Abstract
Binding energies of different nitrile solvents and their utilization for CuCN formation were investigated through quantum chemical calculations. A pulsed laser ablation in liquid (PLAL) method for CuCN synthesis was developed herein. Initially, the interaction between the pulsed laser and the Cu-target generated Cu-ions and electrons at the point of contact. The laser beam also exhibited sufficient energy to dissociate the bonds of the respective solvents. In the case of acetonitrile, the oxidized Cu-ions bonded with CN− to produce CuCN with a cube-like surface structure. Other nitrile solvents generated spherically-shaped Cu@graphitic carbon (Cu@GC) nanoparticles. Thus, the production of CuCN was favorable only in acetonitrile due to the availability of the cyano group immediately after the fragmentation of acetonitrile (CH3+ and CN−) under PLAL. Conversely, propionitrile and butyronitrile released large amounts of hydrocarbons, which deposited on Cu NPs surface to form GC layers. Following the encapsulation of Cu NPs with carbon shells, further interaction with the cyano group was not possible. Subsequently, theoretical study on the binding energies of nitrile solvents was confirmed by highly correlated basic sets of B3LYP and MP2 which results were consistent with the experimental outcomes. The findings obtained herein could be utilized for the development of novel metal–polymer materials.
Similar content being viewed by others
Introduction
The production of metal cyanides has attracted considerable interest due to their commercial applications in different industries. For instance, CuCN and AgCN are employed in the fields of pharmacology, gas production, mining, photography, and metal plating1. CuCN has gained particularly wide recognition owing to its unique characteristics, including luminescence, bioactivity, long-range magnetic order, and electron transfer properties2. Moreover, CuCN is also valuable in the crystal engineering field. The CN group acts as a conservative connector to produce a linear –Cu–CN– chain in CuCN. Cu+ ions favor tetrahedral or trigonal coordination in the presence of pyridyl and cyanide groups. This offers the possibility of expanding the arrangement of framework structures due to the strong coordinating properties of the ligands as well as their ability to act as connectors between various metal centers3. The structural properties of CuCN and AgCN are relatively similar. Both compounds exist in the form of more than one polymorph. Unlike other cyanides, they are presumed to form –Cu–CN–Cu–CN– chains4,5. The basic structure of CuCN exhibits static and large thermal displacement of the chain composed of CN or NC groups, which makes the structure formation problematic6. Despite the existence of numerous conventional synthetic routes for the preparation of metal cyanides, they mostly utilize toxic anions (e.g., cyanide), which can result in serious health issues affecting the nervous, vascular, cardiac, visual, metabolic, and endocrine systems7.
Recently, an environmentally friendly approach called pulsed laser ablation in liquid (PLAL) has been proposed as an attractive alternative to the typical chemical methods. The advantages of this surfactant-free technique include the lack of toxicity, speed, and absence of byproducts; therefore, it can be applied for the production of various nano- to submicron-sized materials8. Moreover, PLAL is highly energy efficient and can be employed for the preparation of inorganic/organic colloids without the necessity for chemical reducing agents. PLAL enables the regulation of various properties of materials, e.g., their shape, size, crystalline phase, and chemical composition, by controlling the working parameters, such as the laser wavelength and power, time as well as reaction solvent9,10,11,12. Focusing the intense pulsed laser on the surface of the desired target immersed in a liquid medium results in the creation of cavitation bubbles, which rapidly expand and collapse within a few microseconds to enable the nucleation and formation of the expected materials13. In this work, we employed PLAL in acetonitrile as a facile one-pot technique for the synthesis of CuCN. Considering potential applications, particles, which are stable in organic solvents, are desired because they can be used for the fabrication of novel valuable coordination polymers and other hydrophobic materials.
The present study investigated the conditions of PLAL of Cu in various organic nitrile solvents. The process resulted in the selective and efficient formation of CuCN and graphitic carbon (GC)-encapsulated Cu nanoparticles (NPs). During PLAL in various nitrile solvents, such as acetonitrile, propionitrile, and butyronitrile, the optimum laser wavelength was adjusted to 355, 532, and 1064 nm, respectively. The conducted theoretical studies provided insights into the solvent structures, binding energy, and bond dissociation energy during the reaction. The mechanistic studies based on the obtained results revealed that the laser prompted the fragmentation of acetonitrile into CH3+ and CN−. The latter bound to Cu ions, generating CuCN. Intriguingly, the production of cubic-structured CuCN was favored only in acetonitrile, while other highly ordered propionitrile and butyronitrile solvents generated spherically-shaped Cu@GC NPs. The synthesized CuCN and Cu@GC were characterized by various analytical techniques, including X-ray diffraction (XRD), Fourier transform (FT) Raman spectroscopy, field emission scanning electron microscopy (FE-SEM), energy-dispersive X-ray spectroscopy (EDS), and transmission electron microscopy (TEM). The evaluation confirmed the rapid synthesis of CuCN with a unique surface structure. The outcomes reported herein could be used for the production of new coordination/polymer materials for applications in the fields of catalysis, sensing, and electronics.
Experimental section
Materials
The Cu plate (≥ 99.99%) was purchased from Sigma-Aldrich, USA. Acetonitrile (high-performance liquid chromatography [HPLC] grade, 99.8%) was obtained from Daejung Chemicals & Metals Co., Ltd, South Korea. Propionitrile (> 99.0%) and butyronitrile (> 99.0%) were acquired from TCI, Japan. All materials were used as received.
Synthesis of CuCN by PLAL
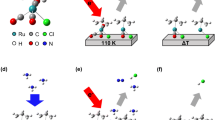
The detailed experimental setup is illustrated as a schematic diagram in Fig. 1. A Cu plate was immersed in a vial containing 10 mL of a nitrile solvent (acetonitrile, propionitrile, or butyronitrile). Subsequently, the PLAL process was performed using a Q-switched Nd: YAG nanosecond laser exhibiting different wavelengths (355, 532, and 1064 nm) at a fixed pulse energy of 80 mJ (Continuum Surelite, SLII-10) for 10 min. To focus the laser beam on the surface of the Cu plate, a focusing lens of f = 30 mm was used. The final CuCN product was collected by centrifugation of the obtained colloidal solutions and dried at ambient temperature.
Material characterization
To evaluate the crystallinity of the prepared materials, XRD analysis was conducted (Bruker, D8 Advance A25) using Cu Kα radiation (λ = 1.54 Å) in the 2θ° range of 20°–60°. The surface morphology, shape, and chemical composition of the synthesized materials were studied by SEM (TESCAN, CLARA) and EDS. Raman spectra were recorded employing a micro-Raman spectrometer with a 632.8 nm He–Ne laser beam as the excitation source (LabRAM HR800, HORIBA, Jobin–Yvon). TEM images were obtained on a 300 kV TF30ST (FEI) microscope. Quantum chemical calculations were performed using the Gaussian 16 program14. Geometry optimization and relative energy calculations for solvents (acetonitrile, propionitrile, and butyronitrile) were conducted by employing a 6-311++ G(d,p)15 basis set using the following two methods: B3LYP16 and MP2 (second-order Moller–Plesset perturbation theory)17. The binding energies of the solvents (acetonitrile, propionitrile, and butyronitrile) were corrected for the basis set superposition error (BSSE)18,19,20 using the counterpoise method. The obtained results were further corrected with zero-point vibrational energy.
Results and discussion
XRD analysis
The formation of materials and their crystallinity were studied by XRD. The XRD patterns of the samples prepared by PLAL of a Cu plate at 355, 532, and 1064 nm in different nitrile solvents are illustrated in Fig. 2a–c. Ablation of the Cu plate at 355, 532, and 1064 nm in propionitrile and butyronitrile led to the formation of Cu NPs. The peaks at 43.2° and 50.4° were consistent with face-centered cubic copper NPs (JCPDS #01-085-1326)21. These peaks coincided with the diffraction of the (111) and (200) planes, respectively. The patterns of the Cu plate ablated at all wavelengths in acetonitrile revealed additional strong peaks at 25.1° and 29.5°, which were ascribed to the rhombohedral crystal phase of CuCN (JCPDS #96-110-1019). These peaks were indexed to the (101) and (110) crystallographic planes22. In addition to CuCN, a trace amount of pure metallic Cu NPs was present in the sample synthesized in acetonitrile, which was confirmed by weak diffraction peaks. However, the amount of Cu NPs in this sample was negligible compared to those in the propionitrile and butyronitrile samples.
XRD patterns of samples synthesized by PLAL of a Cu plate in (i) acetonitrile, (ii) propionitrile, and (iii) butyronitrile at different laser wavelengths of (a) 355, (b) 532, and (c) 1064 nm. Characteristic peaks of metallic Cu at 43.2° and 50.4° denoted as (•) were consistent with JCPDS #01-085-1326. The CuCN peaks at 24.9° and 29.5° (JCPDS #96-110-1019) are indicated by (▪).
FT-Raman analysis
To further identify the molecules and intermolecular bonds, the samples prepared by PLAL of a Cu plate in various nitrile solvents were analyzed by FT-Raman spectroscopy. The FT-Raman spectra of all samples obtained at different laser wavelength (355, 532, and 1064 nm) are shown in Fig. 3a–c. As it can be seen, all spectra exhibited D- and G-bands, which are characteristic of GC. Thus, the D-band originated from a second-order process and described disordered carbon. The presence of GC layers was confirmed by the G-band arising as a result of the Raman scattering process in graphene23. The existence of D- and G-bands indicated the formation of pure metallic Cu NPs covered with GC layers during PLAL in nitrile solvents. More pronounced peaks were detected for the samples synthesized in propionitrile and butyronitrile, suggesting the generation of a high amount of Cu NPs. Notably, the spectra of the samples ablated in acetonitrile at 355, 532, and 1064 nm displayed an additional distinctive peak at 2175 cm−124. This peak was attributed to the copper(I) cyanide species. The ν(CN) mode consists of A1g + Eg + T1u, where A1g is a high intense Raman active mode and T1u is an IR active mode25. The C \(\equiv\) N− stretching vibration mode is commonly used as a structural and environmental probe in a variety of applications and can be found in the high frequency region of around 2000 cm−125,26. The observed FT-Raman results were in good agreement with the XRD analysis.
FT-Raman spectra of samples synthesized by PLAL of a Cu plate in (i) acetonitrile, (ii) propionitrile, and (iii) butyronitrile at different laser wavelengths of (a) 355, (b) 532, and (c) 1064 nm. Peaks at 1350 and 1590 cm−1 correspond to the D- and G-bands of the sp2 hybridized carbon, respectively. They were attributed to GC shells on the surface of spherical Cu NPs. The characteristic CuCN peak at 2170 cm−1 is indicated by (▪).
SEM analysis
The surface morphology and shape of the synthesized materials were studied by SEM. The SEM images of the CuCN sample synthesized in acetonitrile at different laser wavelengths are shown in Fig. 4a–c. The morphology of all samples formed in acetonitrile exhibited micro-sized cubes, which might be due to structural integrity during complex formation, and this behavior is inherent in all the metal–organic complexes3. The elements, from which the materials were composed, were further investigated by EDS. It was determined that the obtained microstructures consisted of Cu, C, and N (Fig. 4c(i–iii)). These results further supported the formation of CuCN in acetonitrile regardless of the laser wavelength. In addition, some spherical NPs were found among cubic CuCN (Fig. 4). The NPs were responsible for the characteristic signals of metallic Cu. Figure 4d illustrates the SEM image of spherical copper NPs obtained by PLAL of a Cu plate in acetonitrile at a wavelength of 355 nm. The EDS elemental mapping images of the sample revealed the presence of Cu and C atoms (Fig. 4d(i–ii)), which confirmed that the spherical Cu NPs were covered with carbon shells due to availability of alkyl carbons in the solvent. Thus, the graphitic carbon layers covered on the surface of micro-sized Cu spheres further prevented the aggregation and growth of the particles even after several days of aging. However, during the PLAL proceses, the micro-sized spherical particles were formed due to the Ostwald ripening process27. The existence of Cu@GC NPs was also confirmed by XRD and FT-Raman analyses.
Furthermore, the SEM images of samples prepared in propionitrile and butyronitrile are shown in Fig. 5. In contrast to the surface morphology of the material obtained in acetonitrile, in the case of the samples prepared in propionitrile and butyronitrile, the majority of the particles were spherical and no cubical structures were observed. It was found that the final shape of the particles in propionitrile or butyronitrile was not affected by the laser wavelength. The EDS analysis (Fig. 5c(i–ii),f(i–ii)) confirmed that similarly to the spherical particles found in acetonitrile, the spherical NPs in the samples prepared in propionitrile or butyronitrile were composed of Cu and C. Notably, the amount of Cu@GC NPs in propionitrile was significantly higher than in butyronitrile28.
TEM analysis
TEM analysis was performed to further confirm the surface structure of the samples prepared by PLAL with 1064 nm, and the resulting images are shown in Fig. 6. The images of Cu@GC in Fig. 6c–f are clearly demonstrated the carbon layers (red lines) covered on the surface of Cu spheres with a lattice distance of 3.35 Å. The carbon shells appeared as thick as the previous works with Au@GC and Pt@C particles obtained by PLAL8,11,29.
Theoretical calculations
Experimental analysis confirmed the formation of CuCN only in acetonitrile, which was supported by the conducted theoretical studies. Overall, analysis of all of the experimental results was in full agreement with the theoretical calculations.
During the PLALprocesses, particle formation and organic solvent decomposition occur simultaneously under the harsh conditions of the plasma plume. The difference in metal source and liquid composition can lead to a variety of materials29. Hence, the fragmentation of solvent molecules in the PLAL process occurs due to the difference in binding energy between atoms, considered as a solvent effect. Based on the theoretical calculations summarized in Table 1, it was clear that the formation of CuCN was favorable in acetonitrile, due to the presence of cyano groups immediately after fragmentation of the solvent molecules. As the bond between C1 and C2 dissociated, a methyl cation (CH3+) and a cyanide anion (CN−) were formed (Fig. 7). The electron configuration of Cu is [Ar]3d104s1, where the filled d-subshell promotes the formation of stable complexes with ligands by π-back bonding22. The back donation of the ligand to the metal through sigma (σ) bonding and further transfer of the electron density back into the π orbitals of the ligand have also been reported30. The CN− species are entirely negatively charged due to the presence of a lone pair of electrons on either side of the atom. Consequently, a strong complexation occurs between CN− and Cu+. Moreover, most of the Cu ions produced during PLAL bind to cyano groups due to the strong affinity of these moieties for each other. This is caused by dπ–pπ* back donation of the d10 Cu(I) orbitals to the low-lying π* orbitals of CN−31. In addition to the formation of CuCN in acetonitrile, some spherical Cu NPs covered with GC shells were observed due to the presence of methyl groups in the solvent. Nonetheless, the carbon source was insufficient to produce many spherical Cu@GC NPs in acetonitrile.
Structures of organic nitrile solvents showing the separated bonds, for which the binding energy (BE) was calculated. BEa—bond between the C1 and C2 atoms (C1–C2 in all nitriles), BEb—bond between two carbon atoms (C2–C3 in propionitrile or C3–C4 in butyronitrile), BEc—bond between the C3 and C2 carbon atoms (C3–C2) in butyronitrile.
Another interesting observation was made when propionitrile and butyronitrile were used as solvents for PLAL of Cu plate. Structural analysis revealed the formation of spherical NPs composed of Cu and C. The characteristic peaks of Cu as well as D- and G-bands of GC were detected in the XRD patterns and FT-Raman spectra of these samples, respectively. Moreover, the characteristic peaks corresponding to CuCN were not detected in the following spectra, and no traces attributed to cubic particles were observed during the FE-SEM analysis. To explain these outcomes, we conducted theoretical calculations of the binding energies (Table 1). Bonds between carbon atoms C1–C2 and C2–C3 were considered (Fig. 7). Both C1–C2 and C2–C3 exhibit distinct binding energy (BEa and BEb, respectively). The values of BEb, i.e., the energy between the C2 and C3 atoms in propionitrile, and BEc in butyronitrile were lower. These bonds underwent dissociation during PLAL, releasing a large amount of alkyl carbons, which further deposited on the surface of Cu NPs to form GC layers. Once Cu NPs were encapsulated in carbon shells, further interactions with the cyano groups were not possible. The GC layers hindered the growth of Cu NPs and prevented external impact, enhancing the stability of the material. Thus, the shapes of all particles synthesized in propionitrile and butyronitrile were spherical. Accordingly, the formation of CuCN upon the elongation of the carbon chain in organic nitriles was less likely. It was also confirmed that the binding energies of C–C and C≡N in acetonitrile were 530.78 and 1167.64 kJ/mol. Hence, it was determined that the C≡N bond was stronger than the C–C bond (Fig. 8, Table 2). Consequently, it was predicted that CuCN was formed by the cleavage of the C–C bond in acetonitrile during PLAL. The theoretical calculations of the bond energies fully supported the experimental results of this study on the predominance of CuCN in acetonitrile and the absence of CuCN in the other two nitriles through comparison.
Three different laser wavelengths (1064, 532, and 355 nm) were used for the material preparation in three different organic nitriles (acetonitrile, propionitrile, and butyronitrile). The basic differences among the laser wavelengths are as follows. When a focused beam of 1064 nm wavelength is used in PLAL, the temperature of plasma plumes is higher than the case of 532 and 355 nm, while the electron densities in the plasma plume are higher at shorter wavelength. In addition, in the case of ablation at 355 nm, the plasma plume size is smaller and the expansion rate is slower32,33,34. Based on these behaviors, unique results were obtained by the formation of CuCN when using acetonitrile as a solvent at all three wavelengths due to its lower carbon content than the other solvents and direct presence of CN− groups after solvent fragmentation. On the other hand, in the case of propionitrile and butyronitrile, CuCN was not formed even at high electron density of laser ablation because the binding energy of BEb or/and BEc is much lower than that of BEa (Fig. 7 and Table 1). Thus, the formation of carbon layers is much more favorable than that of CN− groups, which inhibits the formation of CuCN in propionitrile and butyronitrile.
Conclusions
In summary, a facile one-pot synthesis of CuCN was performed using PLAL in acetonitrile. Theoretical studies on the formation of CuCN were conducted using quantum chemical calculations by performing basic sets of B3LYP and MP2. It was found that the formation CuCN was favorable only in acetonitrile, which was attributed to the high availability of the CN− moiety immediately following the fragmentation of acetonitrile under PLAL. In contrast, the use of highly ordered nitrile solvents, such as propionitrile and butyronitrile, resulted in a release of a high amount of alkyl carbons, which further deposited on the surface of Cu NPs to form GC layers (Cu@GC). The formation of cubic CuCN and spherical Cu@GC structures was examined by XRD, FT-Raman, FE-SEM, EDS, and TEM. The results of the present study involving PLAL-assisted synthesis of CuCN with a unique surface structure can be utilized for the development of novel metal–polymer materials for applications in various fields.
Data availability
The data which support for this research findings are available and can provided based on the request to the corresponding authors.
References
Saadatmand, M. et al. Optimisation of brass plating condition in plating of patented steel wire. Surf. Eng. 27, 19–25. https://doi.org/10.1179/026708410X12459349719972 (2011).
Deng, H. et al. Synthesis of new copper cyanide complexes via the transformation of organonitrile to inorganic cyanide. Inorg. Chem. 47, 5866–5872. https://doi.org/10.1021/ic800164b (2008).
Liang, S.-W. et al. Hydrothermal synthesis and crystal structure of a novel cyanide-bridged double helical copper(I) coordination polymer [Cu3(CN)3(phen)]n. Inorg. Chem. Commun. 9, 1312–1314. https://doi.org/10.1016/j.inoche.2006.08.012 (2006).
Gupta, M. et al. Lattice dynamics and thermal expansion behavior in metal cyanides, MCN (M=Cu, Ag, Au): Neutron inelastic scattering and first principles calculations. Phys. Rev. B 93, 134307. https://doi.org/10.1103/PhysRevB.93.134307 (2015).
Hibble, S. & Chippindale, A. The threading of [(CuCN)2(μ-4,4′-bpy)] sheets by CuCN chains. Z. Anorg. Allg. Chem. 631, 542–545. https://doi.org/10.1002/zaac.200400445 (2005).
Hibble, S. et al. Copper(I) cyanide: A simple compound with a complicated structure and surprising room-temperature reactivity. Angew. Chem. (Int. ed Engl.) 43, 628–630. https://doi.org/10.1002/anie.200352844 (2004).
Simeonova, F. P. et al. Hydrogen Cyanide and Cyanides: Human Health Aspects (World Health Organization, 2004).
Lee, S. H. et al. Selective synthesis of Au and graphitic carbon-encapsulated Au (Au@GC) nanoparticles by pulsed laser ablation in solvents: Catalytic Au and acid-resistant Au@GC nanoparticles. Appl. Surf. Sci. 506, 145006. https://doi.org/10.1016/j.apsusc.2019.145006 (2020).
Lee, S. J. et al. Efficient recovery of palladium nanoparticles from industrial wastewater and their catalytic activity toward reduction of 4-nitrophenol. Chemosphere 262, 128358. https://doi.org/10.1016/j.chemosphere.2020.128358 (2021).
Lee, S. J. et al. Plasmonic ZnO/Au/g-C3N4 nanocomposites as solar light active photocatalysts for degradation of organic contaminants in wastewater. Chemosphere 263, 128262. https://doi.org/10.1016/j.chemosphere.2020.128262 (2021).
Begildayeva, T. et al. Production of copper nanoparticles exhibiting various morphologies via pulsed laser ablation in different solvents and their catalytic activity for reduction of toxic nitroaromatic compounds. J. Hazard. Mater. 409, 124412. https://doi.org/10.1016/j.jhazmat.2020.124412 (2020).
Lee, S. J. et al. Eco-friendly synthesis of lignin mediated silver nanoparticles as a selective sensor and their catalytic removal of aromatic toxic nitro compounds. Environ. Pollut. 269, 116174. https://doi.org/10.1016/j.envpol.2020.116174 (2020).
Jung, H. J. & Choi, M. Y. One-pot synthesis of graphitic and nitrogen-doped graphitic layers on nickel nanoparticles produced by pulsed laser ablation in liquid: Solvent as the carbon and nitrogen source. Appl. Surf. Sci. 457, 1050–1056. https://doi.org/10.1016/j.apsusc.2018.07.036 (2018).
Frisch, M.J. et al. Gaussian 16 Rev. B.01 (Wallingford, CT, 2016).
Ditchfield, R. et al. Self-consistent molecular-orbital methods. IX. An extended Gaussian-type basis for molecular-orbital studies of organic molecules. J. Chem. Phys. 54, 724–728. https://doi.org/10.1063/1.1674902 (1971).
Becke, A. D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652. https://doi.org/10.1063/1.464913 (1993).
Head-Gordon, M. et al. MP2 energy evaluation by direct methods. Chem. Phys. Lett. 153, 503–506. https://doi.org/10.1016/0009-2614(88)85250-3 (1988).
Balabin, R. M. Enthalpy difference between conformations of normal alkanes: Intramolecular basis set superposition error (BSSE) in the case of n-butane and n-hexane. J. Chem. Phys. 129, 164101. https://doi.org/10.1063/1.2997349 (2008).
Dunning, T. H. A road map for the calculation of molecular binding energies. J. Phys. Chem. A 104, 9062–9080. https://doi.org/10.1021/jp001507z (2000).
Liu, B. & McLean, A. D. Accurate calculation of the attractive interaction of two ground state helium atoms. J. Chem. Phys. 59, 4557–4558. https://doi.org/10.1063/1.1680654 (1973).
Leung, A. H. M. et al. Cu/M:ZnO (M = Mg, Al, Cu) colloidal nanocatalysts for the solution hydrogenation of carbon dioxide to methanol. J. Mater. Chem. A 8, 11282–11291. https://doi.org/10.1039/D0TA00509F (2020).
Wang, J., Collins, M. F. & Johari, G. P. CuCN: An orientational glass. Phys. Rev. B 65, 180103. https://doi.org/10.1103/PhysRevB.65.180103 (2002).
Kaniyoor, A. & Sundara, R. A Raman spectroscopic investigation of graphite oxide derived graphene. AIP Adv. 2, 032183–032183. https://doi.org/10.1063/1.4756995 (2012).
Lukey, G. C. et al. Raman study on the speciation of copper cyanide complexes in highly saline solutions. Hydrometallurgy 53, 233–244. https://doi.org/10.1016/S0304-386X(99)00047-X (1999).
Kettle, S. F. A. et al. The vibrational spectra of the cyanide ligand revisited: the ν(CN) infrared and Raman spectroscopy of Prussian blue and its analogues. J. Raman Spectrosc. 42, 2006–2014. https://doi.org/10.1002/jrs.2944 (2011).
Han, J., Blackburn, N. J. & Loehr, T. M. Identification of the cyanide stretching frequency in the cyano derivative of copper/zinc-superoxide dismutase by IR and Raman spectroscopy. Inorg. Chem. 31, 3223–3229. https://doi.org/10.1021/ic00041a011 (1992).
Zhang, D., Gökce, B. & Barcikowski, S. Laser synthesis and processing of colloids: Fundamentals and applications. Chem. Rev. 117, 3990–4103. https://doi.org/10.1021/acs.chemrev.6b00468 (2017).
Hao, C., Xiao, F. & Cui, Z. Preparation and structure of carbon encapsulated copper nanoparticles. J. Nanopart. Res. 10, 47–51. https://doi.org/10.1007/s11051-007-9218-6 (2008).
Zhang, D. et al. Carbon-encapsulated metal/metal carbide/metal oxide core–shell nanostructures generated by laser ablation of metals in organic solvents. ACS Appl. Nano Mater. https://doi.org/10.1021/acsanm.8b01541 (2018).
Nelin, C. J., Bagus, P. & Philpott, M. The nature of the bonding of CN to metals and organic molecules. J. Chem. Phys. 87, 2170–2176. https://doi.org/10.1063/1.453142 (1987).
Romao, C. et al. A high-pressure micro-Raman spectroscopic study of copper cyanide, CuCN. J. Mater. Sci. 45, 2518–2520. https://doi.org/10.1007/s10853-010-4232-y (2010).
Moscicki, T., Hoffman, J. & Szymanski, Z. The effect of laser wavelength on laser-induced carbon plasma. J. Appl. Phys. 114, 083306. https://doi.org/10.1063/1.4819892 (2013).
Shaikh, N. M. et al. Diagnostics of cadmium plasma produced by laser ablation. J. Appl. Phys. 100, 073102. https://doi.org/10.1063/1.2357864 (2006).
Barthélemy, O. et al. Influence of the laser parameters on the space and time characteristics of an aluminum laser-induced plasma. Spectrochim. Acta, Part B 60, 905–914. https://doi.org/10.1016/j.sab.2005.07.001 (2005).
Acknowledgements
This research was supported by Korea Basic Science Institute (National research Facilities and Equipment Center) grant funded by the Ministry of Education (No. 2019R1A6C1010042). Prof. M. Y. Choi and Dr. J. Theerthagiri acknowledge the financial support from National Research Foundation of Korea (NRF), (2019R1A2C1009871, 2019H1D3A1A01071209). Dr. S. J. Lee acknowledge the financial support from National Research Foundation of Korea (NRF-2020R1I1A1A01065748). SEM, EDS, and micro-Raman instruments were used in the Core-Facility Center for Photochemistry & Nanomaterials.
Author information
Authors and Affiliations
Contributions
T.B.—conceptualization, investigation, writing, and data curation; A.A.—investigation, writing, and data curation; S.S.N.—formal analysis and data curation; S.J.L.—investigation, and data curation; J.T.—writing-review and editing; T.H.K.—conceptualization and data curation; M.Y.C.—funding acquisition, project administration, and supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Begildayeva, T., Ahn, A., Naik, S.S. et al. Facile one-pot synthesis of CuCN by pulsed laser ablation in nitrile solvents and mechanistic studies using quantum chemical calculations. Sci Rep 11, 14389 (2021). https://doi.org/10.1038/s41598-021-93768-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-93768-7
This article is cited by
-
Impact of viscosity of liquid on nanoparticles synthesized by laser ablation in liquid: An experimental and theoretical investigation
Applied Physics A (2023)
-
Fundamentals and comprehensive insights on pulsed laser synthesis of advanced materials for diverse photo- and electrocatalytic applications
Light: Science & Applications (2022)
-
Diverse nanomaterials synthesized by laser ablation of pure metals in liquids
Science China Physics, Mechanics & Astronomy (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.