Abstract
Basic research suggests some contributing mechanisms underlying asthma might at the same time benefit patients with asthma against sepsis, while the potential protective effect of comorbid asthma on prognosis of sepsis has not been well studied in clinical research. The study aimed to assess the association between comorbid asthma and prognosis in a cohort of patients admitted to intensive care unit (ICU) with severe sepsis. Patients with severe sepsis admitted to ICUs were included from the MIMIC-III Critical Care Database, and categorized as patients without asthma, patients with stable asthma, and patients with acute exacerbation asthma. The primary study outcome was 28-day mortality since ICU admission. Difference in survival distributions among groups were evaluated by Kaplan–Meier estimator. Multivariable Cox regression was employed to examine the association between comorbid asthma and prognosis. A total of 2469 patients with severe sepsis were included, of which 2327 (94.25%) were without asthma, 125 (5.06%) with stable asthma, and 17 (0.69%) with acute exacerbation asthma. Compared with patients without asthma, patients with asthma (either stable or not) had a slightly younger age (66.73 ± 16.32 versus 64.77 ± 14.81 years), a lower proportion of male sex (56.81% versus 40.14%), and a lower median SAPS II score (46 versus 43). Patients with acute exacerbation asthma saw the highest 28-day mortality rate (35.29%), but patients with stable asthma had the lowest 28-day mortality rate (21.60%) when compared to that (34.42%) in patients without asthma. Consistent results were observed in Kaplan–Meier curves with a p-value for log-rank test of 0.016. After adjusting for potential confounding, compared to being without asthma, being with stable asthma was associated with a reduced risk of 28-day mortality (hazard ratio (HR) 0.65, 95% confidence interval (CI) 0.44–0.97, p = 0.0335), but being with acute exacerbation asthma was toward an increased risk of 28-day mortality (HR 1.82, 95% 0.80–4.10, p = 0.1513). E-value analysis suggested robustness to unmeasured confounding. These findings suggest comorbid stable asthma is associated with a better prognosis in critically ill patients with severe sepsis, while acute exacerbation asthma is associated with worse prognosis.
Similar content being viewed by others
Introduction
Chronic comorbid conditions are common in critically ill patients, and most of them have a negative impact on survival1,2. Asthma is a common and noncommunicable disease of the lungs, which was estimated to affect 235 million individuals globally in 20173, although the prevalence might vary between regions4,5,6,7. An increase in prevalence of asthma has been observed in some countries8,9,10,11, but the disease itself generally has a good prognosis if its symptoms are well controlled to reduce the risk of asthma exacerbations, which is consistent with the decrease in age-standardized death rates from asthma in most countries in the past two decades12. However, it remains unclear about the potential impact of comorbid asthma on prognosis of critically ill patients.
Sepsis is a clinical syndrome with physiologic, biologic, and biochemical abnormalities caused by a dysregulated host response to infection, which leads to life-threatening organ dysfunction13. About 30% of patients admitted to general intensive care units (ICUs) and about 80% of ICU patients with infection were found to meet the criteria of sepsis14,15, although the results might differ when different criteria were used. The management of sepsis is still challenging, with a mortality rate ranging from 10 to 50%16,17,18.
Basic research suggests some contributing mechanisms underlying asthma are essential normal host response to pathogens and thus might at the same time benefit patients with asthma against sepsis. Parasitic infection is found to improve survival from septic peritonitis by driving type 2 helper T-cell (Th2) polarization and enhancing mast cell responses to bacteria in mice19. Higher circulating Th2 levels are also observed in survivors than that in patients died from Staphylococcus aureus infection20, while Th2 immune responses is the major contributing mechanism underlying asthma21. In addition to Th2 pathway, studies on non-Th2 pathways also suggest potential benefits of asthma on prognosis of infection22,23. As the primary sensors of invading pathogens, Toll-like receptors plays a pivotal role in airway allergic inflammation24. Activation of interleukin-17 (IL-17) is involved in the pathology of asthma, which might play an important role in inducing neutrophil migration to the infection focus and therefore reduce the spread of infection25. In addition, a reverse association between asthma and sepsis has also been reported, which indicates that polymorphisms in the myosin light chain kinase gene that confers risk of severe sepsis are associated with a lower risk of asthma26.
However, the potential protective effect of comorbid asthma on prognosis of sepsis has not been well studied in clinical research. A study with a large sample size reveals that diseases associated with an overactive type 2/Th2 immune response including asthma are markedly and significantly underrepresented among septic patients27. An observational study reports comorbid asthma is associated with lower risk of sepsis-mortality28, which is against findings (i.e., negative effect) from previous studies29,30. Considering that there is limited evidence from clinical research that specially investigate the potential impact of asthma on prognosis of critically ill patients with sepsis, and that inconsistent results have been reported, the study aimed to examine the association between comorbid asthma and prognosis of critically ill patients with severe sepsis with an improved study design.
Methods
Data source
The study used data from the Medical Information Mart for Intensive Care (MIMIC) III (v1.4). It is a large, freely-available database comprising de-identified health-related data associated with over forty thousand patients who stayed in critical care units of the Beth Israel Deaconess Medical Center between 2001 and 201231. Access to the data was approved after completing the Collaborative Institutional Training Initiative (CITI) program “Data or Specimens Only Research”. The study was exempt from approval from the institutional review board of the Massachusetts Institute of Technology (no. 0403000206) due to the retrospective design, lack of direct patient intervention, and the security schema for which the reidentification risk was certified as meeting safe harbor standards by Privacert (Cambridge, MA). Informed consent was waived for the same reason. The study was performed in accordance with the Declaration of Helsinki.
Study population
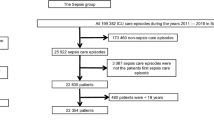
The study included patients admitted to ICU with a diagnosis record of severe sepsis, which was identified by International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code 995.92 (severe sepsis) or 785.52 (septic shock). Since a patient may have more than one hospitalization record in the database, we only included the first ICU admission during the first hospitalization in the database. Patients less than 18 years old at ICU admission and those who stayed in the first ICU hospitalization for less than 24 h were excluded. The inclusion of the patients is presented in Fig. 1.
Exposure
The study population was categorized as three groups, namely patients without asthma, patients with stable asthma, and patients with acute exacerbation asthma. Asthma was identified based on ICD-9-CM codes. Stable asthma was identified using codes 493.00 (Extrinsic asthma, unspecified), 493.10 (Intrinsic asthma, unspecified), 493.20 (Chronic obstructive asthma, unspecified), 493.81 (Exercise induced bronchospasm), 493.82 (Cough variant asthma), and 493.90 (Asthma, unspecified type, unspecified). Acute exacerbation asthma was identified using codes 493.01 (Extrinsic asthma with status asthmaticus), 493.02 (Extrinsic asthma with (acute) exacerbation), 493.11 (Intrinsic asthma with status asthmaticus), 493.12 (Intrinsic asthma with (acute) exacerbation), 493.21 (Chronic obstructive asthma with status asthmaticus), 493.22 (Chronic obstructive asthma with (acute) exacerbation), 493.91 (Asthma, unspecified type, with status asthmaticus), and 493.92 (Asthma, unspecified type, with (acute) exacerbation).
Outcomes
28-day mortality since ICU admission was the main study outcome, and ICU mortality was the secondary study outcome. To identify 28-day mortality, patients were followed from the date of first ICU admission to 28 days after the first ICU admission, or the date of death, whichever came first. To identify ICU mortality, patients were followed from the date of first ICU admission to date of discharge of first ICU hospitalization, or the date of death, whichever came first. Length of ICU stay (for the first ICU hospitalization) was also calculated in the study.
Covariates
Several variables at baseline (i.e., the date of first ICU admission) were studied as covariates, including age, sex, ethnicity, marital status, type of admission (elective or urgent), Simplified Acute Physiology Score (SAPS) II, Sequential Organ Failure Assessment (SOFA) score, mechanical ventilation on first day, renal replacement therapy on first day, and various comorbidities, including congestive heart failure, cardiac arrhythmias, valvular disease, pulmonary circulation disorder, chronic obstructive pulmonary disease, peripheral vascular disorder, hypertension, paralysis, other neurological disease, uncomplicated diabetes, complicated diabetes, hypothyroidism, renal failure, liver disease, peptic ulcer, acquired immune deficiency syndrome, lymphoma, metastatic cancer, solid tumor, rheumatoid arthritis, coagulopathy, obesity, weight loss, fluid and electrolyte disorders, blood loss anemia, deficiency anemia, alcohol abuse, drug abuse, psychoses, and depression. The above variables were identified using codes from the MIMIC Code Repository32.
Statistical analysis
Variables were described as mean ± standard deviation, median (25–75% percentile) or number (percentage) when appropriate. Comparisons between groups were examined by Kruskal–Wallis test or Chi-squared test (or Fisher's exact test for cells with small number)33. Difference in survival distributions among groups were evaluated by Kaplan–Meier estimator. Multivariable Cox regression and multivariable logistic regression were employed to examine the association of comorbid asthma with 28-day mortality and ICU mortality, respectively. We predefined two models to adjust for confounding. Model 1 was adjusting for age, sex, ethnicity, marital status, type of admission, SAPS II, mechanical ventilation on first day, and renal replacement therapy on first day. Model 2 was adjusting for Model 1 and the various comorbidities mentioned above. To evaluate the potential bias for unmeasured confounding, the E-value was calculated34 which quantified the required magnitude of an unmeasured confounder that could negate the observed association between comorbid asthma and prognosis. A p-value less than 0.05 is considered as statistically significant. All the analyses were performed using Empower(R) (www.empowerstats.com; X&Y solutions, Inc., Boston, MA) program.
Results
Demographic and clinical characteristics of the patients
A total of 2469 patients with severe sepsis were included finally. The average age of the study population was 66.62 ± 16.24 years, and 55.85% (1379/2469) were male. Among the study population, 73.31% (1810/2469) were white, and 95.95% (2369/2469) were admitted to ICU urgently; 2327 (94.25%) were without asthma, 125 (5.06%) with stable asthma, and 17 (0.69%) with acute exacerbation asthma. Compared with patients without asthma, patients with asthma (either stable or not) had a slightly younger age (66.73 ± 16.32 versus 64.77 ± 14.81 years), a lower proportion of male sex (56.81% versus 40.14%), and a lower median SAPS II score (46 versus 43). Overall, the three groups showed similar baseline characteristics, where statistically significant difference was observed only in sex, ethnicity, chronic obstructive pulmonary disease, obesity, and depression. Detailed results are presented in Table 1.
Clinical outcomes of the patients
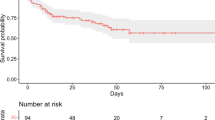
As presented in Table 2, patients with acute exacerbation asthma saw the highest 28-day mortality rate (35.29%, 6/17), but patients with stable asthma had the lowest 28-day mortality rate (21.60%, 27/125) when compared to that (34.42%, 801/2327) in patients without asthma. Consistent results were observed in Kaplan–Meier curves with a p-value for log-rank test of 0.016 (Fig. 2). Similar to 28-day mortality, the highest ICU mortality was seen in patients with acute exacerbation asthma (41.18%, 7/17), and the lowest was seen in patients with stable asthma (16.00%, 20/125). There was no statistically significant difference in the length of ICU stay between groups (p = 0.714).
Association between comorbid asthma and prognosis in the patients
After adjusting for potential confounding, compared to being without asthma, being with stable asthma was associated with a reduced risk of 28-day mortality (hazard ratio (HR) 0.65, 95% confidence interval (CI) 0.44–0.97, p = 0.0335, adjusting for Model 2), but being with acute exacerbation asthma was toward an increased risk of 28-day mortality (HR 1.82, 95% 0.80–4.10, p = 0.1513, adjusting for Model 2). Results for ICU mortality were similar, which showed being with stable asthma was associated with a reduced risk of ICU mortality (odds ratio (OR) 0.57, 95% CI 0.33–0.98, p = 0.0406), but being with acute exacerbation asthma was statistically significantly associated with an increased risk of ICU mortality (OR 4.01, 95% CI 1.35–11.97, p = 0.0127). Detailed results are presented in Tables 3 and 4. The primary findings of the associations between comorbid stable asthma and the studied clinical outcomes were robust, unless there was an unmeasured confounding factor associated with 28-day mortality with an adjusted HR of 2.03 or an unmeasured confounding factor associated with ICU mortality with an adjusted OR of 1.98.
Discussion
In the study, we assessed the associations between comorbid asthma and prognosis of ICU patients with severe sepsis, indicating that comorbid stable asthma is associated with better prognosis in ICU patients with severe sepsis, while comorbid acute exacerbation asthma is associated with worse prognosis. Our study for the first time examined and distinguished the difference in the roles between stable asthma and acute exacerbation asthma, which increases knowledge in this field. It suggests that there could be potential benefit of comorbid asthma on prognosis of sepsis, but the potential protective effect might only exist in patients with stable asthma.
Based on results in our study, for ICU patients with severe sepsis, when compared to those without asthma, the risk of 28-day mortality decreases by more than 30% in those with stable asthma, and the risk of ICU mortality decreases by more than 40%. Considering the prognosis of sepsis especially severe sepsis is yet to improve17, this finding is, to some extent, inspiring. It suggests that as what we speculated from basic researches, the mechanisms underlying asthma at the same time provides a protective effect on patients suffering from severe infection24,25,35. This gives future researches a direction, and it is promising to improve prognosis by avoiding sepsis-related outcomes in people without asthma when the underlying mechanisms are better understood. Our findings are consistent with a few currently available clinical researches, although some differences between these studies should be noticed. The study from Krishack et al27 found the prevalence of asthma in the septic patients was lower than that in the non-septic patients (14% versus 19%). In our study, the prevalence of asthma in the patients with severe sepsis was 5.8%, which was also lower than that in the general ICU patients (6.7%)36, but it should be noted that the patient population investigated in the study by Krishack et al.27 was not restricted to ICU patients only. The study from Zein et al.28 included a much larger sample size compared with ours by using five independent datasets, but only age, sex, ethnicity, income, and comorbidities were considered as confounding factors when assessing the association. Our study specially included patients with severe sepsis only, which could be a reason for a much higher average age (66 versus 55 years) of the study population when compared to Zein et al.’s study28. To identify sepsis by ICD codes has been proved to be a challenge, so we only included patients with severe sepsis since the two ICD codes we used for identification of severe sepsis have been reported to have a positive predictive value of 100% and a specificity of 100%37. Our study also took severity of asthma into consideration, and results of our study indicate that unlike stable asthma, comorbid acute exacerbation asthma significantly increases the risk of ICU mortality by about 3 folds. This finding is important, because it might partly explain why controversial conclusions were drawn from different studies that investigated asthma and clinical outcomes28,30, although relevant evidence is still very limited. In addition, it suggests that the potential mechanisms of the protective effect could also be related to the use of medication that led to a well control of symptoms of asthma. Apart from the confounding factors considered in Zein et al.’s study28, the illness severity score SAPS II was also adjusted in our study, which would greatly increase the strength of our study, since it has been reported to be associated with prognosis of sepsis patients38 and therefore relieve the concern about potential confounding bias.
Although our study cannot provide evidence on the mechanisms underlying the findings, it is interesting to mention that the potential protective effect of comorbid asthma could also exist in other disease. A propensity-score matched cohort study found asthma was associated with lower mortality in patient with myocardial infarction39. The mechanism was suspected to be that Th2 responses (including IL-4 and IL-5) and non-Th2 responses (IL-17), which were involved in the pathogenesis of asthma, might be protective against atherosclerosis and myocardial infarction. Similar findings were also reported in patients with Coronavirus Disease 2019 (COVID-19). Li et al.40 analyzed 548 hospitalized COVID-19 patients and reported a prevalence of asthma of 0.9%, which was markedly lower than that (6.4%) in the adult population in the same region. Avdeev et al.41 reported a prevalence of asthma of 1.8% among 1307 ICU patients with COVID-19. Similar findings were also found in the study from Zhang et al.42. Although interpretation of these results should be cautious, because the study designs of these two studies were not appropriate to assess the potential impact of comorbid asthma on prognosis, they did provide some clues to research in this field.
Our study had some limitations. First, only severe sepsis patients were included, it remains unknown whether the findings can be directly applied to other conditions, such as less severe sepsis or the general ICU patients with infection. This seems possible, since a large observational study found that comorbid type 2 immune diseases (including asthma) was associated with lower risk of hospital mortality in an unselected cohort of patients Hospitalized for Acute Infection43. Second, although various confounding factors were considered in the study, unmeasured confounding cannot be ruled out. Due to data limitation, confounding such as smoking history and medication use were not adjusted. However, based on results of the E-value analysis, an unmeasured confounder was unlikely to explain the entirety of the observed protective effect of comorbid stable asthma. Third, measurement bias is another concern. In the study, exposure and various covariates were mainly identified by ICD codes, which was not further validated in the study due to the nature of a retrospective study design. We think this could not be a serious issue, since a female predominance was observed in the asthma patients, and there was a significantly higher proportion of comorbid obesity in the asthma patients when compared to those without asthma, which was consistent with investigations from other studies44,45 and suggest the quality of data used in our study was somewhat reliable. Last, the limitation on the sample size (especially for patients with acute exacerbation asthma) made further subgroup analysis unavailable in the study, but a further investigation on the association in patients with sepsis caused by different pathogens will increase the knowledge in this field.
Conclusion
Comorbid stable asthma is associated with a better prognosis in critically ill patients with severe sepsis, while acute exacerbation asthma is associated with worse prognosis. Further researches on understanding the underlying mechanisms are warranted and may discover new intervention to improve the management of sepsis.
References
Esper, A. M. & Martin, G. S. The impact of comorbid [corrected] conditions on critical illness. Crit. Care Med. 39(12), 2728–2735 (2011).
Huang, W., Xie, R., Hong, Y. & Chen, Q. Association between comorbid chronic obstructive pulmonary disease and prognosis of patients admitted to the intensive care unit for non-COPD reasons: A retrospective cohort study. Int. J. Chron. Obstruct. Pulmon. Dis. 15, 279–287 (2020).
WHO. 10 Facts on Asthma (WHO, 2017).
Lai, C. K. et al. Global variation in the prevalence and severity of asthma symptoms: Phase three of the International Study of Asthma and Allergies in Childhood (ISAAC). Thorax 64(6), 476–483 (2009).
Variations in the prevalence of respiratory symptoms, self-reported asthma attacks, and use of asthma medication in the European Community Respiratory Health Survey (ECRHS). Eur. Respir. J. 9, 687–695 (1996).
Patel, S. P., Jarvelin, M. R. & Little, M. P. Systematic review of worldwide variations of the prevalence of wheezing symptoms in children. Environ. Health. 7, 57 (2008).
National Cooperative Group on Childhood A, Institute of Environmental H, Related Product Safety CCfDC, Prevention, Chinese Center for Disease C, Prevention. Third nationwide survey of childhood asthma in urban areas of China. Zhonghua Er Ke Za Zhi 51(10), 729–735 (2013).
Maio, S. et al. Respiratory symptoms/diseases prevalence is still increasing: A 25-yr population study. Respir. Med. 110, 58–65 (2016).
Backman, H. et al. Increased prevalence of allergic asthma from 1996 to 2006 and further to 2016-results from three population surveys. Clin. Exp. Allergy. 47(11), 1426–1435 (2017).
de Korte-de, B. D. et al. Stabilizing prevalence trends of eczema, asthma and rhinoconjunctivitis in Dutch schoolchildren (2001–2010). Allergy 70(12), 1669–1673 (2015).
Kim, B. K. et al. Allergies are still on the rise? A 6-year nationwide population-based study in Korea. Allergol. Int. 65(2), 186–191 (2016).
The Global Asthma Report 2018. Auckland, New Zealand: Global Asthma Network (2018). http://globalasthmareport.org/resources/Global_Asthma_Report_2018.pdf. (Accessed 21 July 2021).
Singer, M. et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 315(8), 801–810 (2016).
Abe, T. et al. Epidemiology of sepsis and septic shock in intensive care units between sepsis-2 and sepsis-3 populations: Sepsis prognostication in intensive care unit and emergency room (SPICE-ICU). J. Intens. Care 8, 44 (2020).
Shankar-Hari, M., Harrison, D. A., Rubenfeld, G. D. & Rowan, K. Epidemiology of sepsis and septic shock in critical care units: Comparison between sepsis-2 and sepsis-3 populations using a national critical care database. Br. J. Anaesth. 119(4), 626–636 (2017).
Kaukonen, K. M., Bailey, M., Pilcher, D., Cooper, D. J. & Bellomo, R. Systemic inflammatory response syndrome criteria in defining severe sepsis. N. Engl. J. Med. 372(17), 1629–1638 (2015).
Kaukonen, K. M., Bailey, M., Suzuki, S., Pilcher, D. & Bellomo, R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000–2012. JAMA 311(13), 1308–1316 (2014).
Miller, R. R. 3rd. et al. Multicenter implementation of a severe sepsis and septic shock treatment bundle. Am. J. Respir. Crit. Care Med. 188(1), 77–82 (2013).
Sutherland, R. E. et al. Parasitic infection improves survival from septic peritonitis by enhancing mast cell responses to bacteria in mice. PLoS ONE 6(11), e27564 (2011).
Krishack, P. A. et al. Protection against Staphylococcus aureus bacteremia-induced mortality depends on ILC2s and eosinophils. JCI Insight. https://doi.org/10.1172/jci.insight.124168 (2019).
Caminati, M., Pham, D. L., Bagnasco, D. & Canonica, G. W. Type 2 immunity in asthma. World Allergy Organ. J. 11(1), 13 (2018).
Manni, M. L., Robinson, K. M. & Alcorn, J. F. A tale of two cytokines: IL-17 and IL-22 in asthma and infection. Expert Rev. Respir. Med. 8(1), 25–42 (2014).
Aujla, S. J. & Alcorn, J. F. T(H)17 cells in asthma and inflammation. Biochim. Biophys. Acta 1810(11), 1066–1079 (2011).
Zakeri, A. & Russo, M. Dual role of toll-like receptors in human and experimental asthma models. Front. Immunol. 9, 1027 (2018).
Freitas, A. et al. IL-17 receptor signaling is required to control polymicrobial sepsis. J. Immunol. 182(12), 7846–7854 (2009).
Gao, L. et al. Polymorphisms in the myosin light chain kinase gene that confer risk of severe sepsis are associated with a lower risk of asthma. J. Allergy Clin. Immunol. 119(5), 1111–1118 (2007).
Krishack, P. A. et al. Preexisting type 2 immune activation protects against the development of sepsis. Am. J. Respir. Cell Mol. Biol. 57(5), 628–630 (2017).
Zein, J. G., Love, T. E. & Erzurum, S. C. Asthma is associated with a lower risk of sepsis and sepsis-related mortality. Am. J. Respir. Crit. Care Med. 196(6), 787–790 (2017).
Bang, D. W. et al. Asthma and risk of non-respiratory tract infection: A population-based case-control study. BMJ Open 3(10), e003857 (2013).
Talbot, T. R. et al. Asthma as a risk factor for invasive pneumococcal disease. N. Engl. J. Med. 352(20), 2082–2090 (2005).
Johnson, A. E. et al. MIMIC-III, a freely accessible critical care database. Sci. Data. 3, 160035 (2016).
Johnson, A. E., Stone, D. J., Celi, L. A. & Pollard, T. J. The MIMIC code repository: Enabling reproducibility in critical care research. J. Am. Med. Inform. Assoc. 25(1), 32–39 (2018).
Zhang, Z. Univariate description and bivariate statistical inference: The first step delving into data. Ann. Transl. Med. 4(5), 91 (2016).
Haneuse, S., VanderWeele, T. J. & Arterburn, D. Using the E-value to assess the potential effect of unmeasured confounding in observational studies. JAMA 321(6), 602–603 (2019).
Verhoef, P., Greenberg, J., Hrusch, C., Krishack, P. & Sperling, A. 998: Type 2/th2 inflammatory responses protect against the mortality of Staphylococcus aureus infection. Crit. Care Med. 43(12), 251 (2015).
Shen, Y. et al. Impact of chronic respiratory diseases on re-intubation rate in critically ill patients: A cohort study. Sci. Rep. 11(1), 8663 (2021).
Iwashyna, T. J. et al. Identifying patients with severe sepsis using administrative claims: Patient-level validation of the angus implementation of the international consensus conference definition of severe sepsis. Med. Care 52(6), e39–e43 (2014).
Sharma, S., Gupta, A., Virmani, S. K. & Lal, R. Assessment and comparison of 3 mortality prediction models SAPS II, APACHE II and SOFA for prediction of mortality in patients of sepsis. Int. J. Adv. Med. https://doi.org/10.18203/2349-3933ijam20171476 (2017).
Tashtish, N. et al. Asthma is associated with lower risk of mortality in patients admitted with acute myocardial infarction. Chest 156(4), A37–A38 (2019).
Li, X. et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J. Allergy Clin. Immunol. 146(1), 110–118 (2020).
Avdeev, S. et al. Low prevalence of bronchial asthma and chronic obstructive lung disease among intensive care unit patients with COVID-19. Allergy 75(10), 2703–2704 (2020).
Zhang, J. J. et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy 75(7), 1730–1741 (2020).
Verhoef, P. A., Bhavani, S. V., Carey, K. A. & Churpek, M. M. Allergic immune diseases and the risk of mortality among patients hospitalized for acute infection. Crit. Care Med. 47(12), 1735–1742 (2019).
Leynaert, B. et al. Gender differences in prevalence, diagnosis and incidence of allergic and non-allergic asthma: A population-based cohort. Thorax 67(7), 625–631 (2012).
Peters, U., Dixon, A. E. & Forno, E. Obesity and asthma. J. Allergy Clin. Immunol. 141(4), 1169–1179 (2018).
Author information
Authors and Affiliations
Contributions
Z.L. conceived the research question and designed the study. J.H. and J.Z. contributed equally to this work, who performed the statistical analysis and prepared the first draft of the manuscript. F.W. and J.L. partly performed the analysis. Q.C. extracted the data and verified the analysis. All authors revised and approved the final version of the manuscript for submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huang, J., Zhang, J., Wang, F. et al. Association between comorbid asthma and prognosis of critically ill patients with severe sepsis: a cohort study. Sci Rep 11, 15395 (2021). https://doi.org/10.1038/s41598-021-93907-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-93907-0
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.