Abstract
This systematic review assessed the effectiveness of ozone (O3) in the color change of in-office tooth bleaching in vital teeth (TB) and the sensitivity control. Only randomized controlled clinical trials were included. Seven databases were used as primary search sources, and three additional sources were searched to capture the "grey literature" partially. The JBI tool was used to assess the risk of bias. TB was assessed using the ΔELab color change metric comparing tooth color pre- and post-bleaching. We meta-analyzed the ΔELab estimates per method and calculated the absolute standardized mean difference using random-effect models. The GRADE approach assessed the certainty of the evidence. The ΔELab estimates ranged from 1.28 when the O3 was used alone to 6.93 when combined with hydrogen peroxide (HP). Two studies compared O3 and HP alone, but their TB was similar (SMD = − 0.02; 95%CI: − 0.54; 0.49). The bleaching effectiveness for the combination of O3 + HP compared to HP was similar (SMD = 0.38; 95%CI: − 0.04; 0.81). Thus, based on the available literature, our findings suggest that O3 is not superior to the conventional technique using HP on the change of tooth color. The O3 did not present sensitivity when used alone. When O3 was used in combination with HP, patients reported hypersensitivity only when O3 was applied before HP, i.e., no sensitivity was perceived when O3 was applied after HP.
Similar content being viewed by others
Introduction
Tooth bleaching of vital teeth has become popular over the last decades despite the adverse effects associated with the procedure, such as tooth sensitivity1,2,3,4,5,6,7,8, gingival irritation2,6,8 morphology changes on the enamel surface9, the inflammatory response of the pulp tissue2,10,11,12,13, reduction of the metabolism and cell viability14, changes in vascular permeability15, increased marginal micro infiltration in the tooth/restoration interface2, and microhardness reduction of restorative materials16. Besides these adverse effects, studies have shown that the chemical components of bleaching gels may have cytotoxic and carcinogenic effects2,8,17.
The most common adverse effect after tooth bleaching therapy is tooth sensitivity, with a mean prevalence of 70% in patients during and after the procedure5. Such sensitivity may be related to the use of bleaching gels, which are made of hydrogen peroxide (HP)1,5,6,12. This material has low molecular weight and can spread through enamel and dentin, promoting tooth bleaching but potentially damaging pulp cells11. The free radicals formed by the dissociation of HP are mainly responsible for the toxicity of this compound because its oxidative reactions may cause damage to odontoblasts and decrease their metabolic activity2,3,5.
Ozone (O3) is a natural gas formed by three oxygen atoms, and it has been used for medical therapies since World War I18,19. Currently, health professionals use ozone therapy20 for the treatment of several pathologies due to its high oxidation power, immune response, circulatory stimulation, analgesic and anti-inflammatory properties, and parasitological effect21,22,23. In dentistry, O3 effectively controls infections caused by viruses, protozoa, fungi, and bacteria18,21. Moreover, it seems to promote tissue repair and healing processes24, prevention of dental caries22,23,25, remineralization of the tooth surface22,25, treatment of oral ulcers22, treatment of gingivitis and periodontitis24, pain control22,25,26, endodontic treatment27, halitosis19,21, temporomandibular disorders19,21, complementary treatment of non-carious cervical lesions and tooth sensitivity28,29,30, and tooth bleaching28,29,30,31,32.
Using O3 for tooth bleaching is safe in conditions in which diffusion is an important factor, such as in hard dental tissues, as it works on their organic substances and can be used, for instance, to reduce tetracycline staining28. However, the effectiveness of ozone therapy in tooth bleaching may depend on the application time, bleaching gel concentration, and gas flow rate33. There is still no consensus in the literature on the best usage protocol for O3 and HP for tooth bleaching. Thus, this systematic review aims to evaluate whether O3 can improve the clinical performance of tooth bleaching in vital teeth. The authors worked with the following hypotheses: (1) O3 can promote color change in tooth bleaching better than HP, (2) O3 associated with HP accelerates the effect of color change in tooth bleaching, and (3) O3 reduces tooth sensitivity caused by tooth bleaching.
Methods
Protocol and registration
This systematic review followed the recommendations listed in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols (PRISMA)34 and the Cochrane guidelines35. The protocol of this systematic review was registered in the International Prospective Register of Systematic Reviews (PROSPERO), under number CRD42018099190 (https://www.crd.york.ac.uk/prospero/).
Study design and eligibility criteria
The systematic review aimed to answer the following guiding question, based on the PICO strategy: Do patients treated with tooth bleaching in vital teeth (patients) with ozone therapy (intervention) have improved clinical results of color change and tooth sensitivity (outcome) when compared to the conventional treatment with HP (control)?
Only randomized clinical trials (RCTs) reporting the use of O3 alone or combined with HP gel as one of their study groups for tooth bleaching were included. There was no restriction of year, language, and publication status.
The exclusion criteria were: 1) studies not related to the topic; 2) reviews, observational studies, letters to the editor/editorials, personal opinions, books/book chapters, reports, conference abstracts, and theses; 3) laboratory studies; and 4) case reports and case series.
Sources of information, search and study selection
Cochrane, Embase, LILACS, PubMed, SciELO, Scopus, and Web of Science were the primary databases used for searching the studies. The OATD, OpenThesis, and OpenGrey databases were used to partially capture the "grey literature". The Medical Subject Headings (MeSH), Health Sciences Descriptors (DeCS), and Embase Subject Headings (Emtree) resources were used for selecting the keywords. The Boolean operators "AND" and "OR" were used to enhance the research strategy through several combinations (Table 1). A manual search was also performed through a systematized analysis of the references of the studies that had previously achieved the eligibility step. The search was performed in May 2020. The results obtained from the primary databases were initially exported to EndNote Web (Clarivate Analytics, Philadelphia, USA), excluding the duplicates. Then, they were exported to Microsoft Word (Microsoft Ltd, Washington, USA) as well as the results obtained in the grey literature, in which the remaining duplicates were removed manually.
Before selecting the studies, a calibration exercise was performed among the reviewers. Subsequently, exclusion by titles (first phase), by abstracts (second phase), and by reading the full articles (third phase) was performed. All phases were independently evaluated by two evaluators (LD and MDMAC), and, in case of doubt or disagreement, a third evaluator (LRP) was always consulted to make a final decision.
Data collection
Prior to data extraction, both reviewers (LD and MDMAC) were calibrated by extracting the data from one article and comparing it with the third reviewer, with expertise in dental bleaching and systematic reviews. The reviewers extracted the following information: identification of the study (author, year, location), sample characteristics (number of patients, distribution by sex, and average age), characteristics of sample collection and processing (groups, materials used, application time and follow-up, teeth assessed), specific results: quantification of 1) color change using ΔELab, CIELab (a, b, and L) and 2) dentin sensitivity using the Visual Analogue Scale (VAS). We evaluated whether the studies respected the ethical criteria for the research development according to the current law in the countries of origin, whether the previous signature of the consent form was collected, whether the CONSORT was used as a guideline, and whether the studies were registered in databases of clinical trials. Lastly, the analysis and the results (bleaching effectiveness, O3 effectiveness in bleaching, O3 influence on sensitivity) were analyzed. In case of doubt regarding the data presented in the results of the studies, the authors were contacted.
Risk of individual bias of the studies
The JBI Manual for Evidence Synthesis36 (LD and MDMAC) assessed each domain independently regarding the potential risk of bias, as recommended by the PRISMA statement34.
Each study was categorized according to the percentage of positive answers to the questions. The risk of bias was considered “High” when the study obtained 49% or less “yes” answers, “Moderate” when the study obtained 50% to 69% of “yes” answers, and “Low” when the study reached more than 70% of “yes” score.
Summary measures and meta-analysis
In order to assess bleaching effectiveness, the CIELab (L, a, b) system for measuring color difference was explored. From these data, the delta E (ΔELab), which measures the color change between the pre- and post-bleaching periods for all bleaching methods, was calculated. As some studies did not provide the ΔELab, calculation, the estimate was calculated by the CIE76 formula: \({\Delta E}_{Lab}=\sqrt{\Delta {L}^{2}+\Delta {a}^{2}+\Delta {b}^{2}}\)37.
A meta-analysis with a random-effects model was performed using the Stata 16.0 software (StataCorp., College Station, TX, USA). The ΔELab estimates from the different methods were compared by absolute standardized mean differences (SMD) to compare the bleaching effectiveness. We did not meta-analyzed the VAS measures since only one study28 would be eligible, since the remaining studies29,30 did not show any variability on the VAS scale comparing comparing the pre- and post-bleaching periods.
Certainty of evidence collection
The certainty of evidence and strength of recommendation were assessed with the Grading of Recommendation, Assessment, Development, and Evaluation (GRADE) tool38. The GRADE pro-GDT software (http://gdt.guidelinedevelopment.org) was used for summarizing the results. This assessment was based on study design, methodological limitations, inconsistency, indirect evidence, imprecision, and other considerations. The quality of evidence was characterized as high, moderate, low, or very low38.
Results
Study selection
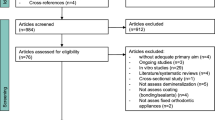
A total of 12,703 results were found in ten electronic databases, including “gray literature”, in the first phase of the study selection. After analysis, only 17 studies were eligible for full-text analysis. The references of the 17 potentially eligible studies were evaluated, and no additional articles were selected. After reading the entire text, 13 studies did not meet the inclusion criteria and were eliminated: twelve were literary reviews, and one was a congress summary. Thus, four studies were included in this review (Fig. 1).
Characteristics of eligible studies
The studies were published between 2016 and 2018 and were performed in Jordan28,29,30 and Turkey32. All studies28,29,30,32 respected the ethical criteria for research development recommended in each country of origin, applying a consent form for all volunteers participating in the study. Only one study30 mentioned using the CONSORT as a guideline, and none of the studies clarified whether they were registered in clinical trial databases.
The total sample included 129 patients treated with tooth bleaching, with 57 in the control group treated with 38% HP and 72 treated with bleaching with O3. From the latter, 29 were treated with O3 alone, while 43 were treated with O3 associated with HP. From all patients, 77 were women, and 52 were men. The age of the patients in each study ranged between 24 and 50 years28, 20 to 35 years32, 20 to 35 years30, and 19 and 33 years29.
All studies used methods of color analysis, as follows: Spectrophotometer32, Colorimeter Konica28,29,30, and Vita Classical28,29,30. The color assessment was registered only in the maxillary dental arch at the following times: initial (before bleaching started)28,29,30,32, after bleaching (24 h)28,29,30, and immediately after bleaching and 48 h later32. Table 2 shows detailed characteristics of the eligible studies.
Risk of individual bias of the studies
Two eligible studies28,32 had a “moderate” risk of bias or methodological quality while two studies28,30 “low” risk of bias. Table 3 shows detailed information on the risk of bias of the studies included. Item 1 was marked as “Unclear” in two studies because the randomization method was not explicit28,32. Item 2 was marked as “Unclear” in one study because it did not describe the steps followed for hiding the sequence until attributing the interventions30, and marked as “No” in three studies28,29,32 because randomization was not explained. As for item 3, two studies were marked as “No” because they did not describe the baseline29,32. In item 4, two studies did not inform about participants blinding29,32. All four studies were marked as “No” in item 5 because they did not blind the operators28,29,30,32. In item 6, only one study was marked as “No” because it did not blind the evaluator from the result32. All studies were marked as “Not applicable” in item 9 because there was no participant dropout and the follow-up time was rather short28,29,30,32.
Specific results of the eligible studies
One of the studies assessed the result of color change in tooth bleaching immediately after applying the products and 48 h later32, while the remaining studies performed this assessment 24 h after the procedure28,29,30. These three studies also measured tooth sensitivity after bleaching28,29,30.
In all studies and all experimental groups, the results of color change in tooth bleaching were positive for whitening the teeth, changing the initial color. Bleaching with O3 presented statistically similar results to the groups using HP in the studies28,29,30.
Bleaching with HP (control group) induced tooth sensitivity in all studies analyzed, and ozone therapy applied alone or after the use of HP was able to eliminate the painful symptomatology and reduce the time of gel application without changing bleaching effectiveness. The ΔELab was pre-informed in only one study32 and calculated for the others using the CIE76 formula, as mentioned by Gaurav37.
Synthesis of results and meta-analysis
Table 4 shows the results of color change and tooth sensitivity for each study. Although all groups achieved positive ΔELab estimates, indicating effective bleaching, there was high variability between study results. The ΔELab estimates ranged from 1.28 when the ozone therapy was used alone to 6.93 when combined with HP.
Figure 2 shows the comparison between the bleaching effectiveness of ozone therapy and HP alone. Only two studies compared these agents, which achieved a similar bleaching effectiveness (SMD = − 0.02; 95%CI: − 0.54; 0.49). On a similar note, comparing the effectiveness of O3 and HP combined to HP alone showed that bleaching effectiveness was also similar between the techniques (SMD = 0.38; 95%CI: − 0.04; 0.81) (Fig. 3).
Regarding tooth sensitivity, ΔVAS scores comparing pre- and post-bleaching periods ranged from 0.0 to 3.2. The highest sensitivity score among all studies (ΔVAS = 3.20) was reported in the group treated with O3 followed by HP. Two other studies using a similar combination but applying HP before the ozone reported no tooth sensitivity (Table 4).
Certainty of evidence
The GRADE tool assessed two outcomes (Bleaching effectiveness—O3 vs. H2O2 and Bleaching effectiveness O3 + H2O2 vs. H2O2). All outcomes were categorized as a very low level of certainty, which means the true effect is likely to be substantially different from the estimated effect. The two outcomes were downgraded in two levels due to risk of bias (limitations in randomization and blindness), imprecision (wide credible intervals and a low number of participants), and publication bias (three out of four articles were performed by the same research group). Table 5 shows more details for each outcome.
Discussion
This study aimed to assess the effect of O3 on color change in tooth bleaching alone and combined with the HP-based bleaching gel, and reduction of tooth sensitivity from the bleaching process in vital teeth. The hypothesis that O3 is more effective in the color change in tooth bleaching than HP was rejected, considering that the results between the different methods were statistically similar. It is worth noting that ΔELab is an important parameter used to assess the effectiveness of bleaching techniques39,40, as values over 1.22 are considered perceptible to the human eye, and color changes over 2.66 are considered acceptable41,42. All the studies included in this review reported color change perceptible to the human eye (1.28—1.66)32 or acceptable (3.08—6.93)28,29,30 for teeth compared before and after the bleaching therapy. These data are compatible with the studies of laboratory and clinical research28,33,43,44,45. The study by Aykut-Yetkiner and colleagues (2017) presented the lowest ΔELab values (1.66 and 1.28), and this is the only study with values classified as perceptible32. This result may be related to the older age of patients, which may affect the result of color change in bleaching40 compared to the other studies28,29,30.
The bleaching ability has been associated with the oxidative effect of free radicals, released by the breakdown of HP through the formation of hydroxyl and perhydroxyl radicals, superoxide anions, and HP anions, converting the chromophores within hard dental tissues into simpler structures or changing their optical properties. This reflects more light and changes the appearance of the tooth to a lighter shade3,5,8. However, a more recent study suggested that HP might whiten normal dentin by oxidizing the benzene ring of aromatic amino acids in dentin phosphoprotein (DPP), which is the main non-collagenous protein located in the organic–inorganic interface and responsible for the fluorescence and color of normal dentin46. Moreover, HP can change the translucency property of enamel that became slightly opaquer after bleaching47. The O3 is an unstable gas that rapidly releases nascent oxygen molecules to form oxygen. Additionally, O3 can oxidize the components responsible for tooth discoloration, as chromophore groups may be broken by ozone, forming smaller molecules and resulting in a tooth bleaching effect by one of three mechanisms (bonding mechanism, substitution mechanism, or cleavage mechanism)28,29. Both mechanisms seem to have similar bleaching effectiveness, as observed in all studies, because there was no statistical difference between the bleaching techniques and protocols used.
The second hypothesis of the study was rejected. The association of O3 with HP does not potentiate the bleaching effect of HP. Although the highest ΔELab values were observed in the groups with such association (6.93, 5.85, 5.3), they were not statistically significant in none of the eligible studies. Thus, although O3 immediately provides a high amount of OH and O* compounds, such an amount cannot increase the bleaching effect with HP. It is worth noting that the decomposition of HP is slow, so its effectiveness becomes more evident for the in-office technique when at least two clinical sessions are performed48. The four eligible studies28,29,30,32 showed that the in-office technique was performed in a single session, showing effective results and clinically perceptible ΔELab values. However, the follow-ups were performed in a short time (immediate and 24 and 48 h), which complicates the analysis of the rebound effect39 that might show a different response from that obtained in the studies.
Another factor worth mentioning is that three of the eligible studies28,29,30 used HP for 20 min, which is different from the manufacturer's recommendation, and they still obtained acceptable values (3.41, 3.08, 3.15) of color change. Perhaps further studies may be performed to verify whether this reduction in application time might result in bleaching ability similar to the time indicated by the manufacturers, which is usually twice the one used in the eligible studies12,13. The reduction of application time would be an important factor that could reduce total chair time and the risk and intensity of tooth sensitivity12 because bleaching-induced damage of the dental tissue is cumulative and proportional to the amount of HP that reaches the pulp10,12,13,49.
Tooth sensitivity is a major clinical factor that should be considered during and after tooth bleaching, as current studies show that medications used to reduce this painful symptomatology are not effective3,7,49,50,51. The study that used O3 before HP showed a perceptible increase in pain sensitivity after bleaching compared with the control group, which leads to the perception that the previous use of O3 would both intensify the oxidative power of the gel and increase its diffusion power through the dental tissues, causing pain. Tooth sensitivity is caused by the increase in tooth permeability, changing hydraulic conductance, and dentin intratubular fluid movement, thus providing greater contact between bleaching agents and odontoblastic extensions and pulp tissue, intensifying and providing sensitivity1,2,3,6,7,12,13,49. Two studies described lower sensitivity for the group treated with HP followed by O3, while another study described higher sensitivity for the group treated with O3 followed by HP. Thus, the order in which the products are applied might be relevant for preventing teeth sensitivity during the bleaching process.
These same studies also show that the use of O3 alone does not cause tooth sensitivity as a side effect of whitening and that O3 associated and used after HP was effective in preventing such an uncomfortable side effect when using PH in high concentrations28,29,30. This confirms the third and last hypothesis. This factor can be explained by the anti-inflammatory, antioxidant, and analgesic properties of O3, which potentially restrict the inflammatory pathways. It has been known that O3 is able to neutralize the neurochemical mediators related to pain sensitivity, to inactivate cyclooxygenase by reducing the release of prostaglandins, and to facilitate the metabolization and elimination of inflammatory mediators1,28,29,30.
The side effects resulting from the use of bleaching gels show the need for alternatives that are more biologically compatible with tooth bleaching treatment. Studies reported that the deleterious effects to the dental pulp affected by technique protocol1,12,13, gel concentration49,52, and secondary components of the bleaching gel formula existent in the commercial product, such as stabilizers, thickeners, dyes, preservatives, and even gel viscosity that reaches the dental pulp might be responsible for affecting the level of diffusion and/or cytotoxicity8,17. The manufacturers neither describe nor provide such products.
Our study is not free of limitations, which include some studies performed by the same author, the limited number of RCTs in the literature, the short follow-up period, and the small number of participants per group in the eligible studies. Further studies with a higher number of participants ought to be performed, considering the extensive variability in the ΔELab results between the groups (1.28–6.93). Another factor would be the follow-up time, as studies with longer follow-up time would be more interesting, considering there is a difference in the behavior of the values presented in the short and long terms (rebound effect) for the different products in several studies40,48. The standardization of time of ozone use is also something to consider because the studies presented different usage periods, ranging from 128,29,30 to 4032 min, without showing differences for the bleaching effect. The last limitation is related to the parameters of color assessment used in the studies because there are current assessment criteria such as WI and ΔE00 that are already established in the literature38 and considered more perceptive clinically. Such parameters would be ideal to complement the results found in this review, but they could not be calculated because one of the eligible studies did not present isolated L, a, and b values, and they were not even provided by the authors after being contacted via e-mail.
One aspect for consideration in the use of ozone therapy is the need for a financial investment to acquire the ozone generating equipment and the need for caution in handling due to the toxicity of the gas in the respiratory system, which requires technical training before use. However, the equipment would have other clinical uses18,20,21,22,23,24,25,26,27 that are not highlighted in this review. The machine allows ozonizing liquids such as water and serum for use in dental procedures, as well as oil18,19. During bleaching, although O3 did not potentiate the use of HP, it was able to reduce tooth sensitivity to zero, which is one of the greatest challenges and side effects of the technique with HP. Considering such properties and clinical findings for ozone, studies directed to patients presenting clinical conditions considered limiting to conventional tooth bleaching, such as tetracycline staining, tooth sensitivity, and presence of non-carious cervical lesions (NCCL), would be relevant, thus observing their effectiveness and therapeutic clinical response.
Certainty of evidence and clinical implications
The evidence obtained with this systematic review and meta-analysis was classified as a very low certainty. This result may be explained mainly because of the lack of studies in the literature assessing the use of ozone for bleaching vital teeth. The imprecision found in the pooled estimates reflects the lack of available literature, as the number of participants included in the meta-analysis is one of the factors affecting the confidence interval of the pooled estimates. Moreover, three of the four included studies were published by the same group of researchers (potential risk of publication bias), showing the lack of studies on the topic in other locations in the world. In this context, one way to expand the certainty in estimates regarding the applicability of ozone for vital teeth bleaching is to perform further studies with a higher number of participants by different research groups that comprise different samples.
Other factors that contributed to downgrading the certainty of evidence were methodological limitations and inconsistency among the studies. As in other complementary therapies such as laser therapy, there is still no consensus regarding the optimal protocol for using ozone therapy to bleaching of vital teeth. As a consequence of such a de-standardization, the estimates of the effect of the studies were conflicting. Thus, further studies should establish a protocol of ozone application with strict and adequate methodologies.
Based on the current evidence, the strength of clinical recommendation for the use of ozone therapy for bleaching vital teeth is weak in favor of intervention. This recommendation was based on three main aspects: (1) The low certainty of evidence; (2) The effect estimates of effect found in the meta-analysis were not superior to ozone therapy for any of the outcomes; 3) The cost and investment required for the clinical use of ozone therapy.
Based on limited evidence, the use of O3 (alone or associated) was not superior to the conventional use of HP for the bleaching of vital teeth. Moreover, O3 cannot intensify the bleaching action of HP, but it showed positive effects for sensitivity.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Pontes, M. et al. Effect of bleaching gel concentration on tooth color and sensitivity: A systematic review and meta-analysis. Oper. Dent. 45, 265–275. https://doi.org/10.2341/17-376-L (2020).
Tredwin, C. J., Naik, S., Lewis, N. J. & Scully, C. Hydrogen peroxide tooth-whitening (bleaching) products: Review of adverse effects and safety issues. Br. Dent. J. 200, 371–376. https://doi.org/10.1038/sj.bdj.4813423 (2006).
Faria-E-Silva, A. L., Nahsan, F. P., Fernandes, M. T. & Martins-Filho, P. R. Effect of preventive use of nonsteroidal anti-inflammatory drugs on sensitivity after dental bleaching: A systematic review and meta-analysis. J. Am. Dent. Assoc. 146, 87-93.e81. https://doi.org/10.1016/j.adaj.2014.10.007 (2015).
Gonçalves, M. L. L. et al. In-office tooth bleaching for adolescents using hydrogen peroxide-based gels: Clinical trial. Braz. Dent. J. 28, 720–725. https://doi.org/10.1590/0103-6440201701516 (2017).
Kielbassa, A. M., Maier, M., Gieren, A. K. & Eliav, E. Tooth sensitivity during and after vital tooth bleaching: A systematic review on an unsolved problem. Quintessence Int. 46, 881–897. https://doi.org/10.3290/j.qi.a34700 (2015).
Rezende, M. et al. Tooth sensitivity after dental bleaching with a desensitizer-containing and a desensitizer-free bleaching gel: A systematic review and meta-analysis. Oper. Dent. 44, E58–E74. https://doi.org/10.2341/17-253-L (2019).
Ribeiro, J. S. et al. Novel in-office peroxide-free tooth-whitening gels: Bleaching effectiveness, enamel surface alterations, and cell viability. Sci. Rep. 10, 10016. https://doi.org/10.1038/s41598-020-66733-z (2020).
Colares, V. L. P. et al. Hydrogen peroxide-based products alter inflammatory and tissue damage-related proteins in the gingival crevicular fluid of healthy volunteers: A randomized trial. Sci. Rep. 9, 3457. https://doi.org/10.1038/s41598-019-40006-w (2019).
Kawamoto, K. & Tsujimoto, Y. Effects of the hydroxyl radical and hydrogen peroxide on tooth bleaching. J. Endod. 30, 45–50. https://doi.org/10.1097/00004770-200401000-00010 (2004).
Benetti, F. et al. In vivo study of the action of a topical anti-inflammatory drug in rat teeth submitted to dental bleaching. Braz. Dent. J. 29, 555–561. https://doi.org/10.1590/0103-6440201802177 (2018).
Cintra, L. T. et al. Evaluation of an experimental rat model for comparative studies of bleaching agents. J. Appl. Oral Sci. 24, 171–180. https://doi.org/10.1590/1678-775720150393 (2016).
Acuña, E. D. et al. In-office bleaching with a commercial 40% hydrogen peroxide gel modified to have different pHs: Color change, surface morphology, and penetration of hydrogen peroxide into the pulp chamber. J. Esthet. Restor. Dent. https://doi.org/10.1111/jerd.12453 (2019).
Balladares, L. et al. Effects of ph and application technique of in-office bleaching gels on hydrogen peroxide penetration into the pulp chamber. Oper. Dent. 44, 659–667. https://doi.org/10.2341/18-148-L (2019).
Soares, D. G., Basso, F. G., Scheffel, D. S., Hebling, J. & de Souza Costa, C. A. Responses of human dental pulp cells after application of a low-concentration bleaching gel to enamel. Arch. Oral Biol. 60, 1428–1436. https://doi.org/10.1016/j.archoralbio.2015.06.014 (2015).
Ferreira, V. G. et al. Tooth bleaching induces changes in the vascular permeability of rat incisor pulps. Am. J. Dent. 26, 298–300 (2013).
Tong, L. S., Pang, M. K., Mok, N. Y., King, N. M. & Wei, S. H. The effects of etching, micro-abrasion, and bleaching on surface enamel. J Dent Res 72, 67–71. https://doi.org/10.1177/00220345930720011001 (1993).
Llena, C. et al. Comparison of diffusion, cytotoxicity and tissue inflammatory reactions of four commercial bleaching products against human dental pulp stem cells. Sci. Rep. 9, 7743. https://doi.org/10.1038/s41598-019-44223-1 (2019).
Bocci, V. A. Scientific and medical aspects of ozone therapy. State of the art. Arch. Med. Res. 37, 425–435. https://doi.org/10.1016/j.arcmed.2005.08.006 (2006).
Saini, R. Ozone therapy in dentistry: A strategic review. J. Nat. Sci. Biol. Med. 2, 151–153. https://doi.org/10.4103/0976-9668.92318 (2011).
Bianco, E., Maddalone, M., Porcaro, G., Amosso, E. & Baldoni, M. Treatment of osteoradionecrosis of the jaw with ozone in the form of oil-based gel: 1-year follow-up. J. Contemp. Dent. Pract. 20, 270–276 (2019).
Nogales, C. G., Ferrari, P. H., Kantorovich, E. O. & Lage-Marques, J. L. Ozone therapy in medicine and dentistry. J. Contemp. Dent. Pract. 9, 75–84 (2008).
Al-Omiri, M. K., Alhijawi, M., AlZarea, B. K., Abul Hassan, R. S. & Lynch, E. Ozone treatment of recurrent aphthous stomatitis: A double blinded study. Sci. Rep. 6, 27772. https://doi.org/10.1038/srep27772 (2016).
Grocholewicz, K. et al. Effect of nano-hydroxyapatite and ozone on approximal initial caries: A randomized clinical trial. Sci. Rep. 10, 11192. https://doi.org/10.1038/s41598-020-67885-8 (2020).
Taşdemir, Z., Alkan, B. A. & Albayrak, H. Effects of ozone therapy on the early healing period of deepithelialized gingival grafts: A randomized placebo-controlled clinical trial. J. Periodontol. 87, 663–671. https://doi.org/10.1902/jop.2016.150217 (2016).
Lynch, E. Evidence-based caries reversal using ozone. J. Esthet. Restor. Dent. 20, 218–222. https://doi.org/10.1111/j.1708-8240.2008.00183.x (2008).
Kazancioglu, H. O., Kurklu, E. & Ezirganli, S. Effects of ozone therapy on pain, swelling, and trismus following third molar surgery. Int. J. Oral Maxillofac. Surg. 43, 644–648. https://doi.org/10.1016/j.ijom.2013.11.006 (2014).
Atabek, D., Bodur, H., Yalçin, G. & Kalayci, Ş. Effects of oxidative irrigants on root dentin structure: Attenuated total reflection Fourier transform infrared spectroscopy study. Oral Health Dent. Manag. 13, 753–756 (2014).
Al-Omiri, M. K., Hassan, R. S., AlZarea, B. K. & Lynch, E. Effects of combining ozone and hydrogren peroxide on tooth bleaching: A clinical study. J. Dent. 53, 88–93. https://doi.org/10.1016/j.jdent.2016.08.002 (2016).
Al-Omiri, M. K., Lamfon, H. A., Al Nazeh, A. A., Kielbassa, A. M. & Lynch, E. Randomized clinical trial on the comparison of bleaching outcomes using either ozone or hydrogen peroxide. Quintessence Int. 49, 625–634. https://doi.org/10.3290/j.qi.a40783 (2018).
Al-Omiri, M. K., Al Nazeh, A. A., Kielbassa, A. M. & Lynch, E. Randomized controlled clinical trial on bleaching sensitivity and whitening efficacy of hydrogen peroxide versus combinations of hydrogen peroxide and ozone. Sci. Rep. 8, 2407. https://doi.org/10.1038/s41598-018-20878-0 (2018).
Al Habashneh, R., Alsalman, W. & Khader, Y. Ozone as an adjunct to conventional nonsurgical therapy in chronic periodontitis: A randomized controlled clinical trial. J. Periodont. Res. 50, 37–43. https://doi.org/10.1111/jre.12177 (2015).
Aykut-Yetkiner, A. et al. Color assessment after bleaching with hydrogen peroxide versus ozone: A randomized controlled clinical trial. Gen. Dent. 65, e12–e17 (2017).
Santana, M. S. et al. Dental bleaching with ozone: Effects on color and enamel microhardness. Acta Odontol. Latinoam 29, 68–75 (2016).
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G. & Grop, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 8, 336–341. https://doi.org/10.1016/j.ijsu.2010.02.007 (2010).
Higgins J. P. T. et al. Cochrane handbook for systematic reviews of interventions version 6.0 (updated July 2019). Cochrane. www.training.cochrane.org/handbook.
Aromataris, E. & Munn, Z. (Eds). JBI Manual for Evidence Synthesis (JBI, 2020). https://synthesismanual.jbi.global.
Sharma, G. Digital Color Imaging Handbook 41–45 (2003)
Balshem, H. et al. GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol. 64, 401–406 (2011).
Kury, M. et al. Color change, diffusion of hydrogen peroxide, and enamel morphology after in-office bleaching with violet light or nonthermal atmospheric plasma: An in vitro study. J. Esthet. Restor. Dent. 32, 102–112. https://doi.org/10.1111/jerd.12556 (2020).
Rezende, M. et al. Staining power of natural and artificial dyes after at-home dental bleaching. J. Contemp. Dent. Pract. 20, 424–427 (2019).
Ishikawa-Nagai, S., Yoshida, A., Sakai, M., Kristiansen, J. & Da Silva, J. D. Clinical evaluation of perceptibility of color differences between natural teeth and all-ceramic crowns. J. Dent. 37(Suppl 1), e57-63. https://doi.org/10.1016/j.jdent.2009.04.004 (2009).
Douglas, R. D., Steinhauer, T. J. & Wee, A. G. Intraoral determination of the tolerance of dentists for perceptibility and acceptability of shade mismatch. J. Prosthet. Dent. 97, 200–208. https://doi.org/10.1016/j.prosdent.2007.02.012 (2007).
Can-Karabulut, D. C. & Karabulut, B. Shear bond strength to enamel after power bleaching activated by different sources. Eur. J. Esthet. Dent. 5, 382–396 (2010).
Zanjani, V. A. et al. Bleaching effect of ozone on pigmented teeth. Dent. Res. J. (Isfahan) 12, 20–24. https://doi.org/10.4103/1735-3327.150295 (2015).
Al-Omiri, M. K., Abul Hassan, R. S., AlZarea, B. K. & Lynch, E. Comparison of dental bleaching effects of ozone and hydrogen peroxide: An ex vivo study. Am. J. Dent. 29, 251–254 (2016).
Jiang, T. et al. Hydrogen peroxide might bleach natural dentin by oxidizing phosphoprotein. J. Dent. Res. 97, 1339–1345. https://doi.org/10.1177/0022034518784260 (2018).
Kwon, S. R., Wang, J., Oyoyo, U. & Li, Y. Evaluation of bleaching efficacy and erosion potential of four different over-the-counter bleaching products. Am. J. Dent. 26, 356–360 (2013).
Bersezio, C. et al. One-year bleaching efficacy using two HP products with different pH: A double-blind randomized clinical trial. J. Esthet. Restor. Dent. 31, 493–499. https://doi.org/10.1111/jerd.12505 (2019).
Markowitz, K. Pretty painful: Why does tooth bleaching hurt?. Med. Hypotheses 74, 835–840. https://doi.org/10.1016/j.mehy.2009.11.044 (2010).
Almassri, H. N. S., Zhang, Q., Yang, X. & Wu, X. The effect of oral anti-inflammatory drugs on reducing tooth sensitivity due to in-office dental bleaching: A systematic review and meta-analysis. J. Am. Dent. Assoc. 150, e145–e157. https://doi.org/10.1016/j.adaj.2019.05.023 (2019).
Peixoto, A. C. et al. Preemptive use of piroxicam on tooth sensitivity caused by in-office bleaching: A randomized clinical trial. Braz. Dent. J. 30, 498–504. https://doi.org/10.1590/0103-6440201902762 (2019).
Carey, C. M. Tooth whitening: What we now know. J. Evid. Based Dent. Pract. 14(Suppl), 70–76. https://doi.org/10.1016/j.jebdp.2014.02.006 (2014).
Funding
This study was also financed in part by CAPES—Finance Code 001. We are also thankful for the support of CNPq (Council for Scientific and Technological Development—Brazil)—Finance Code 307808/2018-1.
Author information
Authors and Affiliations
Contributions
L.D., G.R.S., C.B., M.D.M.A.C., G.G.N. and L.R.P. designed research; L.D., M.D.M.A.C. and G.R.S. conducted research and analyzed data; L.D., L.R.P., G.G.N., C.B., M.D.M.A.C. and G.R.S. wrote the paper; L.R.P. and G.R.S. evidence certainty assessment; C.B. statistic data; G.G.N. and C.B. analyzed and interpreted data; L.D., M.D.M.A.C., G.R.S., L.R.P., G.G.N. and C.B. revised successive drafts of the manuscript. L.R.P. had primary responsibility for final content. All authors read and approved the final manuscript and all authors agree to be accountable for all aspects of work ensuring integrity and accuracy.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dietrich, L., de Assis Costa, M.D.M., Blumenberg, C. et al. A meta-analysis of ozone effect on tooth bleaching. Sci Rep 11, 13177 (2021). https://doi.org/10.1038/s41598-021-92733-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-92733-8
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.