Abstract
Influenza is an important cause of severe illness and death among patients with underlying medical conditions and in the elderly. The aim of this study was to investigate factors associated with ICU admission and death in patients hospitalized with severe laboratory-confirmed influenza during the 2017–2018 season in Catalonia. An observational epidemiological case-to-case study was carried out. Reported cases of severe laboratory-confirmed influenza requiring hospitalization in 2017–2018 influenza season were included. Mixed-effects regression analysis was used to estimate the factors associated with ICU admission and death. A total of 1306 cases of hospitalized severe influenza cases were included, of whom 175 (13.4%) died and 217 (16.6%) were ICU admitted. Age 65–74 years and ≥ 75 years and having ≥ 2 comorbidities were positively associated with death (aOR 3.19; 95%CI 1.19–8.50, aOR 6.95, 95%CI 2.76–1.80 and aOR 1.99; 95%CI 1.12–3.52, respectively). Neuraminidase inhibitor treatment and pneumonia were negatively associated with death. The 65–74 years and ≥ 75 years age groups were negatively associated with ICU admission (aOR 0.41; 95%CI 0.23–0.74 and aOR 0.30; 95%CI 0.17–0.53, respectively). A factor positively associated with ICU admission was neuraminidase inhibitor treatment. Our results support the need to investigate the worst outcomes of hospitalized severe cases, distinguishing between death and ICU admission.
Similar content being viewed by others
Introduction
Influenza affects 10–20% of the unvaccinated population each year and is a leading cause of severe illness and death among patients with underlying medical conditions and in people aged ≥ 65 years1. Worldwide, annual epidemics are estimated to result in about 3 to 5 million cases of severe illness and about 290,000 to 650,000 deaths caused by respiratory conditions2. Most influenza infections are self-limiting, requiring no healthcare visits, but a proportion of cases present severe complications and require hospitalization and ICU admission.
ICU admission and death have been considered as the worst outcomes for severe cases of influenza and sometimes only a category including those patients requiring ICU admission or who died is considered to identify the factors associated with severity3,4. However, because some differences have been found according to the outcome considered5,6, it seems reasonable to look at these different outcomes in a separate analysis.
In the 1999–2000 season, a sentinel surveillance program of influenza and other viral diseases based on physicians of primary healthcare centers was initiated in Catalonia, a region in the northeast of Spain.
In the 2010–2011 season, the surveillance of these diseases was improved by adding information about severe hospitalized influenza cases that were laboratory-confirmed provided by sentinel hospitals. In the 2017–2018 season there were 14 public hospitals, 13 attending adult and pediatric patients and one attending only pediatric patients. The hospitals participating in this sentinel system cover a population of 4,950,851, which represents 66.2% of the whole population7.
In Catalonia, annual influenza vaccination is recommended to persons aged > 60 years, to pregnant women, and to patients with chronic medical conditions in whom influenza could have severe consequences. Antiviral treatment with neuraminidase inhibitors (NAI) is administered only to hospitalized patients with severe presentation and to those in which individual assessment make it advisable because of high risk for severe complications8.
Studies carried out in different geographical areas during the 2017–2018 influenza season showed increased severity in all age groups9,10,11,12,13. In Catalonia, according to the healthcare demand, the age-adjusted cumulative incidence rate in the 2017–2018 influenza season was 2295.26 per 100,000 habitants. Influenza B virus accounted for 63% of influenza viruses detected. The predominant lineage was Yamagata whereas the previous season was, with scarce circulation, Victoria lineage; H1N1 influenza A strains were similar to previous seasons, while a proportion of H3N2 influenza A circulating strains were different from the previous season. The difference in circulating influenza viruses, both AH3N2 and B, respect to the strains contained in the vaccine, could be responsible for a greater number of severe cases affecting specially the elderly14.
The aim of this study was to investigate factors associated with ICU admission and death in patients hospitalized with severe laboratory-confirmed influenza during the 2017–2018 season.
Results
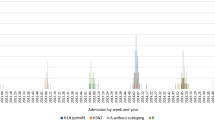
A total of 1306 cases of hospitalized severe influenza cases were included, 56.9% (743/1306) were male, the mean of age was 68.0 ± 20.7 and 34.3% (445/1299) had received the influenza vaccine. 518 were influenza A virus cases and 788 were influenza B virus cases; of the influenza A cases that were subtype, 101 were H1 and 80 were H3; no H5 was found. 175 patients (13.4%) died and 217 (16.6%) were admitted to the ICU (Fig. 1).
There was no collinearity or interaction between variables.
Demographic and clinical characteristics of patients who died or survived are shown in Table 1. Age 65–74 and ≥ 75 years, having ≥ 2 comorbidities were positively associated with death and nosocomial infection (aOR 3.19; 95%CI 1.19–8.50, aOR 6.95, 95%CI 2.76–1.80, aOR 1.99; 95%CI 1.12–3.52 and aOR 1.91; 95%CI 1.16–3.17, respectively). NAI treatment, length of stay and pneumonia were negatively associated with death (aOR 0.23; 95%CI 0.14–0.38, aOR 0.51; 95%CI 0.35–0.74 and aOR 0.64; 95%CI 0.43–0.94, respectively). No coinfections with other influenza-type viruses were detected.
Factors associated with death according to patient groups are summarized in Fig. 2. The factors with the highest positive associations with death were chronic renal disease and chronic liver disease in the 50–64 years age group, chronic cardiovascular disease in the 65–74 years age group and having ≥ 2 comorbidities in the ≥ 75 years age group. Male sex was associated with death only in the 50–64 years age group.
Factors associated with death in hospitalized severe influenza patients according to age group, influenza type, patients admitted to ICU and patients with pneumonia. *Hemoglobinopathy, severe neuromuscular disease or cognitive dysfunction. COPD: chronic obstructive pulmonary disease, NAI: neuraminidase inhibitors.
In influenza A virus cases, the factors positively associated with death were age ≥ 75 years and immune deficiency, while NAI treatment was negatively associated with death.
In influenza B virus cases, factors positively associated with death were age ≥ 75 years, chronic liver disease, having ≥ 2 comorbidities and nosocomial infection. Length of stay and NAI treatment was negatively associated with death.
In patients admitted to the ICU, the proportion of death was 24.9% (54/217) and factors positively associated with death were age ≥ 75 years, having ≥ 2 comorbidities and nosocomial infection.
In patients who presented pneumonia, the proportion of death was 12.6% (105/831) and factors positively associated with death were ≥ 2 chronic medical conditions, age ≥ 75 years, chronic liver disease and nosocomial infection. Length of stay and NAI treatment was negatively associated with death.
The demographic and clinical characteristics of patients admitted to the ICU or not are shown in Table 2. Age < 15 years, 65–74 years or ≥ 75 years were negatively associated with ICU admission (aOR 0.29; 95%CI 0.11–0.76, aOR 0.41; 95%CI 0.23–0.74 and aOR 0.30; 95%CI 0.17–0.53, respectively) as was pneumonia (aOR 0.61; 95%CI 0.41–0.89). NAI treatment and length of stay was positively associated with ICU admission (aOR 2.12; 95%CI 1.19–4.13 and aOR 6.54; 95%CI 4.17–10.24, respectively).
Factors associated with ICU admission according to patient subgroup are summarized in Fig. 3. Chronic vascular disease was positively associated with ICU admission only in the 65–74 years age group.
In influenza A virus cases, factors associated positively with ICU admission were morbid obesity and chronic liver disease. Factors negatively associated with ICU admission were the < 15 years and ≥ 75 years age groups.
In influenza B virus cases, factors negatively associated were the 65–74 years and ≥ 75 years age groups.
In patients with pneumonia, length of stay and NAI treatment was associated positively with ICU admission and factors negatively associated with ICU admission were the < 15 years, 65–74 years and ≥ 75 years age groups.
Discussion
The results of this study show that the proportion of death in hospitalized severe influenza cases was 13.4%, lower than the 18.8% found by Liu et al.15 in the 2016–2018 season in severe patients in Taiwan, but higher than the 9.2% found by Kolosova et al.16 in Russia in hospitalized severe patients in the 2017–2018 season, the 6.5% found in all hospitalized patients in China in the same 2017–2018 season10, and the 5.5% found by Natheglian et al. in hospitalized severe patients during 2015–2018 in Iran12.
The risk of death increased in the 65–74 years and ≥ 75 years age groups, coinciding with the findings of most studies reviewed12,17,18,19,20,21,22,23, although some authors found no differences in mortality according to age24.
Among the factors investigated, COPD was associated with death in the 15–49 years age group, coinciding with the results found by Walker et al. in a study carried out in New Zealand between 2012 and 2015 where the outcome did not distinguish between death and ICU admission25. Immunodeficiency was associated with death in the 15–49 years age group in influenza A and B virus cases and in all age groups in influenza A virus cases, and although we do not have the precise information, the association in the first age group was probably was associated with HIV infection, which has been described as a factor of severity for influenza1, especially for influenza A cases26,27. Czaja et al.28 in the United States during the 2010–2011 to 2014–2015 seasons found that, in hospitalized influenza cases, immunosuppression was also associated with death or transfer to a hospice.
Male sex was only associated with death in the 50–64 years age group. Some authors have found male sex was associated with a higher risk of death12 but, coinciding with our results, Lina et al. in the United States in the 2017–2018 season found that the death was more frequent in males than females in the 50–64 years age group29. Zou et al.13 in a study carried out in influenza-related cases in China in the 2017–2018 season found an association between male sex and death in patients with influenza-related pneumonia, but we did not.
As reported by other authors10,25,26,30, we found that chronic renal disease was associated with death, but only in the 50–64 years age group.
Nosocomial infection was associated with death. A study carried out in France in 2016–2017 season also found that nosocomial influenza was associated with mortality31.
Having received seasonal influenza vaccine was associated with a higher risk of death in the crude analysis but not in the adjusted analysis. This finding could be explained because vaccinated could attend to the hospital more frequently than do unvaccinated subjects as suggested by Casado et al.32 in a study carried out in older adults in 2013–2014 and 2014–2015 season. However, residual confounding by comorbid conditions could not be ruled out as suggested by other authors33 because patients with comorbidities (79% in our study) are more likely to access healthcare resources and subsequently have more opportunities to receive the influenza vaccine. On the other hand, as suggested by Gutiérrez-González et al.17 because only hospitalized severe cases are included in the study, the protection for fatal episodes might be underestimated.
We should also highlight that influenza B virus was the most prevalent in the 2017–2018 influenza season in Catalonia and that the effectiveness of vaccination was only 14% (95%CI 0–47) against influenza B virus cases because the circulating lineage Yamagata was not included in vaccines used14.
NAI treatment was a preventive factor against death in all patients considered globally, in the age groups studied, and in influenza A and B virus cases, as other authors have reported20. However, some authors34,35 found no association between NAI treatment and death. Groenveld et al., in a study carried out in the Netherlands in the 2013–2016 seasons, found that NAI did not avoid death during the hospital stay but did avoid a composite endpoint including 30-day mortality and ICU admission36.
In patients with pneumonia, we also found that NAI treatment was a protective factor against death, without differences according to the influenza virus type. Chen et al., in a study carried out in influenza A-related pneumonia cases in China from 2013 to 2018, found that early NAI treatment was protective against death37 and that protection was higher in influenza B-related pneumonia cases38. Zou et al. found no association between NAI treatment and death and proposed that a double dose of oseltamivir should be administered in patients with pneumonia13.
The proportion of hospitalized severe influenza cases admitted to the ICU was 16.6% (17.8% for influenza A cases and 15.9% for influenza B cases), figures close to the 15.4% (23% for influenza A cases and 12.7% for influenza B cases) obtained by Stahl et al.39 in Germany in the same influenza season, and the 13.4% for influenza A cases and 17.8% for influenza B cases obtained by Assaf-Casals et al.24 in Lebanon during 2008–2016. Other authors studying severe patients have found higher figures29, but the differences in criteria for hospitalization might, at least in part, explain the differences.
The highest proportion of ICU admission was found in the 50–64 years age group, and declined thereafter, with the lowest proportion (10.4%) in the ≥ 75 years age group. This finding was also observed by Oliva et al. in a study carried out in hospitalized severe cases in Spain in the 2010–2011 to 2015–2016 seasons, who found that the 15–64 years age group had the highest proportion of ICU admission19. Beumer et al. found differences in ICU admission according to age: the highest proportion was in the 50–65 years age group and the proportion fell in patients aged ≥ 65 years30. A French study by Pivette et al. in 2012–2017 also found a reduction in ICU admissions in elderly patients with influenza40.
Mazagatos et al.41 in a Spanish study carried out in the 2010–2011 to 2015–2016 seasons found that 38.9% of hospitalized pregnant women who presented severe influenza were admitted to the ICU, but only 7 pregnant women were included in our study with 2 (29%) requiring ICU admission, and therefore this finding cannot be evaluated. In a systematic review by Mertz et al.23 pregnancy as a risk factor was not well studied because of the 56 studies included in the revision only one study having data on this. Coinciding with Walker et al.25, ICU admission was associated with cardiovascular disease in patients aged 65–74 years. Our results and those of Beumer et al. in a study carried out in 2015–2016 in the Netherlands also show a positive association between cardiovascular disease and the risk of ICU admission30.
We found that morbid obesity was not associated with ICU admission or death, these findings were also observed by Atamna el al. in the study carried out in Israel in 2017–2018 season42. In contrast with the results found by Segaloff et al. in a study carried out in Michigan in seasons 2012–2012 and 2012–2013 in adults43. Chronic vascular disease in the 65–74 years age group and morbid obesity in influenza A cases were associated with ICU admission, in contrast to Beumer et al., who found no association with obesity30.
ICU admission was required in 18.1% of patients with influenza-related pneumonia, lower than the 22.4% obtained in Chinese studies by Chen et al. during 2013–201944 and Zou et al. in the 2027–2018 season13. As in all patients considered globally, we found that in patients with pneumonia ICU admission was negatively associated with older age.
When death was the considered outcome, NAI treatment, whether administered in the first 48 h after symptom onset or > 48 h after symptom onset had a protective effect. However, NAI treatment administered > 48 h after symptom onset was associated with ICU admission, a result found in a previous study of including six influenza seasons45; this might be because the severity of patients candidates for ICU admission are treated with NAI if they had not been treated before.
Influenza causes a range of outcomes in children, including severe disease, ICU admission and death3,9. The case fatality rates of influenza in children range between 0 and 4.9%6. In the present study, no deaths were registered in children and no differences in the severity, measured as ICU admission, was found between influenza A and B viruses, coinciding with the findings of other authors46,47.
This study, like all observational studies, has strengths and limitations. The main strength is that there are few studies comparing specific outcomes of severity (death on the one hand and ICU admission on the other) and, therefore, our results may help to identify differences between the two outcomes. Another strength is that all cases were laboratory-confirmed and, therefore, the distribution of independent variables corresponded to real severe influenza cases.
The first limitation is that hospitals participated voluntarily, which could lead to selection bias. However, because the hospitals participating in the surveillance system of severe cases cover more than 66% of the Catalan population, the proportion of patients living in municipalities of ≤ 10,000 inhabitants is 12.8%, similar to the total of Catalonia, where the proportion of the population living in municipalities of ≤ 10,000 inhabitants is 18.5% and we used a mixed-effect logistic regression model with hospitals as a random intercept, we believe the results maybe extensible to hospitalized severe cases in Catalonia. Another limitation is that we only studied one influenza season, when the inclusion of several seasons is recommended due to differences in circulating types and subtypes from one season to another. However, because the 2017–2018 influenza season showed high severity, determining the outcomes of severity in this specific season may be interest. A third limitation is that the small number of pregnant women and children did not permit any conclusions about these two key risk groups for influenza severity.
The final adjusted model was constructed taking into account the propensity score built with variables that included the most important comorbidities, and it seems unlikely that the results suffered a large bias. Nevertheless, some residual confounding cannot be ruled out.
In conclusion, our results support the need to investigate the worst outcomes in hospitalized severe cases of influenza, distinguishing between death and ICU admission. We found that older age was a risk factor for death but was negatively associated with ICU admission. By contrast, while NAI treatment both before and after 48 h of symptom onset prevented death, NAI treatment ≥ 48 h after symptom onset was more frequent in patients admitted to the ICU, probably reflecting medical attitudes to patients requiring ICU admission.
Material and methods
Study design
An observational, epidemiological case-to-case study was carried out in patients hospitalized due to severe acute influenza infection. A severe case of laboratory-confirmed influenza virus infection was defined as a case requiring hospitalization due to pneumonia, septic shock, multi-organ failure, acute respiratory distress, death or any other severe condition including ICU admission, or who developed these clinical signs during hospitalization for other reasons8. The diagnosis was confirmed by RT-PCR and/or culture on nasopharyngeal swab samples.
Respiratory tract samples were processed at each hospital laboratory within 24 h of receipt. A 300 μl aliquot was taken for total nucleic acid extraction and eluted in 25 μl of RNase-free elution buffer using the automatic QIAsymphony system (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Subsequently, two specific one-step multiplex real-time PCR using Stratagene Mx3000P QPCR Systems (Agilent Technologies, Santa Clara, CA, USA) were carried out for typing A/B influenza virus (sensitivity was 10 and 103 copies/μl, respectively) and subtyping influenza A virus (sensitivity was 102, 103 and 10 copies/μl for H1, H3 and H5 RNA, respectively)48. For H1 positive detections to identify H1N1pdm09, specific in-house primers were used.
Data collected
Reported cases of severe laboratory-confirmed influenza requiring hospitalization in the 2017–2018 influenza season were included.
For each reported case, trained interviewer collected the following variables: demographic data (age, sex, size of municipally), medical conditions (COPD, morbid obesity [BMI > 40 kg/m2 to classify obesity for adults and for children obesity is defined as a BMI at or above the 95th percentile for children and teens of the same age and sex], diabetes, chronic renal disease, immune deficiency [HIV infection or other], chronic cardiovascular disease, chronic liver disease, hemoglobinopathy, severe neuromuscular disease or cognitive dysfunction), and clinical data (date of symptom onset, type of virus [A or B], pneumonia, seasonal influenza vaccination status, antiviral treatment with NAI and its date, ICU admission and in-hospital mortality). Cases were considered vaccinated if they had received a dose of influenza vaccine ≥ 14 days before symptom onset. The primary source of information was the medical record.
Statistical analysis
We assessed the associations between death or UCI admission and the independent variables (sociodemographic variables, virus type and clinical characteristics). Possible interactions between independent variables were analyzed by logistic regression. Independent variables were checked for collinearity using the variance inflation factor.
Crude and adjusted odds ratio (OR) and their corresponding 95% confidence intervals (CI) were constructed using a mixed-effects logistic regression model with the variable hospital as a random intercept. Adjusted OR (aOR) were calculated using propensity scores, which were estimated by logistic regression with age, sex, comorbidities, pneumonia, seasonal influenza vaccination, type of virus and NAI treatment as independent variables. The propensity score was used as a continuous covariate in the mixed-effects logistic regression model.
The analysis was performed using the SPSS v.25 statistical package and R v3.5.0 statistical software (http://cran.r-project.org).
Ethical considerations
Data used in the analysis were collected under routine public health surveillance activities as part of the legislated mandate of the Health Department of Catalonia, the competent authority for the surveillance of communicable diseases, which is officially authorized to receive, treat and temporally store personal data on cases of infectious diseases49. All data were fully anonymized. All study activities formed part of public surveillance and were thus exempt from institutional board review of Health Department of Government of Catalonia and did not require informed consent.
Data availability
The data are available from the corresponding author on reasonable request.
Change history
12 October 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41598-021-99937-y
References
Treanor, J. J. Influenza viruses, including avian influenza and swine influenza. In Principles and Practice of Infectious Diseases 9th edn (eds Bennett, J. E. et al.) 2143–2176 (Elsevier, 2020).
World Health Organization. Influenza (Seasonal). (Accessed 15 Oct 2020) https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal) (2018).
Chaudhari, P. P., Monuteaux, M. C., Pannaraj, P. S., Khemani, R. G. & Bachur, R. G. Age-stratified risk of critical illness in young children presenting to the emergency department with suspected influenza. J. Pediatr. 215, 132–138 (2019).
Wong, P. L. et al. The effect of age on clinical characteristics, hospitalization and mortality of patients with influenza-related illness at a tertiary care centre in Malaysia. Influenza. Other. Respir. Viruses. 14, 286–293 (2020).
Martínez, A. et al. Risk factors associated with severe outcomes in adult hospitalized patients according to influenza type and subtype. PLoS ONE 14, e0210353 (2019).
Tuckerman, J., Misan, S., Crawford, N. W. & Marshall, H. S. Influenza in children with special risk medical conditions: A systematic review and meta-analysis. Pediatr. Infect. Dis. J. 38, 912–919 (2019).
Basile, L. et al. Seasonal influenza surveillance: Observational study on the 2017–2018 season with predominant B influenza virus circulation. Vacunas 20, 53–59 (2019).
Sub-direcció General de Vigilància i Resposta a Emergències de Salut Pública. Pla d’actuació a Catalunya enfront d’una infecció per virus de la grip en fase post-pandèmica. Barcelona (Spain): Agència de Salut Pública de Catalunya. (Accessed 21 Oct 2020) https://salutpublica.gencat.cat/web/.content/minisite/aspcat/vigilancia_salut_publica/20180118_grip_postpan_2010.pdf (2018).
Doyle, J. D. & Campbell, A. O. Pediatric influenza and illness severity: What is known and what questions remain?. Curr. Opin. Pediatr. 31, 119–126 (2019).
Fu, X. et al. Clinical characteristics and outcomes during a severe influenza season in China during 2017–2018. BMC. Infect. Dis. 19, 668 (2019).
Gavigan, P. & McCullers, J. A. Influenza: Annual seasonal severity. Curr. Opin. Pediatr. 31, 112–118 (2019).
Nateghian, A. et al. Demographic, clinical, and virological characteristics of patients with a laboratory-confirmed diagnosis of influenza during three consecutive season, 2015/2016–2017/2018, in the Islamic Republic of Iran. J. Clin. Virol. 124, 104281 (2020).
Zou, Q. et al. Influenza A-associated severe pneumonia in hospitalized patients: Risk factors and NAI treatments. Int. J. Infect. Dis. 92, 208–213 (2020).
Sub-direcció General de Vigilància i Resposta a Emergènies de Salut Pública. Pla d’informació de les infeccions respiratòries agudes a Catalunya. Balanç de la temporada gripal 2017–2018. (Accessed 26 March 2021) https://scientiasalut.gencat.cat/bitstream/handle/11351/4321/pidirac_pla_informacio_infeccions_respiratories_agudes_catalunya_balan%c3%a7_temporada_gripal_2017_2018.pdf?sequence=1&isAllowed=y (Agència de Salut Pública de Catalunya, 2018).
Liu, W. D., Yeh, C. Y., Shih, M. C. & Sheng, W. H. Clinical manifestations and risk factors for mortality of patients with severe influenza during the 2016–18 season. Int. J. Infect. Dis. 95, 347–351 (2020).
Kolosova, N. P. et al. Severe cases of seasonal influenza in Russia in 2017–2018. PLoS ONE 14, e0220401 (2019).
Gutiérrez-González, E. et al. Effect of vaccination, comorbidities and age on mortality and severe disease associated with influenza during the season 2016–2017 in a Spanish tertiary hospital. J. Infect. Public. Health. 12, 486–491 (2019).
Hauge, S. H., Bakken, I. J., de Blasio, B. F. & Haberg, S. E. Burden of medically attended influenza in Norway 2008–2017. Influenza. Other. Respir. Viruses. 13, 240–247 (2019).
Oliva, J. et al. Estimating the burden of seasonal influenza in Spain from surveillance of mild and severe influenza disease, 2010–2016. Influenza. Other. Respir. Viruses. 12, 161–170 (2018).
Tekin, S. et al. Predictors of fatality in influenza A virus subtype infections among inpatients in the 2015–16 season. Int. J. Infect. Dis. 81, 6–9 (2019).
Barnes, S. T. et al. Mortality estimates among adult patients with severe acute respiratory infections from two sentinel hospitals in southern Arizona, United States, 2010–2014. BMC. Infect. Dis. 18, 78 (2018).
Wu, P. et al. A joint analysis of influenza-associated hospitalizations and mortality in Hong Kong, 1998–2013. Sci. Rep. 7, 929 (2017).
Mertz, D. et al. Populations at risk for severe or complicated influenza illness: Systematic review and meta-analysis. BMJ 347, f5061 (2013).
Assaf-Casals, A. et al. The burden of laboratory-confirmed influenza infection in Lebanon between 2008 and 2016: A single tertiary care center experience. BMC. Infect. Dis. 20, 339 (2020).
Walker, T. A. et al. Risk of severe influenza among adults with chronic medical conditions. J. Infect. Dis. 221, 183–190 (2020).
Adlhoch, C. et al. Determinants of fatal outcome in patients admitted to intensive care units with influenza, European Union 2009–2017. Open. Forum. Infect. Dis. 6, ofz462 (2019).
Pecego, A. C. et al. Etiology, clinical, and epidemiological characteristics of severe respiratory infection in people living with HIV. Int. J. STD. AIDS. 31, 100–108 (2020).
Czaja, C. A. et al. Age-related differences in hospitalization rates, clinical presentation, and outcomes among older adults hospitalized with influenza-U.S. Influenza hospitalization Surveillance Network (FluSurv-NET). Open. Forum. Infect. Dis. 6, ofz225 (2019).
Lina, B. et al. Complicated hospitalization due to influenza: Results from the Global Hospital Influenza Network for the 2017–2018 season. BMC. Infect. Dis. 20, 465 (2020).
Beumer, M. C. et al. Influenza virus and factors that are associated with ICU admission, pulmonary co-infections and ICU mortality. J. Crit. Care. 50, 59–65 (2019).
Naudion, P., Lepiller, Q. & Bouiller, K. Risk factors and clinical characteristics of patients with nosocomial influenza A infection. J. Med. Virol. 92, 1047–1052 (2020).
Casado, I. et al. Repeated influenza vaccination for preventing severe and fatal influenza infection in older adults: A multicenter case-control study. CMJA. 190, E3–E12 (2018).
Zivich, P. N. et al. Influenza vaccination status and outcomes among influenza-associated hospitalizations in Columbus, Ohio (2012–2015). Epidemiol. Infect. 145, 3284–3292 (2017).
Fica, A. et al. Severe acute respiratory infections (SARI) from influenza in adult patients in Chile: The experience of a sentinel hospital. Rev. Panam. Salud. Pública. 43, e1 (2019).
Dou, L. et al. Decreased hospital length of stay with early administration of oseltamivir in patients hospitalized with influenza. Mayo. Clin. Proc. Innov. Qual. Outcomes. 4, 176–182 (2020).
Groeneveld, G. H. et al. Effectiveness of oseltamivir in reduction of complications and 30-day mortality in severe seasonal influenza infection. Int. J. Antimicrob. Agents. 56, 106155 (2020).
Chen, L., Han, X., Li, Y. L., Zhang, C. & Xing, X. The impact of early neuraminidase inhibitor therapy on clinical outcomes in patients hospitalised with influenza A-related pneumonia: A multicenter, retrospective study. BMC. Infect. Dis. 20, 628 (2020).
Chen, L., Han, X., Li, Y. L., Zhang, C. & Xing, X. Impact of early neuraminidase inhibitor treatment on clinical outcomes in patients with influenza B-related pneumonia: A multicenter cohort study. Eur. J. Clin. Microbiol. Infect. Dis. 39, 1231–1238 (2020).
Stahl, K. et al. Maintenance immunosuppression is associated with better outcome in the 2017/2018 influenza epidemic. Open. Forum. Infect. Dis. 6, ofz381 (2019).
Pivette, M., Nicolay, N., de Lauzun, V. & Hubert, B. Characteristics of hospitalizations with an influenza diagnosis, France, 2012–2013 to 2016–2017 influenza seasons. Influenza. Other. Respir. Viruses. 14, 340–348 (2020).
Mazagatos, C. et al. Exploring the risk of severe outcomes and the role of seasonal influenza vaccination in pregnant women hospitalized with confirmed influenza, Spain, 210/11–2015/16. PLoS ONE 13, e0200934 (2018).
Atamna, A. et al. The impact of obesity on seasonal influenza: A single-center, retrospective study conducted in Israel. Eur. J. Clin. Microbiol. Infect. Dis. https://doi.org/10.1007/s10096-021-04174-w (2021) (in Press).
Segaloff, H. E. et al. The impact of obesity and timely antiviral administration on severe influenza outcomes among hospitalized adults. J. Med. Virol. 90, 212–218 (2018).
Chen, L., Han, X., Li, Y. L., Zhang, C. & Xing, X. Severity and outcomes of influenza-related pneumonia in type A and B strains in China, 2013–2019. Infect. Dis. Poverty. 9, 42 (2020).
Domínguez, A. et al. Effectiveness of antiviral treatment in preventing death in severe hospitalised influenza cases over six seasons. Epidemiol. Infect. 146, 799–808 (2018).
Jané, M. et al. Epidemiological and clinical characteristics of children hospitalized due to influenza A and B in the south of Europe, 2010–2016. Sci. Rep. 9, 12853 (2019).
Mattila, J. M., Vuorinen, T. & Heikkinen, T. Comparative severity of influenza A and B infections in hospitalized children. Pediatr. Infect. Dis. J. 39, 489–493 (2020).
Suwannakarn, K. et al. Typing (A/B) and subtyping (H1/H3/H5) of influenza A viruses by multiplex real-time RT-PCR assays. J. Virol. Methods. 152, 25–31 (2008).
de Catalunya, G. Decret 2013/2015, de 15 de setembre, pel qual es crea la Xarxa de Vigilància Epidemiològica i es regulen els sistemes de notificació de malalties de declaració obligatòria i els brots epidèmics. DOGC. 6958, 1–19 (2015).
Funding
This study was supported by the Programme of Prevention, Surveillance and Control of Transmissible Diseases (PREVICET), CIBER de Epidemiología y Salud Pública (CIBERESP), Instituto de Salud Carlos III, Madrid and the Catalan Agency for the Management of Grants for University Research (AGAUR Grant Number 2017/SGR 1342). The funders had no role in the study design, data collection and analysis, the decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Consortia
Contributions
All the authors participated in the study design, implementation and interpretation. A.D. and N.S. designed the study and drafted the report. N.S. conducted the statistical analysis. L.A., A.N., P.G., N.T., C.R. and M.J. designed and supervised the study, and reviewed the draft report. The authors in The Working Group on Surveillance of Hospitalized Cases of Severe Influenza in Catalonia have participated in the detection of cases, gathering epidemiological information and taking clinical samples and sending samples to the laboratory. All authors reviewed and approved the final version of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: G. Mena and L. Matas were omitted from the Surveillance of Hospitalized Cases of Severe Influenza in Catalonia Working Group in the original version of this Article.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Soldevila, N., Acosta, L., Martínez, A. et al. Behavior of hospitalized severe influenza cases according to the outcome variable in Catalonia, Spain, during the 2017–2018 season. Sci Rep 11, 13587 (2021). https://doi.org/10.1038/s41598-021-92895-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-92895-5
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.