Abstract
Severe Coronavirus disease 2019 (COVID-19) is associated with several pre-existing comorbidities and demographic factors. Similar factors are linked to critical sepsis and acute respiratory distress syndrome (ARDS). We hypothesized that age and comorbidities are more generically linked to critical illness mortality than a specific disease state. We used national databases to identify ICU patients and to retrieve comorbidities. The relative importance of risk factors for 60-day mortality was evaluated using the interaction with disease group (Sepsis, ARDS or COVID-19) in logistic regression models. We included 32,501 adult ICU patients. In the model on 60-day mortality in sepsis and COVID-19 there were significant interactions with disease group for age, sex and asthma. In the model on 60-day mortality in ARDS and COVID-19 significant interactions with cohort were found for acute disease severity, age and chronic renal failure. In conclusion, age and sex play particular roles in COVID-19 mortality during intensive care but the burden of comorbidity was similar between sepsis and COVID-19 and ARDS and COVID-19.
Similar content being viewed by others
Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has overwhelmed intensive care units (ICUs) worldwide beginning in late 2019. The beta coronavirus SARS-CoV-2 enters cells human by binding spike proteins to the angiotensin-converting enzyme 2, a receptor abundantly found on airway epithelial cells, pneumocytes and enterocytes of the small intestine1. The most prominent feature of severe COVID-19 is respiratory failure associated with alveolar inflammation and subsequent fibrosis2. Early reports from China suggested several comorbidities and demographic variables as risk factors for severe disease or death in or outside the ICU3.
The sepsis syndrome comprises a large proportion of ICU bed usage and ICU mortality4 and is commonly defined as a “life-threatening organ dysfunction caused by a dysregulated host response to infection”5. Since 2016 the syndrome is divided into sepsis (formerly severe sepsis) and septic shock with increasing mortality.
Acute respiratory distress syndrome6 (ARDS) is a syndrome of acute lung injury caused by inflammation that leads to pulmonary edema progressing to pulmonary consolidation and, if the inflammation is not resolved, eventually fibrosis. ARDS can be caused by pulmonary processes e.g., pneumonia and inhalation injury or by external inflammation related to, for example, major trauma or non-pulmonary sepsis7.
The outcomes of COVID-19, sepsis and ARDS are intimately correlated with age8,9,10 and, in the cases of COVID-19 and sepsis, also acute disease severity at admission11,12. However, although risk factors for adverse outcome in COVID-19 have been quantified previously, the importance of specific comorbidities in COVID-19 compared to other forms of critical illness have not previously been analyzed13,14,15. Similar risk factors are evident in sepsis and ARDS, and published data do not support the interpretation that ICU patients with COVID-19 are more burdened by comorbidity12,16.
We used the Swedish intensive care registry to compare COVID-19 patients to historical controls with sepsis (i.e. severe sepsis or septic shock) or ARDS to test the relative importance of demographics and comorbidity. We hypothesized that advanced age and comorbidity are signs of reduced physiological compensatory capacity causing patients to be more prone to die within 60 days from admission to critical care for any given illness. Therefore, aging, sex and comorbidity should be equally associated with death in COVID-19, sepsis, or ARDS.
Methods
In this cohort study we aimed to investigate the relative importance of comorbidities, age and sex for the odds of death within 60 days of ICU admission (60-day mortality) in COVID-19, sepsis and ARDS. 60-day mortality is an established mortality measure in COVID-1917. The study was approved by the Regional Ethics Committee of Uppsala (approval no. 2016/421) and the Swedish Ethical Review Authority (approval no. 2020-02144). Informed consent was waived by the same authority because of the nature of the study. We registered the study à priori at ClinicalTrials.gov (NCT04542538) and the research was conducted in accordance with the Declaration of Helsinki with subsequent revisions. Reporting follows the STROBE (strengthening the reporting of observational studies in epidemiology) guidelines18.
Data sources
All general and most specialist ICUs report all admissions to the Swedish intensive care registry (SIR)19,20. The national patient registry (NPR), a research support tool, was established by the Swedish Board of Health and Welfare and reporting is governed by statutory and common law21. We collected data on ICU diagnoses, demographics, ICU care and mortality from the SIR and we received data on comorbidities reported in the five years preceding ICU admission from the in-patient sub-registry of the NPR for all patients.
Disease groups
We compared three groups of adult (age ≥ 18 years) ICU patients diagnosed with COVID-19, sepsis or ARDS. Sepsis was defined as severe sepsis or septic shock according to the Sepsis 2 criteria22, coded with International Statistical Classification of Diseases and Related Health Problems—tenth edition (ICD-10) A49.9, R65.1 or R57.2 in the SIR. ARDS was defined according to the American-European consensus conference on the ARDS definition23, 2011–2015, or the Berlin definition6, 2016 and coded with ICD-10 J80.9 × in the SIR. All ICU-admitted adult COVID-19 patients in Sweden from 6 February 2020 to 16 June 2021 were identified by ICD-10 code U07.1 in the SIR while virtually all Swedish ICU-admitted adult patients with severe sepsis, septic shock or ARDS were identified in the SIR from 2011 to 2016.
Any single Sepsis patient could be included in the ARDS cohort and vice versa. Accordingly, ARDS patients were non-COVID-19 ARDS patients and Sepsis patients were non-COVID-19 Sepsis patients. Patients were only included for their first admission for COVID-19, Sepsis, or ARDS. However, as the COVID-19 group stems from a separate time period a patient could be included in both the COVID-19 and Sepsis or ARDS groups. Exclusion criteria were lack of personal identification number and age < 18 years. ICU care episodes ending and starting in the same 24-h period were merged.
Statistics
Data are reported as medians with interquartile range (IQR) or number with percent in brackets. The primary outcomes were the relative importance of age, sex and comorbidities (Table S1) for 60-day mortality in COVID-19, Sepsis or ARDS.
The relative importance of age, sex, Simplified Acute Physiology Score 3 (SAPS3)24 Box III, and comorbidities were assessed as an interaction with the disease group (COVID-19 or Sepsis and COVID-19 or ARDS) using logistic regression. COVID-19 was compared separately to Sepsis and ARDS. A significant interaction between disease group and a variable indicates a difference in effect between groups for that variable. Because we added age and comorbidities in the models and treatments preceding ICU admission might be related to diagnosis the SAPS3 Box III, representing the acute physiologic derangement at ICU admission, was used. SAPS324 is a risk score initially developed to perform risk adjusted comparisons of hospital mortality in ICU admitted patients between and within ICUSs, but is now widely used and validated also for 30- and 90-day mortality25,26.
We used restricted cubic splines in all continuous variables, age and SPAS3 Box III, as we could not rule out a non-linear relationship with the logit of outcome. To estimate individual risk factor p-values a linear representation of the variable was applied to the model adjusted for the splined variables. We found 14 marginally influential observations in the model on 60-day mortality in COVID-19 and ARDS using the rms-package. We found indications of multicollinearity in relation to age and SAPS3 Box III for all models. SAPS3 data was missing in 414 patients (1.3%), who were excluded from 60-day mortality modelling. Due to an imbalance between groups for the different hospital types, hospital type was added to the models.
Statistical significance was defined as p-value < 0.05 (two-sided). In analysis of crude differences between disease groups we used the Mann–Whitney U-test and Chi2-test as appropriate with Bonferroni-correction because of multiple comparisons. Odds ratios (ORs) were calculated between the 25th and 75th percentiles in variables for which restricted cubic splines were applied, i.e. age and the SAPS3 Box III. Data management and descriptive statistics were performed in SPSS for Windows version 27 (Microsoft Corp., IL, USA). For multiple imputations, regression models and graphics, we used the R Software version 4.0.3 (The R Foundation for Statistical Computing, Vienna, Austria; https://www.r-project.org) with the mice, rms, Hmisc, and forest plot packages.
Sensitivity analyses
The specification and rationale for the performed sensitivity analyses are found in Supplementary Table S2 online.
Results
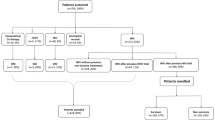
At data acquisition, 7382 consecutive adult COVID-19 ICU patients were enrolled from the SIR. Of the COVID-19 patients, 1389 (19%) were also coded with Sepsis and 5491 (74%) were also coded with ARDS during intensive care. Of the 22,354 adult patients included in the Sepsis group, 1100 were also included in the ARDS group, with a total of 2776 patients, and vice versa (Table 1, Fig. 1). The COVID-19 patients had a numerically lower percentage of women, younger age, a lower SAPS3 and a lower median updated Charlson comorbidity index (CCI)27 than the Sepsis and ARDS patients.
Patient selection flowchart. Patients 18 years or older that were admitted to Swedish ICUs were selected for this study from the Swedish Intensive Care Registry. COVID-19 patients admitted between 6 March and 16 June 2021 were included. Patients with non-COVID-19 sepsis or non-COVID-19 ARDS from 2011 to 2016 were included as controls. COVID-19 Corona virus disease 2019, ICU Intensive care unit, ARDS Acute respiratory distress syndrome.
The 60-day mortality was significantly lower in the COVID-19 patients (27.5%), than in the Sepsis (34.1%) and the ARDS patients (45%). ICU-length of stay was longer for COVID-19 than Sepsis patients and the use of invasive mechanical ventilation was more common in COVID-19 than Sepsis patients, but lower in COVID-19 than ARDS patients. The crude proportion of all studied comorbidities was lower in the COVID-19 than in the Sepsis group. Compared to the ARDS group the COVID-19 group had a lower crude proportion of all studied comorbidities, except type 2 diabetes mellitus (T2DM), chronic renal failure, asthma, and obesity (Table 2). Demographics and comorbidity data of the deceased patients by disease group are summarized in Supplementary Table S4 online.
Logistic modelling
The interaction between the disease group and the individual risk factors in a logistic regression was used to assess the differential effect between COVID-19 and Sepsis, or ARDS. Between COVID-19 and Sepsis the interaction was significant for age (p < 0.001), sex (p < 0.001), and asthma (p = 0.002), indicating a stronger association between age, male sex and asthma with 60-day mortality in COVID-19 than in Sepsis (Fig. 2). In the model on COVID-19 and ARDS the interaction was significant for SAPS3 Box III (p < 0.001), age (p < 0.001) and chronic renal failure (p = 0.001), indicating a stronger association of SAPS3 Box III, age and chronic renal failure to 60-day mortality in COVID-19 than in ARDS (Fig. 3).
Risk factors for 60-day mortality in Sepsis compared to COVID-19. Odds of 60-day mortality with sepsis (a) or COVID-19 (b) based on comorbidity in a logistic regression model. Sepsis is severe sepsis or septic shock without COVID-19. A p-value for interaction < 0.05 denotes a significant interaction of the risk factor with the disease cohort and indicates risk factors with differential effect between sepsis and COVID-19. ICU Intensive care unit, COVID-19 Corona virus disease 2019, p p-value, OR Odds ratio, CI Confidence interval, SAPS3 Box III adjusted Simplified acute physiology score 3 Box III24, COPD Chronic obstructive pulmonary disease. Also in model: Hospital type District—County: 0.84 (0.78–0.90), OR (95% CI) and University—County: 0.92 (0.86–0.98).
Risk factors for 60-day mortality in ARDS compared with COVID-19. Odds of 60-day mortality with ARDS (a) or COVID-19 (b) based on comorbidity in a logistic model. ARDS is ARDS without COVID-19. A p-value for interaction < 0.05 denotes a significant interaction of the risk factor with the disease cohort and indicates risk factors with differential effect between ARDS and COVID-19. ICU Intensive care unit, COVID-19 Corona virus disease 2019, ARDS Acute respiratory distress syndrome, p p-value, OR Odds ratio, CI Confidence interval, SAPS3 Box III adjusted Simplified acute physiology score 3 Box III24, COPD Chronic obstructive pulmonary disease. Also in model: Hospital type District—County: 0.85 (0.73–0.97), OR (95% CI) and University—County: 0.83 (0.75–0.92).
Sensitivity analyses
Results for the sensitivity analyses are summarized in Supplementary Table S3 and presented in detail in Supplementary Tables S5–S15 online. Some differences between the results of the main analyses and those of the sensitivity analyses were seen, however adding a variable denoting time since first inclusion to the model of 60-day mortality in COVID-19 and Sepsis did not affect the inferences (Supplementary Table S5 online). In the analyses where missing SAPS3 Box III was imputed using the mice() function there were no changes in the significance of the interactions (Supplementary Tables S6 and S7 online). In the two sensitivity analyses where patients included in both the Sepsis and ARDS groups were excluded there were no differences for the model including COVID-19 and Sepsis patients (Supplementary Table S8 online). However, in the model on COVID-19 or ARDS the p-value for the interaction between the variable indicting disease group affiliation and age changed from < 0.001 to 0.06 (Supplementary Table S9 online). Excluding the SAPS3 Box III caused no changes in the model on COVID-19 and ARDS, however, the impact of obesity became differential between the COVID-19 and Sepsis groups as the p-value for the interaction with the disease group affiliation variable changed from 0.07 to 0.03 (Supplementary Tables S10 and S11 online). When performing the model on COVID-19 and ARDS without patients with overly influential observations the interaction with asthma became significant as the p-value decreased from 0.07 to 0.03 (Supplementary Table S12 online). All models were performed without the variable denoting hospital type with no change to the results. (Supplementary Tables S13 and S14 online). Finally, length of stay was inversely correlated to age in patients who died within 60 days, and there was a significant interaction with disease group, indicating that end-of-life decisions might have affected differences between outcome in COVID-19 and Sepsis (Supplementary Table S15 online). However, these differences did not affect the main conclusions.
Discussion
The key finding of this study is that almost all comorbidities under investigation were not of greater importance for mortality in COVID-19 compared to in Sepsis and ARDS. This finding is in support of our hypothesis that comorbidities are general risk factors for critical illness mortality, not a specific etiological factor for critical COVID-19 mortality.
While almost all comorbidities under investigation were of similar importance with regard to 60-day mortality in COVID-19 and Sepsis as well as in COVID-19 and ARDS, we found a differential effect for asthma. Asthma showed a stronger association to 60-day mortality in COVID-19 than in Sepsis possibly relating to previous evidence that asthma is associated to a better prognosis in Sepsis28. This finding is also consistent with a previous study in which an independent association was found between asthma and COVID-19 ICU-mortality15. Between COVID-19 and ARDS no differential effect was found for asthma, possibly linked to a common pulmonary pathophysiology in COVID-19 and ARDS29. We also found a differential effect for chronic renal failure showing a protective effect regarding 60-day mortality in ARDS but not in COVID-19.
Of note, our data show a greater association for age to 60-day mortality in COVID-19 than in Sepsis or ARDS. The stronger association of advanced age to 60-day mortality in COVID-19 than in Sepsis and ARDS is in line with several studies recognizing the importance of age in the prognosis of COVID-1912,15,30,31. Moreover, the association to COVID-19 mortality might also be linked to a greater tendency to use of end-of-life decisions in COVID-19 than in Sepsis and ARDS in the aged patients. This tendency is indicated by an interaction between disease group and age in a linear regression model on length of stay in deceased patients presented in Supplemental Table S15 online. We found that the association between female sex and 60-day mortality was higher in Sepsis than in COVID-19. The difference might depend on a higher risk of death in women with Sepsis compared to men, which is under discussion12,32. This contrasts to COVID-19 in which several investigators have found no strong association between sex and ICU mortality15,33,34. However, there are studies where the effect of sex is more pronounced35,36 and the protective effect of female sex in COVID-19 is an area of current investigation37.
The greatest strength of this study is the high-quality datasets on which it is based and the large sample cohorts it examines. A second strength concerns the robustness of our outcome measure, i.e. 60-day mortality where the follow-up can be expected to be complete given the Swedish personal identification number system. A third strength is the low frequency of missing data, which could presumably be missing at random because of the nature of our data. This reasoning implies that model-based imputation can be expected to perform well. We assessed the stability of the results in regard to missing data by performing a sensitivity analysis based on the complete dataset after multiple imputation by chained equations, which did not change the main findings. In addition, possible bias related to Sepsis patients also diagnosed with ARDS, and vice versa, was assessed through sensitivity analyses excluding these patients. This analysis only impacted the effect of the COPD variable in the 60-day mortality models on ARDS and COVID-19 patients. However, excluding all Sepsis patients from the ARDS group meant a reduction in sample size by almost one half. We found indications of multicollinearity in association to SAPS 3. When we performed sensitivity analyzes without SAPS 3 box III the impact on model results was small and thus we feel confident in model stability in this regard. Finally, we address the possible reduced risk-adjusted mortality over time in Sepsis patients defined by the sepsis-2 criteria12,32 using a sensitivity analysis including time as a covariate without effect on the results.
As a registry study, some inherent limitations may be more prominent during the ongoing pandemic. The registries are monitored continuously and amended, but data for the COVID-19 group was reported during the ongoing surges and may include more errors than the historical controls. The unexpectedly low frequency of ARDS- and sepsis-coding in the COVID-19 group is likely due to several causes: (1) in 39% of the patients invasive mechanical ventilation was not performed; and (2) there was an unusually low quality of diagnosis reporting during the peak of the surge. Moreover, our Sepsis group is defined according to the 2001 sepsis definition, which differs somewhat from the sepsis-3 definition5. However, these concerns should not affect data related to the primary outcomes in the study or the registration of exposures relevant to this study. Finally, epidemiological factors may partly explain the observed differences in the distribution of risk factors. Many middle-aged individuals with limited comorbidity have been exposed to and infected with the SARS-COV-2 virus in the community, where some have developed a critical illness. Numerous older individuals with more pronounced comorbidity have practiced strict isolation and may have avoided infection, whereas the very old and frail, usually infected in nursing homes, are seldom admitted to intensive care. Finally, we found indications of multicollinearity in association to age. However, we believe it would be pointless to perform the models without the age variable, as age is such a strong risk factor for organ dysfunction and death in modeling by us and others8,11.
We conclude that the burden of comorbidity is similar for 60-day mortality after ICU admission with COVID-19, Sepsis and ARDS. Age is a more decisive risk factor in COVID-19 than in Sepsis and ARDS.
Data availability
The data that support the findings of this study are available from the respective national registries with restrictions as defined by the General Data Protection Regulation (GDPR), the Swedish Personal Data Act (1998:204), and the licenses with the respective national registries, and so are not publicly available. Data are however available from the authors upon reasonable request after adequate permissions from the Swedish ethical review authority and under the restrictions outlined above.
References
Hamming, I. et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 203, 631–637. https://doi.org/10.1002/path.1570 (2004).
Sadhukhan, P., Ugurlu, M. T. & Hoque, M. O. Effect of COVID-19 on lungs: Focusing on prospective malignant phenotypes. Cancers https://doi.org/10.3390/cancers12123822 (2020).
Zheng, Z. et al. Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis. J. Infect. 81, e16–e25. https://doi.org/10.1016/j.jinf.2020.04.021 (2020).
Sakr, Y. et al. Sepsis in intensive care unit patients: Worldwide data from the intensive care over nations audit. Open Forum Infect. Dis. 5, ofy313. https://doi.org/10.1093/ofid/ofy313 (2018).
Singer, M. et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 315, 801–810. https://doi.org/10.1001/jama.2016.0287 (2016).
Force, A. D. T. et al. Acute respiratory distress syndrome: The Berlin Definition. JAMA 307, 2526–2533. https://doi.org/10.1001/jama.2012.5669 (2012).
Matthay, M. A. et al. Acute respiratory distress syndrome. Nat. Rev. Dis. Primers. 5, 18. https://doi.org/10.1038/s41572-019-0069-0 (2019).
Ho, F. K. et al. Is older age associated with COVID-19 mortality in the absence of other risk factors? General population cohort study of 470,034 participants. PLoS ONE 15, e0241824. https://doi.org/10.1371/journal.pone.0241824 (2020).
Nasa, P., Juneja, D. & Singh, O. Severe sepsis and septic shock in the elderly: An overview. World J. Crit. Care Med. 1, 23–30. https://doi.org/10.5492/wjccm.v1.i1.23 (2012).
Schouten, L. R. A. et al. Increased mortality in elderly patients with acute respiratory distress syndrome is not explained by host response. Intensive Care Med. Exp. 7, 58. https://doi.org/10.1186/s40635-019-0270-1 (2019).
Zettersten, E. et al. Long-term outcome after intensive care for COVID-19: Differences between men and women—A nationwide cohort study. Crit. Care 25, 86. https://doi.org/10.1186/s13054-021-03511-x (2021).
Strandberg, G., Walther, S., Agvald Öhman, C. & Lipcsey, M. Mortality after severe sepsis and septic shock in Swedish Intensive Care Units 2008–2016—A nationwide observational study. Acta Anaesthesiol. Scand. 64, 967–975. https://doi.org/10.1111/aas.13587 (2020).
Grasselli, G. et al. Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern. Med. 180, 1345–1355. https://doi.org/10.1001/jamainternmed.2020.3539 (2020).
Sim, B. L. H. et al. Clinical characteristics and risk factors for severe COVID-19 infections in Malaysia: A nationwide observational study. Lancet Regional Health Western Pacific. https://doi.org/10.1016/j.lanwpc.2020.100055 (2020).
Ahlström, B. et al. The swedish COVID-19 intensive care cohort: Risk factors of ICU admission and ICU mortality. Acta Anaesthesiol. Scand. 65, 525–533. https://doi.org/10.1111/aas.13781 (2021).
Angus, D. C. & van der Poll, T. Severe sepsis and septic shock. N. Engl. J. Med. 369, 840–851. https://doi.org/10.1056/NEJMra1208623 (2013).
Stralin, K. et al. Mortality trends among hospitalised COVID-19 patients in Sweden: A nationwide observational cohort study. Lancet Reg. Health Eur. 4, 100054. https://doi.org/10.1016/j.lanepe.2021.100054 (2021).
von Elm, E. et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Epidemiology 18, 800–804. https://doi.org/10.1097/EDE.0b013e3181577654 (2007).
National Quality Registry for Intensive Care (2019).https://kvalitetsregister.se/englishpages/findaregistry/registerarkivenglish/nationalqualityregistryforintensivecaresir.2175.html Accessed 4 January 2021.
Svenska_intensivvårdsregistret. Svenska intensivvårdsregistrets Årsrapport 2019. (Online, 2020).
The National Patient Register (2019).https://www.socialstyrelsen.se/en/statistics-and-data/registers/register-information/the-national-patient-register/ Accessed 4 January 2021.
Levy, M. M. et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med. 29, 530–538. https://doi.org/10.1007/s00134-003-1662-x (2003).
Bernard, G. R. et al. The American–European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am. J. Respir. Crit. Care Med. 149, 818–824. https://doi.org/10.1164/ajrccm.149.3.7509706 (1994).
Moreno, R. P. et al. SAPS 3—From evaluation of the patient to evaluation of the intensive care unit. Part 2: Development of a prognostic model for hospital mortality at ICU admission. Intensive Care Med. 31, 1345–1355. https://doi.org/10.1007/s00134-005-2763-5 (2005).
Rydenfelt, K. et al. In-hospital vs 30-day mortality in the critically ill—A 2-year Swedish intensive care cohort analysis. Acta Anaesthesiol. Scand. 59, 846–858. https://doi.org/10.1111/aas.12554 (2015).
van der Merwe, E. et al. The SAPS 3 score as a predictor of hospital mortality in a South African tertiary intensive care unit: A prospective cohort study. PLoS ONE 15, e0233317. https://doi.org/10.1371/journal.pone.0233317 (2020).
Quan, H. et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am. J. Epidemiol. 173, 676–682. https://doi.org/10.1093/aje/kwq433 (2011).
Huang, J. et al. Association between comorbid asthma and prognosis of critically ill patients with severe sepsis: A cohort study. Sci. Rep. 11, 15395. https://doi.org/10.1038/s41598-021-93907-0 (2021).
Aslan, A., Aslan, C., Zolbanin, N. M. & Jafari, R. Acute respiratory distress syndrome in COVID-19: Possible mechanisms and therapeutic management. Pneumonia 13, 14. https://doi.org/10.1186/s41479-021-00092-9 (2021).
Petrilli, C. M. et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: Prospective cohort study. BMJ 369, m1966. https://doi.org/10.1136/bmj.m1966 (2020).
Auld, S. C. et al. ICU and ventilator mortality among critically ill adults with coronavirus disease 2019. Crit. Care Med. 48, e799–e804. https://doi.org/10.1097/CCM.0000000000004457 (2020).
Shankar-Hari, M., Harrison, D. A., Rubenfeld, G. D. & Rowan, K. Epidemiology of sepsis and septic shock in critical care units: Comparison between sepsis-2 and sepsis-3 populations using a national critical care database. Br. J. Anaesth. 119, 626–636. https://doi.org/10.1093/bja/aex234 (2017).
Kelada, M., Anto, A., Dave, K. & Saleh, S. N. The role of sex in the risk of mortality from COVID-19 amongst adult patients: A systematic review. Cureus 12, e10114. https://doi.org/10.7759/cureus.10114 (2020).
Suleyman, G. et al. Clinical characteristics and morbidity associated with coronavirus disease 2019 in a series of patients in metropolitan detroit. JAMA Netw. Open 3, e2012270. https://doi.org/10.1001/jamanetworkopen.2020.12270 (2020).
Vahidy, F. S. et al. Sex differences in susceptibility, severity, and outcomes of coronavirus disease 2019: Cross-sectional analysis from a diverse US metropolitan area. PLoS ONE 16, e0245556. https://doi.org/10.1371/journal.pone.0245556 (2021).
Peckham, H. et al. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat. Commun. 11, 6317. https://doi.org/10.1038/s41467-020-19741-6 (2020).
Takahashi, T. & Iwasaki, A. Sex differences in immune responses. Science 371, 347–348. https://doi.org/10.1126/science.abe7199 (2021).
Acknowledgements
Dr. Henrik Renlund, the statistician, is gratefully acknowledged for statistical support.
Funding
Open access funding provided by Uppsala University. Uppsala University Hospital research fund (Institutional funding) and the Centre for Clinical Research at Region Dalarna, Sweden funded this research. The funding sources has had no active part in design, data collection, analysis or manuscript writing.
Author information
Authors and Affiliations
Contributions
B.A., R.F., I.M.L., G.S., M.L., and M.H. conceived and designed the study; B.A. and M.L. acquired the data. B.A. and M.H. analyzed the data; B.A. and M.H. drafted the manuscript; and B.A., R.F., I.M.L., G.S., M.L., and M.H. finalized the manuscript. All authors approved the final manuscript as submitted.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ahlström, B., Frithiof, R., Larsson, IM. et al. A comparison of impact of comorbidities and demographics on 60-day mortality in ICU patients with COVID-19, sepsis and acute respiratory distress syndrome. Sci Rep 12, 15703 (2022). https://doi.org/10.1038/s41598-022-19539-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-19539-0
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.