Abstract
Atherosclerosis has been considered as the main cause of morbidity, mortality, and disability worldwide. The first screening for antigen markers was conducted using the serological identification of antigens by recombinant cDNA expression cloning, which has identified adaptor-related protein complex 3 subunit delta 1 (AP3D1) as an antigen recognized by serum IgG antibodies of patients with atherosclerosis. Serum antibody levels were examined using the amplified luminescent proximity homogeneous assay-linked immunosorbent assay (AlphaLISA) using a recombinant protein as an antigen. It was determined that the serum antibody levels against AP3D1 were higher in patients with acute ischemic stroke (AIS), transient ischemic attack, diabetes mellitus (DM), cardiovascular disease, chronic kidney disease (CKD), esophageal squamous cell carcinoma (ESCC), and colorectal carcinoma than those in the healthy donors. The area under the curve values of DM, nephrosclerosis type of CKD, and ESCC calculated using receiver operating characteristic curve analysis were higher than those of other diseases. Correlation analysis showed that the anti-AP3D1 antibody levels were highly associated with maximum intima-media thickness, which indicates that this marker reflected the development of atherosclerosis. The results of the Japan Public Health Center-based Prospective Study indicated that this antibody marker is deemed useful as risk factors for AIS.
Similar content being viewed by others
Introduction
According to the reports presented to date, atherosclerosis, a chronic inflammatory injury of the arterial wall, may lead to the complications including acute ischemic stroke (AIS) and cardiovascular disease (CVD)1,2,3. Thus, atherosclerosis has been identified as the leading cause of morbidity and mortality worldwide4,5. It was reported that diabetes mellitus (DM) and chronic kidney disease (CKD) have causal roles in induction of atherosclerosis6,7. Atherosclerosis and cancer share many ethiological and mechanistical processes8. Thus, these atherosclerosis-related diseases including AIS, CVD, DM, CKD, and cancer are interrelated more or less with each other. Although many risk factors and biomarkers have been identified for these atherosclerosis-related diseases9,10, their specificity and commonality remain obscure.
Serological analysis of recombinant cDNA expression libraries (SEREX)11,12 is one of the most effective and comprehensive methods to identify antigenic targets for various types of malignant tumors in humans; in fact, it has been used to determine more than 1000 novel tumor antigens11,12. In this study, we were able to find that the serum antibody levels against some of the SEREX antigens were elevated in patients compared with healthy donors (HDs) and reported TROP2, SLC2A1, TRIM21, and myomegalin as antibody biomarkers for esophageal squamous cell carcinoma (ESCC)13,14,15,16. SEREX was also introduced in screening the biomarkers for atherosclerosis and identified antibody biomarkers such as RPA217, PDCD1118,19, MMP1, CBX1, CBX520, DNAJC221, ASXL222, and LRPAP123 for atherosclerotic diseases including AIS and CVD. Notably, the antibody levels against DHPS, ATP2B4, BMP-1, ASXL2, and LRPAP1 were also elevated in patients with ESCC, which suggests the presence of multiple common biomarkers for atherosclerosis and cancer.
In this study, using the SEREX method screening, adaptor-related protein complex 3 subunit delta 1 (AP3D1) was identified as a target antigen recognized by serum IgG antibodies in the sera of patients with atherosclerosis. Next, to evaluate the specificity and commonality among atherosclerosis-related diseases, the levels of serum anti-AP3D1 antibodies in patients with AIS, DM, CVD, CKD and solid cancer were examined.
Methods
Patients and HDs' sera
This study was approved by the Local Ethical Review Board of Chiba University, Graduate School of Medicine (Chiba, Japan) and the review boards of the participating hospitals. All experimental procedures were performed in accordance with the Declaration of Helsinki.
Blood samples were collected from patients who had provided their informed consent. Each serum sample was centrifuged at 2000×g for 10 min at 4°C, and the supernatant was stored at −80°C until use. Repeated thawing and the freezing of samples were avoided.
Serum samples of patients with AIS, transient ischemic attack (TIA), deep and subcortical white matter hyperintensity (DSWMH), asymptomatic cerebral infarction (asymptCI), and chronic-phase cerebral infarction (cCI) were provided by the Chiba Prefectural Sawara Hospital, Chiba Rosai Hospital, and Chiba Aoba Municipal Hospital, and sera of patients with DM and CVD were obtained from Chiba University Hospital. The stroke subtype of each patient was determined according to the criteria of the Trial of Org 10,172 in Acute Stroke Treatment classification system24. In this study, large-artery atherosclerosis or small-artery occlusion (lacune) were included as AIS or cerebral infarction. Serum samples associated with AIS, TIA, and CVD were obtained within 2 weeks after disease onset. Sera of CKD patients were obtained from the Kumamoto cohort25,26, and Chiba University Hospital provided the serum samples of patients with ESCC and colorectal carcinoma (CRC). Sera of HDs were obtained from Chiba University Hospital, Port Square Kashiwado Clinic, and Chiba Prefectural Sawara Hospital. Sample of HDs from Port Square Kashiwado Clinic and Chiba Prefectural Sawara Hospital were selected from individuals who exhibited no abnormalities in cranial magnetic resonance imaging.
Immune screening: serological identification of antigens by recombinant cDNA expression cloning
Initial screening was conducted using the SEREX method as has been described previously13,14,15,16,17,27,28,29. Sera of patients with atherosclerosis were used to search for antigens that could be recognized by the serum IgG antibody. The library used was a Uni-ZAP XR cDNA phage library containing a human microvascular endothelial cell cDNA library (Stratagene; Agilent Technologies, Inc., La Jolla, CA), which was infected into Escherichia coli (E. coli) XL1-Blue MRF′. Proteins were then transferred onto nitrocellulose membranes [NitroBind, Osmonics Inc., Minnetonka, MN)], which were pretreated with 10 mM isopropyl-β-D-thiogalactoside (IPTG) (Wako Pure Chemicals, Osaka, Japan) for 30 min. The membranes were blocked for 1 h with 1% protease-free bovine serum albumin (Wako Pure Chemicals), 20 mM Tris–HCl (pH 7.5), 0.15 M NaCl, and 0.05% Tween-20, treated overnight to 1:2000 diluted sera from the patients, and then incubated for 1 h with 1:5000 diluted alkaline phosphatase-conjugated goat anti-human IgG (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA). Finally, using a color development solution [0.3 mg/ml nitroblue tetrazolium (Wako Pure Chemicals), 0.15 mg/ml 5-bromo-4-chloro-3-indolyl-phosphate (Wako Pure Chemicals), 100 mM Tris–HCl (pH 9.5), 100 mM NaCl, and 5 mM MgCl2], the positive reactions could be identified. The resulting positive antibody were then re-cloned twice in order to obtain monoclonality13,14,15,16,17,27,28,29.
Sequence analysis of identified clones
Monoclonal phage cDNA clones were converted to pBluescript phagemids by in vivo excision using the ExAssist helper phage (Stratagene; Agilent Technologies, La Jolla, CA). Plasmid DNA was obtained from the E. coli SOLR strains transformed by the phagemids. Homology search of the inserted and sequenced cDNAs using a public database provided by the the National Center for Biotechnology Information (https://blast.ncbi.nlm.nih.gov/Blast.cgi) identified the genes.
AP3D1 protein expression, extraction, and purification
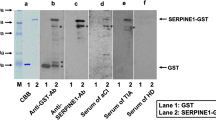
The region of 2490–4347 of the AP3D1 gene was isolated and was recombined into the EcoRI/NotI site of pGEX-4T-1 (GE Healthcare Life Sciences, Pittsburgh, PA), followed by confirmation by DNA sequencing. The E. coli BL-21 transfected with pGEX-4T-1-AP3D1 was then treated with 0.1 mM IPTG at 37°C for 3 h to induce the expression of cDNA products. Then, the cells were lysed in BugBuster Master Mix (Merck KGaA, Darmstadt, Germany). With the Glutathione-Sepharose (GE Healthcare Life Sciences, Pittsburgh, PA) column chromatography according to the manufacturer's instructions, glutathione S‑transferase (GST)‑fused-AP3D1 protein was purified, as has been described previously17,19,20.
AlphaLISA (amplified luminescence proximity homogeneous assay-linked immunosorbent assay)
To evaluate the serum antibody levels, AlphaLISA was used. Initially, AlphaLISA was performed in 384-well microtiter plates (white opaque OptiPlate, PerkinElmer, Waltham, MA, USA) containing either 2.5 µl of 1:100 diluted serum or 2.5 µl of 10 µg/ml of GST and GST-AP3D1 protein (25 mM HEPES, pH 7.4, 0.1% casein, 0.5% Triton X-100, 1 mg/ml dextran-500 and 0.05% Proclin-300). The reaction mixture was then incubated at room temperature for 6–8 h. Secondly, after adding anti-human IgG-conjugated acceptor beads (2.5 µl at 40 µg/ml) and glutathione-conjugated donor beads (2.5 µl at 40 µg/ml), the mixture was further incubated at room temperature in the dark for 7–14 days. Chemical emissions were read on an EnSpire Alpha microplate reader (PerkinElmer) as previously described18,19,20,21,22,23,30. Specific reactions were then calculated by subtracting the alpha photon counts of the GST and buffer control from the counts of the GST-AP3D1 protein.
JPHC cohort analysis
The longitudinal association between plasma AP3D1 levels (using the above AlphaLISA detection antibody levels) and incident AIS were examined in the Japan Public Health Center-based prospective Study (JPHC). The study nested within JPHC cohort31,32, involving approximately 30,000 Japanese individuals aged 40–69 years at the baseline period of 1990–1994 whose plasma were stored. The antibody levels of AP3D1 protein were measured in 202 cases of AIS in the cohort developed between the baseline and 2008, and in 202 controls whose sex, age (within 2 years), date of blood sampling (within 3 months), time since last meal (within 4 h) and study location (Public Health Center area) were matched with the cases. We used a conditional logistic regression model to estimate the odds ratios (ORs) and 95% confidence intervals (CIs) for AIS with respect to the antibody levels of AP3D1 protein.
Statistical analysis
All statistical analyses were conducted using GraphPad Prism 5 (GraphPad Software, La Jolla, CA) and EZR software33. The Kruskal–Wallis test (Mann–Whitney U test with Bonferroni's correction applied) was used to evaluate differences among > 3 groups, and the Mann–Whitney U test was employed to determine significant differences between the two groups. Correlations were calculated using Spearman's correlation analysis and logistic regression analysis. The predictive values of the putative disease markers were assessed using a receiver operating characteristic (ROC) curve analysis, and the cutoff values were set to maximize the sums of sensitivity and specificity. All tests were two-tailed, and P values lower than 0.05 were considered to be statistically significant. The power calculation was performed using G-Power 3.1 software (Heinrich-Heine-Universität Düsseldorf).
Ethics declarations
The present study was approved by the Local Ethical Review Board of Chiba University, Graduate School of Medicine (Chiba, Japan) as well as the review boards of co-operating hospitals or institutes. Serum or plasma was collected from participants who had provided informed consent by following the protocols approved by their institutional ethical committees.
Consent for publication
Not applicable.
Results
Initial screening of AP3D1 antigens using SEREX
As an initial SEREX screening, sera of patients with atherosclerosis were used to search for antigens that could be recognized by serum IgG antibodies, one of which was AP3D1 (accession no. NM_003938.8). The region of 2490–4347 of the AP3D1 gene was then isolated and recombined into the EcoRI/NotI site of pGEX-4T-1, followed by confirmation by DNA sequencing. The cDNA was then expressed in E. coli, purified by affinity chromatography, and employed as an antigen in order to examine the serum antibody levels.
The levels of anti-AP3D1 antibodies were elevated in patients with AIS and TIA
The serum anti-AP3D1-antibody (s-AP3D1-Ab) levels in patients with AIS and TIA were examined using AlphaLISA. AIS and TIA sera were provided by Chiba Prefectural Sawara Hospital, Chiba Rosai Hospital, and Chiba Aoba Municipal Hospital, whereas samples of HDs were obtained from Chiba University, Port Square Kashiwado Clinic, and Chiba Prefectural Sawara Hospital. The average ages [± standard deviations (SDs)] of the HDs and patients with AIS and TIA were 51.85 ± 8.74, 57.99 ± 7.97, and 69.45 ± 11.64 years, respectively (Table 1 upper panel). The levels of s-AP3D1-Abs were determined to be significantly higher in patients with AIS and TIA than those in HDs (Fig. 1a). When the ages of the subjects were matched to 65 years, the s-AP3D1-Ab levels were still significantly higher in patients with AIS than those in HDs (Supplementary Fig. S1a). At a cutoff value equivalent to the average plus two SDs of the HD values, the s-AP3D1-Ab-positive rates in HDs and patients with AIS and TIA were 2.4%, 10.1%, and 10.4%, respectively (Table 1 lower panel). ROC curve analysis revealed that the area under the curve (AUC) values for s-AP3D1-Abs vs. AIS and vs. TIA were 0.616 and 0.662, respectively (Fig. 1b,c). No significant difference was found in the positive rates and the AUC values between AIS and TIA.
Comparing the serum AP3D1-antibody levels between healthy donors (HDs) and patients with AIS and TIA. This figure shows the levels of serum antibodies against AP3D1 (s-AP3D1-Abs) examined using amplified luminescence proximity homogeneous assay-linked immunosorbent assay (AlphaLISA) (a). The bars represent the average and average ± standard deviation (SD). P-values were calculated using the Kruskal–Wallis test (Mann–Whitney U test with Bonferroni's correction applied). ***P < 0.001. The total average values, SDs, cutoff values, positive numbers, positive rates (%), and P-values are summarized in Table 1. A receiver operating characteristic (ROC) curve analysis was performed to assess the abilities of s-AP3D1-Abs in detecting either (b) acute ischemic stroke (AIS) or (c) transient ischemic attack (TIA). The numbers in the figures indicate the cutoff values for marker levels, and the numbers in parentheses indicate sensitivity (left) and specificity (right). P value, area under the curve (AUC), and 95% confidence intervals (95% CI) are also shown. AP3D1, adaptor-related protein complex 3 subunit delta 1. The results of the power calculation using G-Power 3.1 software were 0.9762618 for AIS and 0.9953412 for TIA (Supplementary Table S3).
Elevation of s-AP3D1-Abs levels in patients with DM
The levels of s-AP3D1-Abs were also examined for DM. Sera of HD were obtained from Chiba University, whereas the sera of patients with DM were provided by the Chiba University Hospital. The average ages (± SDs) of the HDs and patients with DM were 45.20 ± 10.95 and 63.12 ± 12.04 years, respectively. The AlphaLISA results revealed that s-AP3D1-Ab levels were significantly higher in patients with DM than in the HDs (Fig. 2a). When the levels of s-AP3D1-Abs were compared between age-matched (60 years) HDs and patients with DM, the levels were also significantly higher in patients with DM than in HDs (Supplementary Fig. S1b). When the positive samples for which the AlphaLISA counts exceeded the cutoff value were scored, the positive rates of s-AP3D1-Abs in the HDs and the patients with DM were 3.7% and 41.8%, respectively (Table 2). The AUC value of s-AP3D1-Abs vs. DM was as high as 0.791 (Fig. 2b). Therefore, it can be concluded that the s-AP3D1-Ab levels were closely associated with DM.
Comparing the levels of serum antibodies against AP3D1 between HDs and patients with DM. The s-AP3D1-Abs levels in HDs and patients with diabetes mellitus (DM) examined using AlphaLISA are shown (a). The bars represent the average and average ± SD. P-values were calculated using the Mann–Whitney U test. ***P < 0.001. The data are summarized in Table 2. ROC curves to assess the ability of s-AP3D1-Abs to predict DM are shown (b). The numbers in the figures are the same as those shown in Fig. 1. The results of the power calculation were shown in Supplementary Table S3.
The s-AP3D1-Abs levels were associated with CVD
For the next step, the antibody levels in samples from CVD patients were examined. The samples of CVD patients were obtained from Chiba University Hospital, and those in HDs were from Chiba University, Port Square Kashiwado Clinic, and Chiba Prefectural Sawara Hospital. The average ages (± SDs) of the HDs and CVD patients were 45.27 ± 11.20 and 66.07 ± 11.32 years, respectively. Compared with HDs, s-AP3D1-Abs levels were significantly higher in patients with CVD (Fig. 3a), and the s-AP3D1-Ab positivity rates in HDs and patients with CVD were 5.1% and 24.0%, respectively (Table 3). ROC curve analysis revealed that AUC of s-AP3D1-Abs for CVD was 0.758 (Fig. 3b).
Comparing the serum AP3D1-Ab levels between HDs and cardiovascular disease (CVD) patients. This figure shows the s-AP3D1-Ab levels in HDs and CVD patients examined using AlphaLISA (a). The bars represent the average and average ± SD. P-values were calculated using the Mann–Whitney U test. ***P < 0.001. The data are summarized in Table 3. ROC curves to assess the ability of s-AP3D1-Abs to predict CVD are shown (b). The numbers in the figures are the same as those shown in Fig. 1. The results of the power calculation were shown in Supplementary Table S3.
The s-AP3D1-Ab levels were closely related to CKD
The antibody levels in the sera of CKD patients were examined, which were assumed to be closely related to atherosclerosis. The sera of the CKD patients were obtained from the Kumamoto cohort22,23, including 145 from patients with diabetic kidney disease (type 1 CKD), 32 from patients with nephrosclerosis (type 2 CKD), and 123 from patients with glomerulonephritis (type 3 CKD). The sera of HDs (82 specimens) were obtained from Chiba University, Chiba Prefectural Sawara Hospital, and the National Hospital Organization of Shimoshizu Hospital. Patients from all three groups of CKD were found to have significantly higher levels of s-AP3D1-Abs compared to that in HDs (Fig. 4a). The s-AP3D1-Ab-positive rates in HDs and patients with type 1, type 2, and type 3 CKD were 4.9%, 27.6%, 37.5%, and 22.8%, respectively (Table 4). ROC curve analysis revealed AUC of s-AP3D1-Abs of type 1, type 2, and type 3 CKD to be 0.791, 0.874, and 0.735, respectively (Fig. 4b–d). Type 2 CKD showed the highest AUC value among all diseases examined.
Comparing the serum AP3D1-antibody levels between HDs and patients with chronic kidney disease (CKD). (a) The s-AP3D1-Ab levels were compared between HDs and patients with diabetic CKD (type 1 CKD), nephrosclerosis (type 2 CKD), and glomerulonephritis (type 3 CKD). Results are presented as described in the legend of Fig. 1. P-values vs. HD controls were calculated using the Kruskal–Wallis test. ***P < 0.001. The data are summarized in Table 4. The total average values, SDs, cutoff values, positive numbers, positive rates (%), and P-values are summarized in Table 4. The ability of s-AP3D1-Abs to (b) predict type 1, (c) type 2, and (d) type 3 CKD was also evaluated using the ROC curve analysis. The results of the power calculation were shown in Supplementary Table S3.
Association of s-AP3D1-Ab levels with ESCC or CRC
The s-AP3D1-Abs levels were also measured in serum samples from the HDs and patients with ESCC or CRC. AlphaLISA results revealed that s-AP3D1Ab levels were significantly higher in patients with ESCC and CRC than in HDs (Fig. 5a). The positivity rates of s-AP3D1-Abs in HDs and patients with ESCC and CRC were 3.1%, 42.2%, and 15.6%, respectively (Table 5). The AUC values were 0.872 and 0.743 for ESCC and CRC, respectively (Fig. 5b,c).
Comparing the serum AP3D1-antibody levels between HDs and patients with digestive organ cancer. This figure shows the (a) s-AP3D1-Ab levels in HDs and patients with esophageal squamous cell carcinoma (ESCC) or colorectal carcinoma (CRC) examined using AlphaLISA. The bars represent the average and average ± SD. P-values were calculated using the Kruskal–Wallis test. ***P < 0.001. The data are summarized in Table 5. ROC curves to assess the ability of s-AP3D1-Abs to predict (b) ESCC and (c) CRC are shown. The numbers in the figures are the same as those shown in Fig. 1. The results of the power calculation were shown in Supplementary Table S3.
Correlation analysis
Correlation analysis of s-AP3D1-Ab levels and subject data was performed using 633 specimens from Chiba Prefectural Sawara Hospital, including 139 samples from HDs, 121 from patients with DSWMH, 17 from patients with asymptCI, 43 from patients with TIA, 226 from patients with AIS, 57 from patients with cCI, and 30 from other diseases. Baseline characteristics of the study subjects in Sawara Hospital cohort are summarized in Supplementary Table S1. Using the Mann–Whitney U test, the s-AP3D1-Ab levels were compared between participants with body mass index (BMI) < 25 and BMI ≥ 25; participants with or without diseases of DM, hypertension (HT), CVD, and dyslipidemia; and between those patients who were smokers or nonsmokers and those who consumed alcohol or not. The analysis showed that the s-AP3D1-Ab levels were significantly higher in the subjects with HT than in those without HT and those with DM than without DM (Table 6). Conversely, no significant differences in s-AP3D1-Ab levels were observed in the other categories.
Performing a logistic regression analysis of the predictors for AIS using the results of the Sawara Hospital was also considered, which included 139 samples from HDs and 226 from patients with AIS. An elevated s-AP3D1-Ab level was associated with an increased risk of AIS as shown by the univariate logistic regression analysis (P < 0.0001). A multivariate logistic regression analysis has identified age, HT, and DM, but not s-AP3D1-Ab, as independent predictors of AIS (Supplementary Table S2).
Next, correlation analysis was performed using a Spearman application in order to determine the correlation between s-AP3D1-Ab levels and subject parameters, including general information such as age, body height, weight, BMI, and the degree of artery stenosis [the maximum intima-media thickness (max IMT)]. The serum s-AP3D1-Ab levels were determined to be closely correlated with age (P < 0.001), max IMT (P < 0.001), blood pressure (BP) (P < 0.001), and smoking period (P < 0.001) (Table 7). Conversely, inverse correlation was observed between s-AP3D1-Ab levels and height, weight, Ca, and low-density lipoprotein cholesterol. Blood glucose and glycated hemoglobin, which are identified as DM markers, were not significantly correlated with the s-AP3D1-Abs levels. These results suggest that s-AP3D1-Ab reflected atherosclerosis and its causal HT and smoking.
JPHC cohort analysis
To determine whether s-AP3D1-Ab marker can be applied to predict the onset of AIS, JPHC cohort samples were examined. The antibody level of AP3D1 protein was positively and strongly associated with the risk of AIS: the ORs (95% CI) were 1.40 (0.75–2.63), 1.97 (1.07–3.65), and 2.28 (1.26–4.13) for the samples with the second, third, and highest quartiles of antibody level, respectively, compared with the lowest quartile (Table 8). These results indicate that the antibody markers against AP3D1 are useful in predicting the onset of AIS.
Discussion
Through the initial SEREX screening, AP3D1 was identified as an antigen recognized by serum IgG in patients with atherosclerosis. The s-AP3D1-Ab levels were higher in patients with AIS, TIA, DM, CVD, CKD, ESCC, and CRC than in the HDs (Figs. 1–5 and Tables 1–5). Meanwhile, to reduce the effect of age, we compared the serum antibody levels of HDs and age-matched patients. The results showed that the s-AP3D1-Ab levels in patients with AIS and DM were significantly higher than those in HDs (Supplementary Fig. S1). Among these diseases, the highest positive rates were observed for ESCC, DM, and type 2 CKD (Tables 1–5). The AUC values for nephrosclerosis type 2 CKD and ESCC, diabetic type 1 CKD, and DM were 0.874 and 0.872, 0.791, and 0.791, respectively, which were higher than other diseases. The comparison using the Mann–Whitney U test revealed that the s-AP3D1-Ab levels were significantly higher in the subjects with DM than in those without DM (Table 6). In contrast, no significant correlation was found between the s-AP3D1-Ab levels and DM markers, including blood glucose and glycated hemoglobin (Table 7). Consequently, the s-AP3D1-Ab levels do not directly reflect DM, but are associated with DM-induced atherosclerotic disorders, which are also related to CKD and cancer. Consistently, Spearman correlation analysis revealed a significant association between s-AP3D1-Ab levels and max IMT (P < 0.001), which reflects arterial stenosis, namely, atherosclerosis (Table 7). The antibody levels significantly correlated with HT (P < 0.0001) (Table 6), which are well-known risk factors for atherosclerosis34. A univariate logistic regression analysis revealed that an elevated AP3D1‑Ab level was associated with an increased risk of AIS (P < 0.0001). A multivariate logistic regression analysis has also identified age, HT, and DM, but not AP3D1‑Ab as independent predictors of AIS (Supplementary Table S2). Therefore, s-AP3D1-Ab marker may discriminate a certain type, if not all, of atherosclerosis caused by HT or DM, leading to the development of AIS and CVD.
AP3D1 is a subunit of the AP3 adaptor-like complex35, which is expressed in the ubiquitous AP3 complex and also in the neuronal form36. AP3 is a heterotetrameric adaptor protein involved in the biogenesis of lysosome-related organelles, such as platelet-dense bodies. Mouse mutants of the null AP3D1 allele were reported to show abnormal bleeding due to the absence of a storage pool of dense platelet particles, raising the possibility that AP3D1 functions as a thrombogenic regulator through platelet function37. Platelets have been identified to play a significant role in hemostatic and thrombotic processes, where abnormal platelet adhesion/activation can lead to the formation of clots (thrombosis)38. Thrombosis is known to be closely associated with atherosclerosis39. Hirokawa et al.40 reported that AP3D1-DOT1L-SF3A2 was identified as a new susceptibility locus for myocardial infarction (MI) by European genome-wide association studies (GWAS), which is consistent with our observation that s-AP3D1-Abs levels were significantly higher in patients with CVD including MI (Fig. 3a, Table 3). On the other hand, Xiao et al. reported that angiotensin II facilitated the binding of AP3D1 with beta-arrestin, which resulted in the activation of AP3D1 as a scaffold protein41. Angiotensin II plays a key role in the pathogenesis of HT42,43,44,45, leading to endothelial dysfunction and atherosclerosis46,47. AP3D1 can possibly mediate angiotensin II-induced HT and atherosclerosis. This was compatible with the results that the s-AP3D1-Ab levels were significantly associated with HT (P < 0.0001) (Table 6) and BP (P < 0.001) (Table 7).
HT is also known as a risk factor for various types of cancers48,49,50,51. Previous reports have proved that angiotensin II is associated with cancer development52,53. Angiotensin II is a major upstream regulator of cancer cachexia54 and can further stimulate angiogenesis and tumor growth of breast cancer55,56. Thus, AP3D1 could also mediate angiotensin II-induced carcinogenesis. Based on the results, s-AP3D1-Abs levels were elevated in cancer patients (Fig. 5).
The progression of atherosclerosis and cancer often takes several years or more and, in the early stages, is sometimes accompanied by low levels of tissue destruction, which can lead to leakage of proteins from the cells. During this repeated leakage of proteins, even low levels of antigens can induce amplified expression of the antibodies57. Thus, antibody markers are deemed more sensitive than antigen markers and may be useful for the early diagnosis of solid cancers including ESCC and CRC. s-AP3D1-Ab marker was closely associated with max IMT, an index of atherosclerosis, which then leads to the onset of AIS and AMI. Thus, predicting these onsets using s-AP3D1-Ab marker is possible. In fact, the results of JPHC cohort analysis indicated that the antibody marker against AP3D1 is useful in predicting the onset of AIS (Table 8). Atherosclerotic AIS and AMI and cancer have been identified as the leading causes of death; thus, the s-AP3D1-Ab marker would be highly useful to reduce its mortality.
As antihypertensive agents, statins, and antiplatelet agents are generally known to prevent the pathogenesis of atherosclerosis58,59,60, the potential modulatory effects of these drugs on s-AP3D1-Ab levels must be considered. Second, as the controls were healthy volunteer donors, potential confounding factors between patients with atherosclerosis, cancers, and controls (age, BMI, HT, DM, and hyperlipidemia) were not adjusted in the analysis of this study. Lastly, physiological testing, such as baPWV, or coronary artery calcification, was not performed to evaluate atherosclerosis in subjects subjected to the s-AP3D1-Ab analysis. Nevertheless, these tests might be expected to confirm the results of this study. The study population included only Japanese patients; thus, further studies are required in patients who are not taking drugs that can affect atherosclerosis and in other ethnic groups. Development of more biomarkers for the early diagnosis of atherosclerotic diseases and early detection of the development of tumors may improve the quality of life.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Weyand, C. M., Berry, G. J. & Goronzy, J. J. The immunoinhibitory PD-1/PD-L1 pathway in inflammatory blood vessel disease. J. Leukoc. Biol. 103, 565–575. https://doi.org/10.1189/jlb.3MA0717-283 (2018) (PMID: 28848042).
Bartoloni, E., Alunno, A. & Gerli, R. Hypertension as a cardiovascular risk factor in autoimmune rheumatic diseases. Nat. Rev. Cardiol. 15, 33–44. https://doi.org/10.1038/nrcardio.2017.118 (2018) (PMID: 28836617).
Kim, J. S. & Bang, O. Y. Medical treatment of intracranial atherosclerosis: An update. J. Stroke 19, 261–270. https://doi.org/10.5853/jos.2017.01830 (2017) (PMID: 29037012).
Lim, S. & Park, S. Role of vascular smooth muscle cell in the inflammation of atherosclerosis. BMB Rep. 47, 1–7. https://doi.org/10.5483/bmbrep.2014.47.1.285 (2014) (PMID: 24388105).
Virmani, R., Kolodgie, F. D., Burke, A. P., Farb, A. & Schwartz, S. M. Lessons from sudden coronary death: A comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 20, 1262–1275. https://doi.org/10.1161/01.atv.20.5.1262 (2000) (PMID: 10807742).
Poznyak, A. et al. The diabetes mellitus-atherosclerosis connection: The role of lipid and glucose metabolism and chronic inflammation. Int. J. Mol. Sci. 21, 1835. https://doi.org/10.3390/ijms21051835 (2020) (PMID: 32155866).
Drüeke, T. B. & Massy, Z. A. Atherosclerosis in CKD: Differences from the general population. Nat. Rev. Nephrol. 6, 723–735. https://doi.org/10.1038/nrneph.2010.143 (2010) (PMID: 20978469).
Tapia-Vieyra, J. V., Delgado-Coello, B. & Mas-Oliva, J. Atherosclerosis and cancer: A resemblance with far-reaching implications. Arch. Med. Res. 48, 12–26. https://doi.org/10.1016/j.arcmed.2017.03.005 (2017).
Carandang, R. et al. Trends in incidence, lifetime risk, severity, and 30-day mortality of stroke over the past 50 years. JAMA 296, 2939–2946. https://doi.org/10.1001/jama.296.24.2939 (2006).
Kubo, M. et al. Secular trends in the incidence of and risk factors for ischemic stroke and its subtypes in Japanese population. Circulation 118, 2672–2678. https://doi.org/10.1161/CIRCULATIONAHA.107.743211 (2008).
Sahin, U. et al. Human neoplasms elicit multiple specific immune responses in the autologous host. Proc. Natl. Acad. Sci. USA 92, 11810–11813. https://doi.org/10.1073/pnas.92.25.11810 (1995).
Chen, Y. T. et al. A testicular antigen aberrantly expressed in human cancers detected by autologous antibody screening. Proc. Natl. Acad. Sci. USA 94, 1914–1918. https://doi.org/10.1073/pnas.94.5.1914 (1997).
Nakashima, K. et al. Serological identification of TROP2 by recombinant cDNA expression cloning using sera of patients with esophageal squamous cell carcinoma. Int. J. Cancer 112, 1029–1035. https://doi.org/10.1002/ijc.20517 (2004).
Kuboshima, M. et al. Identification of a novel SEREX antigen, SLC2A1/GLUT1, in esophageal squamous cell carcinoma. Int. J. Oncol. 28, 463–468. https://doi.org/10.3892/ijo.28.2.463 (2006).
Kuboshima, M. et al. Presence of serum tripartite motif-containing 21 antibodies in patients with esophageal squamous cell carcinoma. Cancer Sci. 97, 380–386 (2006).
Shimada, H. et al. Serum anti-myomegalin antibodies in patients with esophageal squamous cell carcinoma. Int. J. Oncol. 30, 97–103 (2007).
Machida, T. et al. Identification of stroke-associated-antigens via screening of recombinant proteins from the human expression cDNA library (SEREX). J. Transl. Med. 13, 1–11 (2015).
Yoshida, Y. et al. Elevation of autoantibody level against PDCD11 in patients with transient ischemic attack. Oncotarget 9, 8836–8848. https://doi.org/10.18632/oncotarget.23653 (2018).
Yoshida, Y. et al. Elevation of autoantibody in patients with ischemic stroke. Neurol. Med. Chir. (Tokyo) 58, 303–310. https://doi.org/10.2176/nmc.ra.2018-0022 (2018).
Wang, H. et al. Association of serum levels of antibodies against MMP1, CBX1, and CBX5 with transient ischemic attack and cerebral infarction. Oncotarget 9, 5600–5613. https://doi.org/10.18632/oncotarget.23789 (2018).
Yoshida, Y. et al. Elevated levels of autoantibodies against DNAJC2 in sera of patients with atherosclerotic diseases. Heliyon 6, e04661. https://doi.org/10.1016/j.heliyon.2020.e04661 (2020).
Li, S. Y. et al. Association between serum anti-ASXL2 antibody levels and acute ischemic stroke, acute myocardial infarction, diabetes mellitus, chronic kidney disease and digestive organ cancer, and their possible association with atherosclerosis and hypertension. Int. J. Mol. Med. 46, 1274–1288. https://doi.org/10.3892/ijmm.2020.4690 (2020).
Sumazaki, M. et al. Serum anti-LRPAP1 is a common biomarker for digestive organ cancers and atherosclerotic diseases. Cancer Sci. 111, 4453–4464. https://doi.org/10.1111/cas.14652 (2020).
Adams, H. P. Jr. et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 24, 35–41. https://doi.org/10.1161/01.str.24.1.35 (1993).
Nishiura, R. et al. Elevated osteoprotegerin levels predict cardiovascular events in new hemodialysis patients. Am. J. Nephrol. 29, 257–263 (2009).
Komatsu, H. et al. Recent therapeutic strategies improve renal outcome in patients with IgA nephropathy. Am. J. Nephrol. 30, 19–25 (2009).
Kagaya, A. et al. Identification of a novel SEREX antigen family, ECSA, in esophageal squamous cell carcinoma. Proteome Sci. 9, 31. https://doi.org/10.1186/1477-5956-9-31 (2011).
Shimada, H. et al. Elevated serum antibody levels against cyclin L2 in patients with esophageal squamous cell carcinomai. J. Cancer Sci. Ther. 07, 60–66. https://doi.org/10.4172/1948-5956.1000326 (2015).
Shimada, H. et al. Identification of Makorin 1 as a novel SEREX antigen of esophageal squamous cell carcinoma. BMC Cancer 9, 1–9 (2009).
Chen, P. M. et al. Nardilysin is a promising biomarker for the early diagnosis of acute coronary syndrome. Int. J. Cardiol. 243, 1–8 (2017).
Tsugane, S. & Sawada, N. The JPHC study: Design and some findings on the typical Japanese diet. Jpn. J. Clin. Oncol. 44, 777–782. https://doi.org/10.1093/jjco/hyu096 (2014).
Ikeda, A. et al. The combination of Helicobacter pylori- and cytotoxin-associated gene-A seropositivity in relation to the risk of myocardial infarction in middle-aged Japanese: The Japan Public Health Center-based study. Atherosclerosis 230, 67–72 (2013).
Kanda, Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant 48, 452–458. https://doi.org/10.1038/bmt.2012.244 (2013).
Zhu, H., Li, Z., Lv, J. & Zhao, R. Effects of cerebral small vessel disease on the outcome of patients with ischemic stroke caused by large artery atherosclerosis. Neurol. Res. 40, 381–390 (2018).
Simpson, F., Peden, A. A., Christopoulou, L. & Robinson, M. S. Characterization of the adaptor-related protein complex, AP-3. J. Cell Biol. 137, 835–845. https://doi.org/10.1083/jcb.137.4.835 (1997).
Ammann, S. et al. Mutations in AP3D1 associated with immunodeficiency and seizures define a new type of Hermansky-Pudlak syndrome. Blood 127, 997–1006. https://doi.org/10.1182/blood-2015-09-671636 (2016).
Kantheti, P. et al. Mutation in AP-3 δ in the mocha mouse links endosomal transport to storage deficiency in platelets, melanosomes, and synaptic vesicles. Neuron 21, 111–122. https://doi.org/10.1016/s0896-6273(00)80519-x (1998).
Vorchheimer, D. A. & Becker, R. Platelets in atherothrombosis. Mayo Clin. Proc. 81, 59–68 (2006).
Badimon, L. & Vilahur, G. Thrombosis formation on atherosclerotic lesions and plaque rupture. J. Intern. Med. 276, 618–632 (2014).
Hirokawa, M. et al. A genome-wide association study identifies PLCL2 and AP3D1-DOT1L-SF3A2 as new susceptibility loci for myocardial infarction in Japanese. Eur. J. Hum. Genet. 23, 374–380. https://doi.org/10.1038/ejhg.2014.110 (2015).
Xiao, K. et al. Functional specialization of β-arrestin interactions revealed by proteomic analysis. Proc. Natl. Acad. Sci. USA 104, 12011–12016. https://doi.org/10.1073/pnas.0704849104 (2007).
Naik, P., Murumkar, P., Giridhar, R. & Yadav, M. R. Angiotensin II receptor type 1 (AT1) selective nonpeptidic antagonists: A perspective. Bioorg. Med. Chem. 18, 8418–8456. https://doi.org/10.1016/j.bmc.2010.10.043 (2010).
Burnier, M. Angiotensin II type 1 receptor blockers. Circulation 103, 904–912. https://doi.org/10.1161/01.CIR.103.6.904 (2001).
Burnier, M. & Brunner, H. R. Angiotensin II receptor antagonists. Lancet 355, 637–645. https://doi.org/10.1016/S0140-6736(99)10365-9 (2000).
Cappelli, A. et al. Design, synthesis, and biological evaluation of AT1 angiotensin II receptor antagonists based on the pyrazolo[3,4-b]pyridine and related heteroaromatic bicyclic systems. J. Med. Chem. 51, 2137–2146. https://doi.org/10.1021/jm7011563 (2008).
Bellien, J. et al. Epoxyeicosatrienoic acids contribute with altered nitric oxide and endothelin-1 pathways to conduit artery endothelial dysfunction in essential hypertension. Circulation 125, 1266–1275. https://doi.org/10.1161/circulationaha.111.070680 (2012).
Chen, L. et al. Impaired endothelial repair capacity of early endothelial progenitor cells in hypertensive patients with primary hyperaldosteronemia: Role of 5,6,7,8-tetrahydrobiopterin oxidation and endothelial nitric oxide synthase uncoupling. Hypertension 67, 430–439. https://doi.org/10.1161/HYPERTENSIONAHA.115.06597 (2016).
O’Donnell, M. J. et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): A case-control study. Lancet 376, 112–123. https://doi.org/10.1016/S0140-6736(10)60834-3 (2010).
Sanfilippo, K. M. et al. Hypertension and obesity and the risk of kidney cancer in 2 large cohorts of US men and women. Hypertension 63, 934–941. https://doi.org/10.1161/HYPERTENSIONAHA.113.02953 (2014).
Sun, L. M. et al. Hypertension and subsequent genitourinary and gynecologic cancers risk: A population-based cohort study. Medicine (United States) 94, 1–8. https://doi.org/10.1097/MD.0000000000000753 (2015).
Han, H. et al. Hypertension and breast cancer risk: A systematic review and meta-analysis. Sci. Rep. 7, 1–9. https://doi.org/10.1038/srep44877 (2017).
Cambados, N. et al. Angiotensin-(1–7) counteracts the transforming effects triggered by angiotensin II in breast cancer cells. Oncotarget 8, 88475–88487. https://doi.org/10.18632/oncotarget.19290 (2017).
Sobczuk, P., Szczylik, C., Porta, C. & Czarnecka, A. M. Renin angiotensin system deregulation as renal cancer risk factor (Review). Oncol. Lett. 14, 5059–5068. https://doi.org/10.3892/ol.2017.6826 (2017).
Penafuerte, C. A. et al. Identification of neutrophil-derived proteases and angiotensin II as biomarkers of cancer cachexia. Br. J. Cancer 114, 680–687. https://doi.org/10.1038/bjc.2016.3 (2016).
Rodrigues-Ferreira, S. et al. Angiotensin ii facilitates breast cancer cell migration and metastasis. PLoS ONE 7, 1–8. https://doi.org/10.1371/journal.pone.0035667 (2012).
Masamune, A. et al. The angiotensin II type i receptor blocker olmesartan inhibits the growth of pancreatic cancer by targeting stellate cell activities in mice. Scand. J. Gastroenterol. 48, 602–609. https://doi.org/10.3109/00365521.2013.777776 (2013).
Qiu, J., Keyser, B., Lin, Z. T. & Wu, T. Autoantibodies as potential biomarkers in breast cancer. Biosensors 8, 67. https://doi.org/10.3390/bios8030067 (2018).
Kang, B. Y., Wang, W., Palade, P., Sharma, S. G. & Mehta, J. L. Cardiac hypertrophy during hypercholesterolemia and its amelioration with rosuvastatin and amlodipine. J. Cardiovasc. Pharmacol. 54, 327–334. https://doi.org/10.1097/FJC.0b013e3181b76713 (2009).
Hörl, G. et al. Simvastatin efficiently lowers small LDL-IgG immune complex levels: A therapeutic quality beyond the lipid-lowering effect. PLoS ONE 11, 1–23. https://doi.org/10.1371/journal.pone.0148210 (2016).
Aude, Y. W. & Mehta, J. L. Nonplatelet-mediated effects of aspirin. Drugs Today 38, 501–507. https://doi.org/10.1358/dot.2002.38.7.820117 (2002).
Acknowledgements
The authors would like to thank Prof. Masaki Takiguchi and Dr. Xiao-Meng Zhang (Department of Biochemistry and Genetics, Graduate School of Medicine, Chiba University) for supporting our research. We also thank Prof. Kenichiro Kitamura (Department of Internal Medicine 3, University of Yamanashi School of Medicine), Dr. Koichi Kashiwado (Department of Neurology, Kashiwado Hospital), Dr. Takeshi Wada (Department of Internal Medicine, Chiba Aoba Municipal Hospital), Dr. Akiyo Aotsuka (Department of Internal Medicine, Chiba Aoba Municipal Hospital) for providing research materials. Finally, we want to thank Initiative for Realizing Diversity in the Research Environment of Chiba University for supporting English proofreading expense.
Funding
The present study was supported, in part, by research Grants from JSPS KAKENHI Grant Numbers 20K17953, 19K09451, 17K19810, 20K07810, 16K10520, 15K10117, and 15K01842, the Japan Agency for Medical Research and Development (AMED), and Japan Science and Technology Agency (JST).
Author information
Authors and Affiliations
Contributions
S.Y.L., E.K., T.Mac., F.N., H.M., H.S., H.K., Y.I., and T.H. conceived and designed the study. S.Y.L., M.K., S.K., M.S., K.I., H.A., G.T., N.S., and R.N. performed the experiments and acquired the data. S.M., Y.M., M.T., K.Yo., Y.K., H.T., and K.M. contributed reagents, materials, analysis tools or patient data. Y.Y., T.Mat., H.I., S.T., and H.W. analyzed and interpreted the data. M.S., K.Ya., N.S., M.I., S.Y., K.G. performed the statistical analyses. S.Y.L., T.Mac., Y.Y., Y.I., and T.H. drafted the manuscript. All authors gave final approval and agree to be accountable for all aspects of work ensuring integrity and accuracy. All authors confirm that tables and figures were not published previously.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, SY., Yoshida, Y., Kobayashi, E. et al. Serum anti-AP3D1 antibodies are risk factors for acute ischemic stroke related with atherosclerosis. Sci Rep 11, 13450 (2021). https://doi.org/10.1038/s41598-021-92786-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-92786-9
This article is cited by
-
Serum anti-PCK1 antibody levels are a prognostic factor for patients with diabetes mellitus
BMC Endocrine Disorders (2023)
-
Analysis of patients with colorectal cancer shows a specific increase in serum anti-ING1 autoantibody levels
BMC Cancer (2023)
-
Association of high proprotein convertase subtilisin/kexin type 9 antibody level with poor prognosis in patients with diabetes: a prospective study
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.