysis. J. Sleep Res. 18, 148–158 (2009).
Cappuccio, F. P., D’Elia, L., Strazzullo, P. & Miller, M. A. Sleep duration and all-cause mortality: A systematic review and meta-analysis of prospective studies. Sleep 33, 585–592 (2010).
Stenholm, S. et al. Sleep-related factors and mobility in older men and women. J. Gerontol. A Biol. Sci. Med. Sci. 65, 649–657 (2010).
Stenholm, S., Kronholm, K., Bandinelli, S., Guralnik, J. M. & Ferrucci, L. Self-reported sleep duration and time in bed as predictors of physical function decline: results from the InCHIANTI study. Sleep 34, 1583–1593 (2011).
Grandner, M. A. et al. Extreme sleep durations and increased C-reactive protein: Effects of sex and ethnoracial group. Sleep 36, 769-779E (2013).
Irwin, M. R. & Opp, M. R. Sleep health: Reciprocal regulation of sleep and innate immunity. Neuropsychopharmacology 42, 129–155 (2017).
Buysse, D. J. Sleep health: Can we define it? Does it matter?. Sleep 37, 9–17 (2014).
Irwin, M. R. Why sleep is important for health: A psychoneuroimmunology perspective. Annu. Rev. Psychol. 66, 143–172 (2015).
Buysse, D. J., Reynolds, C. F. 3rd., Monk, T. H., Berman, S. R. & Kupfer, D. J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 28, 193–213 (1989).
Carney, C. E. et al. The consensus sleep diary: Standardizing prospective sleep self-monitoring. Sleep 35, 287–302 (2012).
Landry, G. J., Best, J. R. & Liu-Ambrose, T. Measuring sleep quality in older adults: A comparison using subjective and objective methods. Front. Aging Neurosci. 7, 166 (2015).
McCraty, R. & Shaffer, F. Heart rate variability: New perspectives on physiological mechanisms, assessment of self-regulatory capacity, and health risk. Glob. Adv. Health Med. 4, 46–61 (2015).
Chang, C. et al. Association between heart rate variability and fluctuations in resting-state functional connectivity. Neuroimage 68, 93–104 (2013).
Kernbach, J. M. et al. Subspecialization within default mode nodes characterized in 10,000 UK Biobank participants. Proc. Natl. Acad. Sci. U. S. A. 115, 12295–12300 (2018).
Taylor, J. A., Carr, D. L., Myers, C. W. & Eckberg, D. L. Mechanisms underlying very-low-frequency RR-interval oscillations in humans. Circulation 98, 547–555 (1998).
Shaffer, F., McCraty, R. & Zerr, C. L. A healthy heart is not a metronome: An integrative review of the heart’s anatomy and heart rate variability. Front. Psychol. 5, 1040 (2014).
Shaffer, F. & Ginsberg, J. P. An overview of heart rate variability metrics and norms. Front. Public Health. 5, 258 (2017).
Landgraf, D., Shostak, A. & Oster, H. Clock genes and sleep. Pflugers Arch. 463, 3–14 (2012).
Logan, R. W. & McClung, C. A. Rhythms of life: circadian disruption and brain disorders across the lifespan. Nat. Rev. Neurosci. 20, 49–65 (2019).
Massimini, M. et al. Triggering sleep slow waves by transcranial magnetic stimulation. Proc. Natl. Acad. Sci. U. S. A. 104, 8496–8501 (2007).
Ly, J. Q. M. et al. Circadian regulation of human cortical excitability. Nat. Commun. 7, 11828 (2016).
Gundel, A., Drescher, J., Spatenko, Y. A. & Polyakov, V. V. Changes in basal heart rate in spaceflights up to 438 days. Aviat. Space Environ. Med. 73, 17–21 (2002).
Verdecchia, P. et al. Adverse prognostic value of a blunted circadian rhythm of heart rate in essential hypertension. J. Hypertens. 16, 1335–1343 (1998).
Eguchi, K. et al. Nocturnal nondipping of heart rate predicts cardiovascular events in hypertensive patients. J. Hypertens. 27, 2265–2270 (2009).
Cuspidi, C., Tadic, M. & Sala, C. Is the blunted fall in nighttime heart rate a marker of subclinical cardiac damage?. J. Clin. Hypertens. (Greenwich) 19, 410–412 (2017).
Cornelissen, G. et al. Resonance of about-weekly human heart rate rhythm with solar activity change. Biologia (Bratisl.) 51, 749–756 (1996).
Cornelissen, G. et al. Non-photic solar associations of heart rate variability and myocardial infarction. J. Atmos. Solar-Terr. Phys. 64, 707–720 (2002).
Halberg, F. et al. Cycles tipping the scale between death and survival (=“Life”). Prog. Theor. Phys. Supp. 173, 153–181 (2008).
Otsuka, K. et al. Alternating-light-darkness-influenced human electrocardiographic magnetoreception in association with geomagnetic pulsations. Biomed. Pharmacother. 55(Suppl 1), 63s–75s (2001).
Oinuma, S. et al. Graded response of heart rate variability, associated with an alteration of geomagnetic activity in a subarctic area. Biomed. Pharmacother. 56(Suppl 2), 284s–288s (2002).
Foley, L. E., Gegear, R. J. & Reppert, S. M. Human cryptochrome exhibits light-dependent magnetosensitivity. Nat. Commun. 2, 356 (2011).
Thayer, J. F., Hansen, A. L., Saus-Rose, E. & Johnsen, B. H. Heart rate variability, prefrontal neural function, and cognitive performance: the neurovisceral integration perspective on self-regulation, adaptation, and health. Ann. Behav. Med. 37, 141–153 (2009).
Lin, P. et al. Dynamic default mode network across different brain states. Sci. Rep. 7, 46088 (2017).
Demertzi, A. et al. Cortical reorganization in an astronaut’s brain after long-duration spaceflight. Brain Struct. Funct. 221, 2873–2876 (2016).
Pechenkova, E. et al. Alterations of functional brain connectivity after long-duration spaceflight as revealed by fMRI. Front. Physiol. 10, 761 (2019).
Roberts, D. R. et al. Prolonged microgravity affects human brain structure and function. Am. J. Neuroradiol. 40, 1878–1885 (2019).
Acknowledgements
The authors thank Dr. I. Tayama, S. Ishida and T. Aiba from the Space Biomedical Research Group, Japan Aerospace Exploration Agency (JAXA), for cooperation in our study. The authors also acknowledge the cooperation of the astronauts, the engineers, staff and managers of JAXA and NASA. We thank Yohsuke Kamide, Director of the Rikubetsu Space and Earth Science Museum, Professor Emeritus at Nagoya University, Toshio Ozawa, Professor Emeritus at Kochi University and Honorary Director of Tokyo Metropolitan Geriatric Hospital and Institute of Gerontology, and Børre H. Holmeslet, Auroral Observatory of the University of Tromsø, Tromsø, Norway for supporting this study. The help of Larry A. Beaty to improve the English language for greater clarity and readability is greatly appreciated. The JAXA Chronobiology Project was supported by the Japan Aerospace Exploration Agency (S.F., K.M., H.O., C.M.) and the Halberg Chronobiology Fund (G.C., K.O.).
Author information
Authors and Affiliations
Contributions
K.O. and G.C. wrote the first draft of the manuscript and prepared the figures. K.O., H.O. and C.M. designed the study, and S.F. and K.M. contributed to the acquisition of data. K.O., G.C. and Y.K. analyzed the data, and K.O., G.C., S.F., Y.K., K.S., K.M., H.O. and C.M. contributed to the writing and editing of the manuscript. All authors read and contributed to the final version of the manuscripts.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Otsuka, K., Cornelissen, G., Furukawa, S. et al. Astronauts well-being and possibly anti-aging improved during long-duration spaceflight. Sci Rep 11, 14907 (2021). https://doi.org/10.1038/s41598-021-94478-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-94478-w
This article is cited by
-
Methods for assessing change in brain plasticity at night and psychological resilience during daytime between repeated long-duration space missions
Scientific Reports (2023)
-
How Sleep Research in Extreme Environments Can Inform the Military: Advocating for a Transactional Model of Sleep Adaptation
Current Psychiatry Reports (2023)
-
Unconscious mind activates central cardiovascular network and promotes adaptation to microgravity possibly anti-aging during 1-year-long spaceflight
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.