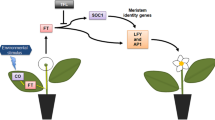
Abstract
It is widely known that during the reproductive stage (flowering), plants do not root well. Most protocols of shoot regeneration in plants utilize juvenile tissue. Adding these two realities together encouraged us to study the role of florigen in shoot regeneration. Mature tobacco tissue that expresses the endogenous tobacco florigen mRNA regenerates poorly, while juvenile tissue that does not express the florigen regenerates shoots well. Inhibition of Nitric Oxide (NO) synthesis reduced shoot regeneration as well as promoted flowering and increased tobacco florigen level. In contrast, the addition of NO (by way of NO donor) to the tissue increased regeneration, delayed flowering, reduced tobacco florigen mRNA. Ectopic expression of florigen genes in tobacco or tomato decreased regeneration capacity significantly. Overexpression pear PcFT2 gene increased regeneration capacity. During regeneration, florigen mRNA was not changed. We conclude that florigen presence in mature tobacco leaves reduces roots and shoots regeneration and is the possible reason for the age-related decrease in regeneration capacity.
Similar content being viewed by others
Introduction
Plant regeneration by rebuilding new organs (organogenesis) results from new organ formation through dedifferentiation of differentiated plant cells and reorganization of cell division to create new organ meristems and new vascular connection between the explant and the newly regenerating organ1,2. Regeneration of a multicellular organism from a segment of adult somatic tissue is a prevalent phenomenon in both plants and animals3. While plant cells are presumed to maintain totipotency, animal cells, except for stem cells, do not. Plant cells that are already differentiated into organs can regenerate whole plants under in vitro conditions4,5. The ratio of the two main growth regulators, auxin and cytokinin, determines regenerating cells’ developmental program. High cytokinin to auxin ratio directs shoot regeneration, while low cytokinin to auxin ratio results in roots6.
Animals contain stem cells that maintain totipotency throughout the animal's life for the purpose of tissue regeneration and repair. In contrast, plants, except for two cells at the apical meristem and two cells at the root meristem, do not have stem cells or totipotent cells circulating. Interestingly, in common with animal stem cells that decline in regenerative ability with age, there is a progressive decline in plant cells’ regenerative ability during their life cycle7,8. In plants, chronological age affects the plant regenerative capability manifested as rooting ability or shoot regeneration9,10. Recently Zhang et al.11 showed that miR156, a chronologically regulated microRNA, regulates shoot regeneration in leaf segments from tobacco plants by allowing the gradual changes in SQUAMOSA PROMOTER BINDING PROTEIN-LIKE (SPL) mRNA levels11. The rise in SPL mRNA levels leads to a progressive drop in the ability to regenerate shoots in tobacco and Arabidopsis explants11. miR156- targeted SPLs were shown to regulate a diverse age-related process, such as embryonic pattern formation, juvenile-to-adult phase transition, the timing of flowering, and shoot regeneration ability11,12,13,14,15,16,17. Thus, the juvenile to adult change is strictly controlled by a regulatory gene network that contains at least miR156 that regulated SPL family members and downstream of SPL like FLOWERING LOCUS T (FT)18.
Research reports in past years have shown that flower buds’ presence reduces rooting, such as in Rhododendron and Camellia, Coleus, Vaccinium, and Taxus19. Even earlier, Wilton20 showed that little or no cambial activity is present in the stems of flowering plants compared to non-flowering plants20. The above effects indicate that the capacity to regenerate roots or shoots and maintain cell division activity in the cambium is associated with the plants' maturity state. Therefore, i it is a common practice in vegetative rooting of cuttings to use as juvenile tissue as possible.
FLOWERING LOCUS T (FT) is a transmissible floral inducer in flowering plants. FT is a critical element in annual plants' competence to flower shortly after emergence; however, perennial plants contain at least two FLOWERING LOCUS T genes with different functions21. Perennials have an extended juvenile vegetative period lasting up to many years of vegetative growth before achieving flowering18,22. After flowering, perennials enter into a yearly cycle of vegetative and reproductive processes. Perennials express at least two versions of FT-like genes to regulate flowering and non-flowering phases. FT1-like and FT2-like (a phosphatidylethanolamine-binding protein family) of perennial plants regulate cellular proliferation and new tissue formation and induce flowering when expressed tobacco or Arabidopsis21,22. However, while FT1-like expression in perennial plants precedes flowering, suggesting it functions as florigen, FT2-like genes are associated with the juvenile and vegetative period21,23. Thus, FLOWERING LOCUS T-like genes coordinate the repeated cycles of vegetative and reproductive growth in perennial like poplar and pear by cycled expression year-round21,24.
Kumar et al.25 demonstrated that Oncidium's flowering is mediated by NO (Nitric Oxide) levels, suggesting that NO controlled the phase transition and flowering process25. Application of sodium nitroprusside (NO donor) to Arabidopsis vtc1 mutant caused late flowering, and expression levels of flowering-associated genes (CO, FT, and LFY) were reduced, suggesting NO signaling is vital for flowering25,26. The induction to flowering or vegetation pattern relies on the balance between the expression levels of genes in the PEBP gene family (phosphatidylethanolamine‐binding protein) like FT1 or FT221,24,27 mutations these homologous genes have different consequences on flowering or vegetative growth. However, it seems that these genes determine the fate of the meristem for vegetative or reproductive growth28.
In the study here, we present evidence to show that the florigen gene levels in tobacco or tomato influence regeneration capacity. Overexpression of pear PcFT2 gene increased regeneration capacity while FT1 or florigen reduces regeneration capacity. During regeneration, tobacco florigen mRNA does not change. We conclude that florigen presence in mature tobacco leaves reduces roots and shoots regeneration. It may be the possible reason for the age-related decrease in regeneration capacity.
Results
Root and shoot regeneration is affected by leaf age
We tested root regeneration from leaf petioles and found that as the leaves mature and the plant approaches flowering, the number of roots regenerated declines (Fig. 1a) as well as percent regeneration (Fig. S1). Root regeneration from mature leaf segments taken from the leaf blade was much lower than juvenile leaf segments (Fig. 1b), as was percent shoot regeneration (Fig. S2a). The number of shoots regenerated from juvenile leaves (leaf number 7-8) was significantly higher than from mature leaves (leaf number 20-21) (Fig. 1c), as was percent shoot regeneration (Fig. S2b). Juvenile leaves regenerate more roots and shoots than mature leaves. The juvenile leaves' tips are much rounded than the mature leaves (Fig. 1d); as the tobacco plant ages, the leaves turn more elongated, which can be seen as the ratio between leaf width to leaf length decreases (Fig. S3). Preventing the effect of leaf age on shoot or root regenerative ability was achieved by using leaves at the same developmental stage, 3–4 days after they reached the final size. Under our conditions in the growth room, tobacco plants flowers at about 20 to 22 leaves.
Effect of leaf age on regeneration. Tobacco leaves were detached at the plant's petiole-stem junction and numbered starting from the first true leaf and were placed in agar supplemented with IBA. (a) The number of roots ± SE regenerated from leaf petioles were counted after 4, 5, and 6 days in IBA each day. The experiment was done in 2 replicates with 5–7 leaves each leaf number. Percent regeneration can be seen in figure S2. (b) The number of roots ± SE regenerated from leaf blades of juvenile (leaf 4 or 8) or mature (leaf 20) leaves. The experiment was done in 3 replicates and each 10–15 leaf segment. Percent regeneration can be seen in Fig. S3. (c) The number of shoots ± SE regenerated from leaf blades was counted after 30 days on the regeneration medium of mature (leaf 20) or juvenile (leaf 8) segments. The experiment was done in 3 replicates, and each had 20–25 leaf segments. Percent regeneration can be seen in Fig. S3. (d) Phenotype difference between mature and juvenile leaves. Statistical analysis was conducted among each color group using the JMP program using Tukey analysis. Different letters depict statistically significant differences between genotypes or treatments (p{f} < 0.001).
Shoot regeneration and flowering are affected by Nitric Oxide
The flowering of tobacco and Arabidopsis plants was affected by a short preincubation of the seedlings in the presence or absence of Nitric Oxide (NO) level modifiers. Growing the seedlings of tobacco (Fig. 2a) or Arabidopsis (Fig. 2b) on media containing the NO synthesis inhibitor DiPhenyleneiodonium (DiPhenyl) prior to planting in pots advances flowering of both plant genotypes (Fig. 2a; 2b). Incubation with NO donor Molsidomine (Molsido) had a non-significant but repeated small flowering delay (Fig. 2a,b). Shoot regeneration was enhanced in plants treated with Molsidomine (Fig. 2c) and inhibited by DiPhenyl in non-transformed SR1 plants. Overexpression of avocado florigen in SR1 plants reduced shoot regeneration (Fig. 2c) while promoting flowering (Fig S2c). DiPhenyl inhibited shoot regeneration in florigen expressing and untransformed plants. However, florigen overexpressing plants were insensitive to treatment with NO donor (Fig. 2c).
Effect of Nitric Oxide donor or inhibitor on flowering and shoot regeneration. (a) Tobacco seedlings at the cotyledon stage were transferred and grown on an agar medium supplemented with 2% sucrose and 5 µM DiPhenyl or 5 µM Molsido. At the seven-leaf stage, seedlings were transferred to soil and grown to flower. The number of leaves at the time flower buds emerged ± SE was counted for tobacco plants, each treated with DiPhenyl or Molsido and control. The experiment was done in 2 replicates with 5–7 plants each. (b) Arabidopsis seedlings at the cotyledon stage were grown on agar medium supplemented with 2% sucrose and 5 µM DiPhenyl or 5 µM Molsido. At a six-leaf stage, seedlings were transferred to soil and grown to flower. The number of leaves at the time flower buds emerged ± SE was counted, each treated with DiPhenyl or Molsido and control. (c) The effect of Nitric Oxide donor (Reg + Molsido) or inhibitor (Reg + DiPhenyl) on the number of shoots ± SE regenerated after 30 days on juvenile leaves segments from non-transformed plants (SR1); plants transformed with a 35S::florigen-like gene from avocado (SR1 + florigen). The experiment was done in 3 replicates, and each had 20–25 leaf segments. Statistical analysis was conducted among each color group using the JMP program and Tukey analysis. Different letters depict statistically significant differences between treatments (p{f} < 0.001). (d) Tobacco seedlings treated with 5 µM DiPhenyl or 5 µM Molsido or none before transfer to soil. The seedlings were transferred to 2% sucrose only or 2% sucrose + 5 µM DiPhenyl or 2% sucrose + 5 µM Molsido agar at the cotyledon stage. All plants recovered from the treatments.
Treating tobacco seedlings with DiPhenyl that inhibits NO synthesis29,30 shows a similar phenotype of lacking NO as in Arabidopsis nos1 mutant31 (Fig. 2d) i.e., impaired growth, yellowish first true leaves, reduces root size, defective abscisic acid-induced stomatal movements, and most importantly, induces early blooming31. Transfer of the tobacco seedlings to the soil without DiPhenyl resulted in rapid greening and growth and early flowering (Fig. 2d). The ratio between leaf width to length did not change after treatment with NO modifiers (Fig. S3a) or overexpression of florigen (Fig. S3b).
FLOWERING LOCUS T mRNA level is influenced by leaf position and NO treatment
The level of mRNA of several FLOWERING LOCUS T (NtFT1, NtFT2, NtFT3, and NtFT4) genes of tobacco was compared between juvenile leaf and mature leaf and juvenile leaves treated with DiPhenyl or Molsido. NtFT4 mRNA (the tobacco florigen) level was high in mature leaves, and DiPhenyl treated juvenile leaves and correlated with flowering. In leaves of plants that do not flower, NtFT4 was not expressed (Fig. 3a). NtFT2 and NtFT1 are expressed in all examined leaves (Fig. 3a), and NtFT3 was only expressed in mature leaves. The NtFT gene family level does not change during the initial regeneration period, but NtTFL1 expression increases (Fig. 3b).
Effect of treatment with Nitric Oxide donor or inhibitor or leaf age on tobacco FLOWERING LOCUS T gene family expression. (a) The expression level of tobacco FLOWERING LOCUS T gene family at the juvenile, mature, juvenile leaf treated with DiPhenyl and juvenile leaf treated with Molsido. Tobacco seedlings at the cotyledon stage were transferred and grown on an agar medium supplemented with 2% sucrose and 5 µM DiPhenyl or 5 µM Molsido. Gene expression was tested in leaves number 7 as juvenile and 20 as mature grown in a sterile condition. mRNA levels of the FLOWERING LOCUS T gene family are expressed as log units. (b) Expression level of tobacco FLOWERING LOCUS T gene family during the seven days induction period of shoots on Reg medium. (c) Visualisation by heatmap of genes whose mRNA expression increase or decreases at the juvenile, mature, juvenile leaf treated with DiPhenyl and juvenile leaf treated with Molsido.
The expression of genes associated with FLOWERING LOCUS T, like TEMPRANILLO or SQUAMOSA PROMOTER BINDING, did not show the pattern exhibited by the tobacco florigen (NtFT4) (Tables S1 and S2).
mRNA expression heatmap shows extensive changes between mature and juvenile leaves (Fig. 3c). The expression of 2792 genes changes between juvenile and mature leaves, while between juvenile leaves treated with DiPhenyl, only 403 genes changed expression, and between juvenile leaves treated with Molsido, only 212 genes changed expression (Fig. S4a). The gene heatmap expression of juvenile leaf treated with DiPhenyl shows specific patterns like the mature leaf. The Molsido treated leaf is similar to juvenile leaves (Fig. 3c). Six different expression patterns, i.e., C1-C6, were distinguished according to similarity or difference to juvenile leaf (Fig. S4b).
Florigen affects the leave’s ability to regenerate
Overexpression of avocado (Presea americana) FLOWERING LOCUS T-like genes (florigen) in tobacco resulted in reduced shoot regeneration capacity (Fig. 4a). The flowering promoting effect of florigen caused a decrease in shoot regeneration in the tomato cotyledon segment overexpressing either the tomato florigen (SFT) or the pepper florigen (CaFT1-LIKE) (Fig. 4b). Mature leaf segments collected at the time flower buds are visible show reduced shoot regeneration in non-transformed plants (Fig. 4c). However, flowering did not affect shoot regeneration in plants transformed with avocado florigen (PaFT1) (Fig. 4c). Overexpression of the pear (PcFT2) rejuvenator gene in tobacco plants resulted in increased shoot regeneration capacity in juvenile leaf segments (Fig. 4d).
Effect of florigen or rejuvenator on shoot regeneration. (a) Number of shoots ± SE was counted for SR1 tobacco leaf segments, each transformed with florigen from avocado or non-transformed (SR1). The experiment was done in 4 replicates from separate transformed plants, each with 20 segments per plate on Reg medium. (b) The number of shoots ± SE was counted after 30 days on the regeneration medium of tomato S. lycopersicon cv M82. Cotyledons segments 20 per plate, four plates per genotype were tested for regeneration ability. sft = M82 plants mutated in florigen gene; M82 = wild type M82 plants; sft + 35::SFT = M82 plants mutated in tomato florigen gene overexpressing a functional tomato florigen under 35S promotor; sft + 35::FT pepper = M82 plants mutated in tomato florigen gene overexpressing a functional Capsicum annum florigen (FT1-like) gene. The number of shoots ± SE was counted for tomato cotyledons segments placed on the regeneration medium. (c) The number of shoots ± SE was counted after 30 days on the regeneration medium of mature (noted as flowering) or juvenile tobacco leaves segments. The experiment was done in 3 replicates, and each had 20–25 leaf segments. (d) Number of shoots ± SE was counted for SR1 tobacco leaf segments from plants overexpressing the pear (Pyrus communis) rejuvenator gene that confers juvenility and non-transformed tobacco (SR1). The experiment was done in 4 replicates from separate transformed plants, each with 20 segments per plate on Reg medium Statistical analysis was conducted using the JMP program and Tukey analysis. Different letters depict statistically significant differences between genotypes or treatments (p{f} < 0.001).
Discussion
We designed this study to analyse the basis of root and shoot regeneration differences between vegetative state (Juvenile) and flowering state (Mature) plant tissues. Past reports described that flower buds’ presence on a plant reduces rooting, for example, in Rhododendron, Camellia, Coleus, Vaccinium, and Taxus19 or inhibit cambial activity in stems of flowering plants20. The differences between juvenile and mature tissues in the capacity to regenerate roots or shoots depend on physiological age. Most shoot or root regeneration protocols vary vastly between plant species; almost all with a few exceptions, use a tissues (or explant) that are juvenile, such as cotyledon, hypocotyl, petiole, or dormant meristem. In both plants and animals, regeneration ability declines with age7,8,11.
FLOWERING LOCUS T (FT) is a small mobile protein that functions as a floral and developmental regulator gene family. FT protein is a critical element in annual plants' competence to flower shortly after emergence; however, perennial plants contain at least two FT genes with different functions in flowering florigen and a rejuvenator21. Perennials plants have an extended juvenile period lasting up to many years of vegetative growth before achieving flowering18,22. After the first flowering period, perennials enter into a yearly cycle of vegetative and reproductive processes. Perennials express at least two versions of FT genes, florigen and a rejuvenator gene. Both are a phosphatidylethanolamine-binding protein family (PEBP gene family) and induced flowering when expressed in tobacco or Arabidopsis21,22. However, while FT1, the florigen expression in perennial plant's expression, precedes flowering, the FT2 or the rejuvenator expression is during the vegetative period 21,23. Thus, florigen and a rejuvenator are both homologs to the A. thaliana gene FLOWERING LOCUS T (AtFT1) gene, coordinating the repeated cycles of vegetative and reproductive growth in woody perennial like poplar24 (Populus spp.) or pear21 by cycling expression year-round. Florigen action in the meristem to induce flowering27,32 functions to transform the leaf into a mature organ changing its shape33 and reducing its capacity to regenerate. While the phase change from vegetative to reproductive growth in a plant is accompanied by changing leaf shape33, we found that inducing early flowering and reducing regeneration ability is not related to this shape change. While the shape changes are correlated with reduced mir156 and increased SPL genes33, this study shows that independently from flowering and other meristematic effects, FT genes function in the leaf tissue as a determinant of juvenility or maturity depending if the rejuvenator or the florigen is expressed without changing the leaf shape that is typical of plant maturity. Examining FT's immediate suspects in meristematic flowering processes did not reveal an expression pattern that is similar to FT mRNA (Table S1 and S2). Thirteen FT-like genes were identified in tobacco34; out of these 13 genes, NtFT4 and NtFT5 were shown to function as florigen35,36. RNA–seq of leaf tissue from mature or juvenile leaves showed that NtFT4 is expressed in mature leaves and not in juvenile leaves, while NtFT5 is not expressed in the leaves. Treating the tobacco leaves with NO modifiers that promoted flowering and inhibited regeneration increased NtFT4 but did not affect the expression of NtFT5 (remained zero). Treatment of tobacco leaves with NO modifiers that inhibited flowering and enhanced regeneration and depressed NtFT4 expression, NtFT5 expression was zero. We, therefore, conclude that there are tissue-specific florigen in tobacco and maybe other plants. Some florigen genes are expressed in the leaves, and some in the stem35.
NO (Nitric Oxide) levels are involved in flowering, as demonstrated in Oncidium's25 and Arabidopsis37. Application of NO donor on Arabidopsis vtc1 mutant caused late flowering, and the expression levels of flowering-associated genes (AtCO, AtFT, and AtLFY) were reduced, suggesting NO signaling is part of flowering25,26. Reducing the amount of NO in Arabidopsis plants promoted flowering, increasing NO inhibited flowering37, as was shown here. In parallel, reducing the amount of NO decreases regeneration, and increasing the amount of cellular NO increases regeneration30 (and in this study). We postulated a connection between NO and FT gene family members; it seems that NO level controls FT mRNA expression and thus flowering and regeneration ability. Our data show that NO level affects florigen mRNA level and, as a consequence, influences regeneration and flowering. A link between flowering and root regeneration (rooting) is known for decades and used in plants' vegetative production of crop plants like trees, vegetables, and flowers. The florigen's mRNA expression level seems to explain why plants at the reproductive stage do not regenerate as well as plants in the vegetative phase when the florigen is not expressed. NO impact on seed aging progression was demonstrated by treating seeds with NO or NO donors that alleviate cell death and aging38. NO also delays tissue senescence38,39. Here we show that the effect of NO on shoot regeneration is in juvenile tissue and not in mature tissue; thus, the effects of NO on aging are not related to the effect of NO on shoot regeneration. It is probably a different and direct effect on regeneration. The main NO target in animal tissue is the soluble enzyme guanylyl cyclase; however, NO in plants is unknown40. The indication that NO affects different plant processes could result from many targets and effectors to NO. They vary according to a temporal and physical location.
Juvenility across kingdoms is associated with enhanced regenerative ability. For example, juvenile plants exhibit a high regenerative capacity; as the plant mature, this capacity declines11, as shown here, and modifying mice's adolescent state affects tissue repair, a type of regeneration41 or juvenile axolotl can regenerate a limb faster than an adult42. These observations show that the juvenility state of the tissue governs plants' and animals' regenerative capacity. Zhang et al.11 speculated that the binding of SPL9 to ARR2 changes the conformation of ARR2, thereby impairing its transcriptional activation toward downstream11. In the flowering cascade, FT is influenced by miR156 and SPL genes43. Thus, as shown here, the florigen protein FT affects regeneration capacity on its own.
Our results revealed that the decrease in shoot and root regeneration in mature plant tissue is correlated with a high florigen expression. The mechanism causing the reduced shoot or root regenerative capacity in old plants and whether FT expression is connected to altered phytohormones response awaits further investigations. Ongoing analysis is the effect of cytokinins and auxin combination on shoot regeneration in mature vs. juvenile leaves. Knox gene family was implicated in FT function27 and in shoot regeneration30. We are currently testing the effect of Knox gene family knockout using crisper technology on shoot regeneration.
Shoot and root regeneration is influenced by many factors, the explants, the culture medium, phytohormones, and gelling agent, to name the most tested. FT genes expression level or presence can be used as a marker for regeneration capacity. FT gene manipulation can increase plant species propagation, especially in recalcitrant and rare and endangered plants.
Materials and methods
Seed decontamination and plant growth
Tobacco Seeds (Nicotiana tabacum L. cv. SR1) or Arabidopsis Colombia are used and grown in our lab for many years. Seeds were cleansed with sodium hypochlorite (0.5% active material) in a 1.7 mL micro-tube (Eppendorf) for 15 min. After sodium hypochlorite treatment, the seeds were washed thrice with sterile water and spread on ½-strength MS medium (Duchefa Co. Haarlem, Netherlands, Product number M0221.0050) on a Petri dishes. After germination, seedlings were transffered to polypropylene Vitro Vent containers (Duchefa, NL; 9.6 cm × 9.6 cm and 9 cm in height) with ½-strength MS medium. Molsido (five µM Molsidomine and five μM DiPhenyleneiodonium chloride) was added to the agar medium when treated with NO effectors. Plants were grown in sterile containers in a growth room with 16 hours of light and eight hours of darkness at 26 °C for weeks until leaves were ready to be harvested for regeneration. Tobacco plants were transformed as described before30,44 with Avocado (Persea Americana), FLOWERING LOCUS T-like plasmids obtained from Ziv et al.45, pear (Pyrus communis) from Frieman et al.21, and pepper (Capsicum annum) from Borovsky et al46. Transgenic tomato (Solanum lycopersicon M82) seeds were obtained from Borovsky et al46.
The effect of NO on regeneration was done by placing leaf segments on Reg medium containing NO donors (5 µM Molsidomine), and NO synthesis inhibitor (5 μM DiPhenyleneiodonium) see Subban et al30.
Leaves preparation and shoot and root regeneration
Leaves were detached from sterile plants, and the midrib was removed. The leaf blade was cut into segments about 25 mm2 (5 mm × 5 mm) and placed on a shoot regeneration (Reg) medium containing standard MS salts as before30,44. Medium was augmented with 30 g l−1 sucrose and 8 g l−1 agar and the following growth regulators: 4.57 μM IAA; 9.29 µM Kinetin, and 4.56 µM Zeatin (all from Duchefa Co.) or on 1 mg l−1 IBA for rooting. At least 20 leaf segments were placed on each petri dish with at least four plates per treatment in all experiments. Shoot regeneration from leaf segments was scored 30–32 days after putting them on the medium; root regeneration from segments was scored 10-15 days after placing the leaf segment on medium. Leaf petiole rooting was scored between 4 to 15 days.
Analyses of variance (ANOVA) were performed with the SAS/JMP software (SAS Institute Inc., Cary, NC, USA). Differences among means were calculated based on the Tukey–Kramer honestly significant difference (HSD) test for three or more treatments and T-test for two treatments30.
The numbers of explants and regenerated shoots or roots were scored. The regenerative capacity is represented by the number of regenerated shoots or roots per explants. At least three independent experiments (biological triplicates) were performed, and in each, at least three samples were tested.
RNA preparation and transcript detection
RNA was isolated from leaf segment using the TRI-reagent (Sigma-Aldrich, St. Louis, MO, USA) according to the manufacturer’s instructions and then treated with DNAse (Turbo DNA-free™, Ambion Waltham, Massachusetts, USA). Total RNA was sent to the Weizmann Institute G-INCPM unit for RNA-seq analysis.
Transcriptome analysis
Raw reads were analyzed by filtering and cleaning procedure. The Trimmomatic tool47 was used to remove Illumina adapters from the reads. FASTX (http://hannonlab.cshl.edu/fastx_toolkit/index.html, version 0.0.13.2) was used next to trim the read-end with quality scores <30, utilising the FASTQ Quality Trimmer, and to eliminate sequences with fewer than 70% base pairs homology with a quality record ≤30 using the FASTQ Quality Filter. The selected reads were mapped to the reference genome of Nicotiana tabacum48 ftp://ftp.solgenomics.net/genomes/Nicotiana_tabacum/edwards_et_al_2017/assembly/) using STAR software49,50. Gene abundance estimation was performed using Cufflinks51 combined with gene annotations from the Sol Genomics Network database (https://solgenomics.net/; ftp://ftp.solgenomics.net/genomes/Nicotiana_tabacum/edwards_et_al_2017/annotation). PCA analysis and Heatmap visualization were performed using R Bioconductor51. Gene expression numbers were calculated as FPKM. Distinction expression analysis was completed using the DESeq2 R package52. Genes that varied from the control more than twofold, with an adjusted p-value of no more than 0.05, were considered differentially expressed53. Venn diagrams were produced using the online tool at bioinformatics.psb.ugent.be/webtools/Venn/. KOBAS 3.0 tool54 http://kobas.cbi.pku.edu.cn/kobas3/?t=1) was applied to find the statistical enrichment of differentially expressed genes KEGG pathway and Gene Ontology (GO).
References
Terzi, M. & Lo Schiavo, F. Somatic embryogenesis. In Plant Tissue Culture: Applications and Limitations (Ed. Bhajwani, S.S.) 54–66 (1990).
Sugiyama, M. Organogenesis in vitro. Curr. Opin. Plant Biol. 2, 61–64 (1999).
Birnbaum, K. D. & Sánchez Alvarado, A. Slicing across kingdoms: Regeneration in plants and animals. Cell 132, 697–710 (2008).
Duclercq, J., Sangwan-Norreel, B., Catterou, M. & De Sangwan, R. S. novo shoot organogenesis: From art to science. Trends Plant Sci. 16, 597–606 (2011).
Sugimoto, K., Gordon, S. P. & Meyerowitz, E. M. Regeneration in plants and animals: Dedifferentiation, transdifferentiation, or just differentiation?. Trends Cell Biol. 2011(21), 212–218 (2011).
Skoog, F. & Miller, C. O. Chemical regulation of growth and organ formation in plant tissues cultured in vitro. Symp. Soc. Exp. Biol. 11, 118–130 (1957).
Ruckh, J. M. et al. Rejuvenation of regeneration in the aging central nervous system. Cell Stem Cell 10, 96–103 (2012).
Porrello, E. R. et al. Transient regenerative potential of the neonatal mouse heart. Science 331, 1078–1080 (2011).
Leal, F. J. & Krezdorn, A. H. Rooting avocado cuttings. Proc. Fla. State Hort. Soc. 77, 358–362 (1964).
Ye, B. et al. AP2/ERF transcription factors integrate age and wound signals for root regeneration. Plant Cell 32, 226–241 (2020).
Zhang, T. Q. et al. An intrinsic microRNA timer regulates progressive decline in shoot regenerative capacity in plants. Plant Cell 27, 349–360 (2015).
Wang, J. W., Czech, B. & Weigel, D. MiR156-regulated SPL transcription factors define an endogenous flowering pathway in Arabidopsis thaliana. Cell 138, 738–749 (2009).
Wu, G. et al. The sequential action of miR156 and miR172 regulates developmental timing in Arabidopsis. Cell 138, 750–759 (2009).
Nodine, M. D. & Bartel, D. P. MicroRNAs prevent precocious gene expression and enable pattern formation during plant embryogenesis. Genes Dev. 24, 2678–2692 (2010).
Bergonzi, S. et al. Mechanisms of age-dependent response to winter temperature in perennial flowering of Arabis alpina. Science 340, 1094–1097 (2013).
Zhou, C. M. et al. Molecular basis of age-dependent vernalization in Cardamine flexuosa. Science 340, 1097–1100 (2013).
Rubio-Somoza, I. et al. Temporal control of leaf complexity by miRNA-regulated licensing of protein complexes. Curr. Biol. 24, 2714–2719 (2014).
Bergonzi, S. & Albani, M. C. Reproductive competence from an annual and a perennial perspective. J. Exp. Bot. 62, 4415–4422 (2011).
Adams, D. G. & Roberts, A. N. Effect of flower buds on rooting response. Oragon Ornament. Nursery Digest. 9, 1–2 (1965).
Wilton, O. C. Correlation of cambial activity with flowering and regeneration. Int. J. Plant Sci. (formally Botanical Gazette) 99, 854–864 (1938).
Freiman, A. et al. Expression of flowering locus T2 transgene from Pyrus communis L. delays dormancy and leaf senescence in Malus × domestica Borkh, and causes early flowering in tobacco. Plant Sci. 241, 164–176. https://doi.org/10.1016/j.plantsci.2015.09.012 (2015).
Mimida, N. et al. Apple FLOWERING LOCUS T proteins interact with transcription factors implicated in cell growth and organ development. Tree Physiol. 31, 555–566 (2011).
Kobayashi, Y., Kaya, H., Goto, K., Iwabuchi, M. & Araki, T. A pair of related genes with antagonistic roles in mediating flowering signals. Science 286, 1960–1962 (1999).
Hsu, C. Y. et al. FLOWERING LOCUS T duplication coordinates reproductive and vegetative growth in perennial poplar. Proc. Natl. Acad. Sci. 108, 10756–10761. https://doi.org/10.1073/pnas.1104713108 (2011).
Kumar, R. S. et al. Nitric oxide participates in plant flowering repression by ascorbate. Sci. Rep. 6, 35246 (2016).
Zhang, Z. W. et al. Nitrogen and Nitric Oxide regulate Arabidopsis flowering differently. Plant Sci. 284, 177–184. https://doi.org/10.1016/j.plantsci.2019.04.015 (2019).
Shalit, A. et al. The flowering hormone florigen functions as a general systemic regulator of growth and termination. Proc. Natl. Acad. Sci. 6, 8392–8397. https://doi.org/10.1073/pnas.0810810106 (2009).
Amaya, I., Ratcliffe, O. J. & Bradley, D. J. Expression of CENTRORADIALIS (CEN) and CEN-like genes in tobacco reveals a conserved mechanism controlling phase change in diverse species. Plant Cell 11, 1405–1418 (1999).
Stuehr, D. J. et al. Inhibition of macrophage and endothelial cell Nitric Oxide synthase by DiPhenyleneiodonium and its analogs. FASEB J. 5, 98–103. https://doi.org/10.1096/fasebj.5.1.1703974 (1991).
Subban, P. et al. Shoot regeneration is not a single cell event. Plants 10, 58. https://doi.org/10.3390/plants10010058 (2021).
Guo, F. Q., Okamoto, M. & Crawford, N. M. Identification of a plant Nitric Oxide synthase gene involved in hormonal signalling. Science 302, 100–103. https://doi.org/10.1126/science.1086770 (2003).
Jaeger, K. E. & Wigge, P. A. FT protein acts as a long-range signal in Arabidopsis. Curr. Biol. 17, 1050–1054 (2007).
Lawrence, E. H. et al. Vegetative phase change in Populus tremula × alba. New Phytol. https://doi.org/10.1111/nph.17316 (2021).
Beinecke, F. A. et al. The FT/FD-dependent initiation of flowering under long-day conditions in the day-neutral species Nicotiana tabacum originates from the facultative short-day ancestor Nicotiana tomentosiformis. Plant J. 96, 329–342. https://doi.org/10.1111/tpj.14033 (2018).
Schmidt, F. J. et al. The major floral promoter NtFT5 in Tobacco (Nicotiana tabacum) is a promising target for crop improvement. Front. Plant Sci. 10, 1666. https://doi.org/10.3389/fpls.2019.01666 (2020).
Harig, L. et al. Proteins from the FLOWERING LOCUS T-like subclade of the PEBP family act antagonistically to regulate floral initiation in tobacco. Plant J. 72, 908–921. https://doi.org/10.1111/j.1365-313X.2012.05125.x (2012).
He, Y. et al. Nitric Oxide represses the Arabidopsis floral transition. Science 305, 1968–1971. https://doi.org/10.1126/science.1098837 (2004).
He, Y., Xue, H., Li, Y. & Wang, X. Nitric Oxide alleviates cell death through protein S-nitrosylation and transcriptional regulation during the ageing of elm seeds. J. Exp. Bot. 69, 5141–5155. https://doi.org/10.1093/jxb/ery270 (2018).
Katarzyna, C. et al. Effect of nitrogen reactive compounds on aging in seed. Front. in Plant Sci. https://doi.org/10.3389/fpls.2020.01011 (2020).
Bruand, C. & Meilhoc, E. Nitric Oxide in plants: pro- or anti-senescence. J. Exp. Bot. 70, 4419–4427. https://doi.org/10.1093/jxb/erz117 (2019).
Shyh-Chang, N. et al. Lin28 enhances tissue repair by reprogramming cellular metabolism. Cell 155, 778–792 (2013).
Young, H. E., Bailey, C. F. & Dalley, B. K. Gross morphological analysis of limb regeneration in postmetamorphic adult Ambystoma. Anat. Rec. 206, 295–306. https://doi.org/10.1002/ar.1092060308 (1983).
Amasino, R. Seasonal and developmental timing of flowering. Plant J. 61, 1001–1013. https://doi.org/10.1111/j.1365-313X.2010.04148.x (2010).
Shaya, F. et al. Expression of mitochondrial gene fragments within the tapetum induce male-sterility by limiting the biogenesis of the respiratory machinery in transgenic tobacco plants. J. Integr. Plant Biol. 54, 115–130 (2012).
Ziv, D., Zviran, T., Zezak, O., Samach, A. & Irihimovitch, V. Expression profiling of FLOWERING LOCUS T-Like Gene in alternate bearing ‘Hass’’ Avocado trees suggests a role for PaFT in Avocado flower induction’. PLoS ONE 9, e110613. https://doi.org/10.1371/journal.pone.0110613 (2014).
Borovsky, Y., Mohan, V., Shabtai, S. & Paran, I. CaFT-LIKE is a flowering promoter in pepper and functions as florigen in tomato. Plant Sci. 301, 110678. https://doi.org/10.1016/j.plantsci.2020.110678 (2020).
Bolger, A. M., Lohse, M. & Usade, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120. https://doi.org/10.1093/bioinformatics/btu170 (2014).
Edwards, K. et al. A reference genome for Nicotiana tabacum enables map-based cloning of homeologous loci implicated in nitrogen utilization efficiency. BMC Genom. 18, 448. https://doi.org/10.1186/s12864-017-3791-6 (2017).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Trapnell, C. et al. Supplementary methods for the paper transcript assembly and quantification by RNA-seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 28, 511–515. https://doi.org/10.1038/nbt.1621 (2010).
Gentleman, R. C. et al. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 5, R80. https://doi.org/10.1186/gb-2004-5-10-r80 (2004).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550. https://doi.org/10.1186/s13059-014-0550-8 (2014).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B Methodol. 57, 289–300 (1995).
Xie, C. et al. KOBAS 2.0: a web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 39, W316–W322 (2011).
Author information
Authors and Affiliations
Contributions
Conceptualization, M.R.; methodology, M.R., A.D.-F; software, A.D.-F; experimental, Y.K., M.F. data curation, A.D.-F.; Original draft preparation, M.R., A.D.-F.; writing, reviewing and editing, M.R.; visualization, M.R. A.D.-F. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kutsher, Y., Fisler, M., Faigenboim, A. et al. Florigen governs shoot regeneration. Sci Rep 11, 13710 (2021). https://doi.org/10.1038/s41598-021-93180-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-93180-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.