Abstract
When two or more parasitoid species, particularly candidates for biocontrol, share the same target in the same temporal window, a complex of behaviors can occur among them. We studied the type of interactions (competition and intraguild predation) that existed between the nymphal parasitoids Anagyrus cachamai and A. lapachosus (Hymenoptera: Encyrtidae), two candidate neoclassical biocontrol agents against the Puerto Rican cactus pest mealybug, Hypogeococcus sp. (Hemiptera: Pseudococcidae). The surrogate native congener host in Argentina, the cactus mealybug Hypogeococcus sp., was studied to predict which species should be released; in the case that both should be released, in which order, and their potential impact on host suppression. In the laboratory we conducted experiments where different densities of the host mealybug were exposed to naive females of A. cachamai and A. lapachosus sequentially in both directions. Experiments were analyzed by combining a series of competitive behavioral and functional response models. A fully Bayesian approach was used to select the best explaining models and calculate their parameters. Intraguild predation existed between A. cachamai, the species that had the greatest ability to exploit the resource, and A. lapachosus, the strongest species in the interference competition. The role that intraguild predation played in suppression of Hypogeococcus sp. indicated that a multiple release strategy for the two biocontrol agents would produce better control than a single release; as for the release order, A. lapachosus should be released first.
Similar content being viewed by others
Introduction
Intraguild predation is a combination of exploitative competition and predation among potential competitors that use the same host or prey1,2, either a uni- or bidirectional interaction. When unidirectional, one of the interacting species is called the intraguild predator (a natural enemy that attacks another natural enemy species) and the other is the intraguild prey (the natural enemy attacked). In bidirectional cases each species fulfils both roles3. Intraguild predation may occur among only predators, only parasitoids, or different combinations of parasitoids and predators. When this interaction occurs between parasitoids and predators, it is typically asymmetrical, the parasitoid is always the subordinate species, a prey. This can have particular relevance in suppression of herbivorous insects since it can impact the population dynamics of both the natural enemies and the pest4. If one species simply kills the other without feeding on it, the interaction is qualified as interspecific killing, an extreme form of interference competition5. To ascertain the potential occurrence of intraguild predation in parasitoid-parasitoid interactions, the experimental design must quantify immediate fitness benefits of the species involved in the interaction. Predation of the intraguild prey should provide direct nutritional and energetic gains for the intraguild predator, which are reflected in increased growth, reproduction, or survival2.
The study of intraguild predation has become relevant in biological control programs because it can have negative consequences on pest mortality6,7. Although this problem could be avoided by the introduction of a single biological control agent, several reasons may promote the release of more than one agent, such as lack of efficacy of the biocontrol agent, low establishment rate8, or simply natural enemies present in the release area that negatively interact with the one agent9.
There is currently no consensus on the role of intraguild predation regarding the success of biological control programs10,11. The first theoretical models developed on intraguild predation analyzed the changes that occurred in the equilibrium of the populations of the intraguild predator, the intraguild prey, and in the resource shared by both (i.e., the pest), as a function of the environmental productivity12,13,14,15,16,17. According to these models, if the intraguild predator is a weaker resource competitor (inferior natural enemy) than the intraguild prey, both can coexist, or even exclude the intraguild prey. In both cases, the equilibrium density of the pest will increase as a result of the reduction of the intraguild prey, the natural enemy that has the greatest ability to exploit the resource (the pest). When the intraguild predator is a superior natural enemy of the exploitative competition, the intraguild predator will exclude the intraguild prey. In this case, there is no negative effect of the intraguild predation on the resulting biological control, and there is no benefit in releasing the intraguild prey to control the pest4. Janssen et al.18 when reviewing the theory of intraguild predation and its consequences on biological control, found that the resulting predictions from the above theoretical models were not confirmed in many of the empirical studies they analyzed. The authors considered, as possible causes of this discrepancy between theory and results, that the theoretical models do not take into account the effect of the antipredatory behavior of the intraguild prey and the pest, the complexity of the food chain, and the temporal and spatial scale analyzed. Mechanistic models of population, community and ecosystem dynamics require the mathematical description of trophic interactions in the form of functional response equations, which allows describing the number of hosts/preys attacked by a parasitoid/predator in relation to host/prey abundance. In fact, intraguild predation is strongly related to functional response. If the shared resource is abundant, the overlap between the intraguild predator and the intraguild prey would be rare, but if the resource is scarce, the interaction is inevitable. Although some studies integrated the inter- and intraspecific competition into the functional response models, there are no models that consider intraguild predation, or multiple interactions19,20. Functional response studies should include behavioral interactions among multiple consumer species or types of resources to improve their predictive power. So, just as population biology has gained enormously from the incorporation of individual processes in population models21, mechanistic understanding in community ecology can be increased by incorporating behavioral studies at the interspecific level into community models via multi specific functional response equations20. In biological control programs, this type of analysis could increase knowledge of interactions of the candidate species with each other and effects of their interactions on control of the pest. This information is of the utmost importance when there are several candidate species being considered in a biological control program since there is a wide range of potential interactions that could influence their performance22.
Parasitoid competition can be extrinsic (adult-adult) or intrinsic (adult-larva or larva-larva)23; it is usually studied through laboratory experiments, where hosts are exposed to parasitic wasps at different sequences and combinations. One of the most common difficulties of these kinds of experiments is to elucidate what happens inside the host24 and the effect of host availability on the ability of the wasps to locate the hosts25. To solve this problem, Bruzzone et al.26 proposed a novel approach that integrates competitive behavioral and functional response models. The models developed allow the description of the competition process of endoparasitoids both on interference (which species is a better interference competitor, if the competitor has advantages by arriving first; and when arriving second, whether the parasitoid avoids, accepts, or prefers the already parasitized hosts), and exploitation (if there are differences in terms of functional response).
The aim of this study was to analyze the type of interactions (competition and intraguild predation) that existed between two parasitoid species, both promising biological control candidates, and the effect of these interactions on mortality of the shared host. To reach the objective, the methodological approach proposed by Bruzzone et al.26 was used. We particularly analyzed the intrinsic interactions (adult-larva and larva-larva) of Anagyrus cachamai Triapitsyn, Logarzo & Aguirre and A. lapachosus Triapitsyn, Aguirre & Logarzo (Hymenoptera: Encyrtidae), candidate agents for the neoclassical biological control of the Harrisia cactus mealybug Hypogeococcus sp. (Hemiptera: Pseudococcidae), a Brazilian native turned invasive pest of the native cacti in Puerto Rico27,28,29. With the information obtained, we predicted if both parasitoid species should be released and the level of their potential impact on the target pest.
Methods
The studies were conducted at Fundación para el Estudio de Especies Invasivas (FuEDEI) facilities, located in Hurlingham, Buenos Aires, Argentina, between April 2015 and February 2017. All the experiments and insect rearing were carried out under laboratory-controlled conditions (25 ± 1 °C, 16:8 L:D, 60–80% RH), except stated otherwise.
Parasitoid rearing
Laboratory studies were carried out with colonies of the parasitoid species A. cachamai and A. lapachosus reared at FuEDEI facilities since 2014 with the methodology described in Aguirre et al.28. Both parasitoids were reared separately on first instar nymphs of Hypogeococcus sp. “Cactaceae host-clade”30, a congener but a different species from the pest Hypogeococcus sp. in Puerto Rico. Mealybug colonies were reared on clean potted plants of Cleistocactus baumannii (Lem.) Lem. (Cactaceae). All observations were conducted under a dissecting microscope at 40×.
Colonies of these two encyrtid species were reared in separate chambers. Four mated females of the same parasitoid species were placed in a plastic cage (2 L) with a hole in the lid (6 cm diameter) covered with polyester gauze for ventilation. The cage contained an excised piece of C. baumannii (20–25 cm long) infested with about 100 first instar nymphs of Hypogeococcus sp. “Cactaceae host-clade”, obtained from separate mealybug colonies. After 72 h, the four female wasps were removed from the cage, and the nymphs exposed to the parasitoids were monitored every 3 days. Once the first pupa was detected, monitoring was conducted daily, and all parasitoid pupae found were removed and transferred to a Petri dish (1.5 cm high × 5.5 cm diameter) covered with plastic food wrap to keep wasps from escaping after emergence. In this way, it was possible to control the wasps’ age, mating, and feeding conditions. The emerged wasps were transferred to a new Petri dish of equal dimensions, with a squashed drop of honey on the bottom, and covered with clear plastic food wrap, either for rearing or experimental purposes. The age of the female parasitoids for the experiments was 24–48 h old; they were fed, mated, and had no previous oviposition experience. From now on, when we mention nymphs of Hypogeococcus sp. exposed to female parasitoids, we refer to first instar nymphs of Hypogeococcus sp. “Cactaceae host-clade” on 20–25 cm long pieces of C. baumannii.
Interspecific parasitoid interaction experiments
These studies were performed by integrating functional response and competition experiments. Consequently, different Hypogeococcus sp. nymph densities were exposed to A. cachamai and A. lapachosus sequentially in both ways. As a result, two functional response curves were obtained for the interaction, one where the nymphs were first exposed to females of A. cachamai and then the same nymphs were exposed to females of A. lapachosus. The second functional response curve was the reciprocal experiment where nymphs were first exposed to A. lapachosus. At the same time, the potential impact of each of the two studied parasitoids on the target pest was estimated with a standard functional response trial conducted with each parasitoid species, ergo the number of hosts attacked as a function of hosts offered to each parasitoid species without interaction was estimated. The functional response curves in the absence of interaction were used as a baseline to the treatments of consecutive exposures, and they also served as a null model in which the absence of interference between the hosts was postulated.
To estimate the functional response curves of the interaction between A. cachamai and A. lapachosus, Hypogeococcus sp. nymphs were exposed at a constant density to a female of one parasitoid species (A. cachamai or A. lapachosus) for 24 h, at the end of which, the female was removed and the nymphs were exposed to a female of the second, different species for another 24 h. The reciprocal exposure for the two wasp species was also performed. Both exposure combinations were conducted at 10 different nymph densities: 10, 20, 30, 40, 50, 60, 70, 80, 90 and 110, with a maximum error of 10% in the number of nymphs per density offered. The densities were determined based on the results of a pilot test, where densities of 80 and 110 nymphs produced a plateau in the curve of the number of nymphs attacked as a function of the density offered. The nymphs exposed to both A. cachamai and A. lapachosus females were monitored every 3 days until all non-parasitized nymphs completed their development and wasps emerged from the parasitized nymphs. For each density tested, the number and species of parasitoids emerged and the number of non-parasitized nymphs was recorded. Functional response of each species in the absence of interaction (baseline) was estimated with an experimental design similar to that explained above, with the difference that nymphs were not exposed to a second female of the alternative species. The densities used for the construction of the functional response curves were 10, 20, 40, 60, 80 and 110 nymphs (with a maximum error of 10% in the number of nymphs per density offered), and each density was replicated five times. The experiments were conducted in vented plastic cages similar to the one described for the parasitoid rearing.
Data analyses
The outcome of the interspecific parasitoid interaction experiments was analyzed through a Bayesian process of model selection. This analysis procedure started with a simple model of type one functional response, adding terms until an optimal model was obtained resulting in a balance between explanatory power and complexity. The result was the identification of the competitive relationship between parasitoids (Bruzzone et al.26; see appendix section for details on the models used (Supplementary).
The main reason for using this approach was the lack of complete control of the number of hosts offered in each trial, and the inability to directly observe the interaction between parasitoid larvae since both parasitoid species are endoparasitoids and the interaction occurred inside very small individual nymphs (0.35 ± 0.06 mm). To address these drawbacks of the experimental setup, we opted to model the number of expected emerging parasitoids using a functional response model to estimate the expected number of hosts attacked by each parasitoid species. Once we had an estimate of the number of hosts attacked, it was possible to estimate the overlap degree of the hosts attacked by both parasitoid species, estimating in turn the competition process between hosts by comparing the number of parasitoids emerged with those expected through different competition models.
First, we estimated the number of nymphs attacked as a function of the nymphs offered; consequently, an estimate of the parasitoid functional response was needed. A host selection model using a set theory was necessary to identify how the female parasitoid chose its hosts, and its output was applied to a well-known model of competition, in this case, the Thurstone/Bradley Terry Model31,32.
The base model of the interspecific parasitoid interaction experiments between the first species to arrive (species 1), and the second to arrive (species 2) was as follows:
where \({R}_{12}(p)={R}_{1}\left(p\right)\cap {R}_{2}\left(p\right)\), where E1/2 is the expected number of species 1 emerged, given that species 2 also attacks the same host, and p is the number of hosts available. R1(p) is the functional response of species 1; R2(p) is the functional response of species 2; and w12 is the proportion of times in which species 1 won in the competition against species 2. The term R10(p) represents the hosts attacked by species 1 that are not attacked by species 2, and R12(p) are the hosts attacked by species 1 that are later attacked by species 2, so R1(p) = R10(p) + R12(p). The whole term \({R}_{1}\left(p\right)\cap {R}_{2}(p)\) is the expected superposition of the functional responses of species 1 and 2.
The functional response model gives the proportion of hosts attacked by each parasitoid, which allowed us to describe the exploitation competition. The competition model provides the proportions of each species of parasitoid that emerges from the hosts attacked by both, which allowed us to analyze whether interference competition or intraguild predation existed. The interference competitive behavior was divided into three dimensions: competitive rating, the effect in the order of arrival to the host in the parasitoid’s competitive strength, and a parasitoid superposition model (an estimator of the degree of utilization/rejection of already parasitized hosts). We also modeled whether host mortality was constant and independent of the number of parasitoids that attacked a host, and if parasitoids were able to differentiate between suitable and unsuitable hosts (Supplementary, Equation (8)). Finally, we performed a stepwise selection of the proposed models in order to find which one had the best balance between explanation of the data (in terms of the likelihood function) and complexity (in terms of number of parameters).
The selection of models and the estimation of parameter distribution were conducted in a Bayesian framework. We performed the model selection procedure using the algorithm Reversible Jump Markov-Chain Monte Carlo, in which the routine automatically “jumped” from one model to another and then selected the best model balancing information and fitting. To achieve this, in each jump, for each additional parameter, the log-likelihood function was penalized with a value of minus two. The first 40,000 iterations of the reversible procedure were discarded as a burn-in model selection, and the last 20,000 were used to calculate the weight of each model in the model averaging procedure. Also, 10,000 iterations of Markov Chain Monte Carlo were performed for each iteration of the Reversible Jump algorithm33, resulting in a total of 60,000,000 iterations. The last 20,000,000 iterations were used to calculate a posteriori distribution of the parameters. Expected vs. observed values were compared using a binomial likelihood function for the number of the parasitoids emerged in relation to the total hosts offered. The a priori distribution of the parameters of the functional response curves were a non-informative uniform distribution between 0 and 1, the same for the multiparasitism index. On the other hand, for the competition parameters, the a priori distribution was a normal distribution with mean 0 and deviance 10 for all the parameters since we did not have a priori information of the variables distribution. As in the studies of competitive behavior, the strength of each species is an “interval scale” and therefore does not have an origin ordinate34. The variable of the species A. cachamai was arbitrarily fixed as 0 and used as a reference of the competitive strength of A. lapachosus (the ordinate of origin was the A. cachamai strength, and the interval unit was the standard deviation of that species’ strength). All analyses were performed using a PyMC library for Bayesian estimation35 in the Python programming language.
Results
Interspecific parasitoid interaction experiments
The results of the laboratory experiments are shown in Table 1. In the absence of interaction, both A. lapachosus and A. cachamai females parasitized around 24% of Hypogeococcus sp. exposed nymphs. In the presence of interaction, A. lapachosus attacked 20.1% of the nymphs offered when A. cachamai females were released first. In the reciprocal experiment, when A. lapachosus was released first, 23.89% of host nymphs were parasitized by A. lapachosus. Parasitism by A. cachamai revealed an attack of 18.94% of the nymphs offered when A. lapachosus females were released first and an attack of 11.51% of the offered nymphs when A. cachamai was released first. The percentage of parasitism produced by a single species was lower than when two species were sequentially introduced in the arena, regardless of the order of release (Table 1).
Data analyses
From the 112 proposed models to analyze the results of the laboratory experiments, eight models were selected via the reversible jump procedure (Table 2). These integrated models comprised between 14 and 16 biological parameters that explained the behavior of wasps when they shared the same host; first instar nymphs of Hypogeococcus sp. All the models selected indicated that A. cachamai and A. lapachosus competed with each other.
When the components of the eight selected models were analyzed, we found that in terms of functional response, all the selected models had generalized type III functional response (GP), the models without host depletion (NHD) were selected in 66.7% of the iterations, and those GP that incorporated Roger’s host depletion correction (HD) were selected for the remaining 33.3% of iterations (Table 2). In the GP models (Supplementary, Equation (1.3)), the attack rate (a) follows a linear relationship to the density of the hosts offered (p). The initial attack rate of an inexperienced female (b) was 0.10 ± 0.03 d−1 for A. cachamai females and 0.11 ± 0.05 d−1 for A. lapachosus females, and no difference was observed between the species. The attack rate was higher for A. lapachosus than for A. cachamai females (0.007 ± 0.001 vs. 0.003 ± 0.001 d−1), while the handling time (H) was shorter for A. cachamai than A. lapachosus females (0.002 ± 0.002 vs. 0.019 ± 0.004 d) (Table 3). Therefore, the handling time of A. cachamai differed by an order of magnitude with respect to that found in A. lapachosus, while the attack rate, as a function of the number of nymphs offered, was in the same order of magnitude (Figs. 1 and 2). The estimated functional response curves indicated that A. lapachosus was the most efficient species at densities of Hypogeococcus sp. nymphs below 83, while above this value, A. cachamai was more efficient (Fig. 3).
Observed functional response of the parasitoid Anagyrus cachamai attacking Hypogeococcus sp. nymphs. Solid line indicates the mean estimation of functional response, grey area indicates its credibility interval, and dashed line indicates the a posteriori credibility interval for individual measurements. White circles are the observed number of emerged parasitoids in interaction experiments, while dark circles are the number of parasitoids emerged in experiments without interaction.
Observed functional response of the parasitoid Anagyrus lapachosus attacking Hypogeococcus sp. nymphs. Solid line indicates the mean estimation of functional response, grey area indicates its credibility interval, and dashed line indicates the a posteriori credibility interval for individual measurements. White circles are the observed number of emerged parasitoids in interaction experiments, while dark circles are the number of parasitoids emerged in experiments without interaction.
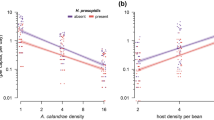
Observed functional response curves of two competing parasitoid species, Anagyrus cachamai and A. lapachosus, attacking Hypogeococcus sp. nymphs. The black line represents the average estimated functional response for A. cachamai and the dotted line for A. lapachosus. The light gray area indicates the credibility interval for the estimated functional response of A. cachamai, and the dark gray area shows the credibility interval for A. lapachosus.
The interference competition behavior models revealed that A. lapachosus was the species that showed the greatest strength of the two species (Table 3). The models that considered the existence of competitive advantage by the order of arrival to the host and incorporated the females’ ability to discriminate between parasitized and unparasitized hosts were selected in 100% of the iterations (Table 2). The species arriving first to the host had a competitive disadvantage over the one that arrived second (Table 3). The competitive strength of the first arrival species was calculated as: µ1 + h1 (µ: strength and h: first arrival term). With this information, we calculated the proportion of times in which the strength of the second species arriving at the host was superior to the strength of the species that arrived first, and therefore won the competition according to Equation (3) (Supplementary). The competitive strength of A. cachamai females when they arrived first to Hypogeococcus sp. nymphs was − 0.9 ± 0.7 (µ1 = 0.0 + h1 = − 0.9; see Table 3). Since the competitive strength of A. lapachosus was 0.2 ± 0.7, there was a mean difference of strength of 1.1 between the two species, indicating that A. lapachosus was able to win a proportion of 0.9 (0.5–1) interactions against A. cachamai when arriving second. When the first arriving parasitoid was A. lapachosus, its competitive strength was − 0.5 ± 1.1 (µ1 = 0.2 + h1 = − 0.7; see Table 3). As the strength of A. cachamai was 0 ± 0 and the difference of strength was − 0.5, when A. cachamai arrived second to the host it was able to win 0.7 (0.3–0.9) of the contests. The behavior of avoidance/random/preference of the already parasitized hosts revealed that A. cachamai females avoided ovipositing in the already parasitized nymphs whereas A. lapachosus females preferred the parasitized hosts (Table 3).
The models that considered rejection by females of unsuitable hosts were selected in 44.4% of the iterations (Table 2). According to this result, a proportion of 0.15 of the nymphs offered to A. cachamai and A. lapachosus females were avoided by both parasitoid species. Models that considered increased mortality caused by multiparasitism were selected in 40.9% of the iterations (Table 2). This meant that some Hypogeococcus sp. nymphs died as a direct effect of A. cachamai or A. lapachosus female oviposition, regardless of parasitoid species. The increase in host mortality was 0.14 ± 0.07 in the first attack, raising this value with the number of attacks (n) as (1 − (0.14 ± 0.07))n (according to Equation (8), Supplementary).
Discussion
Our data provided evidence that A. lapachosus did practice intraguild predation on A. cachamai. The species differed in terms of their functional response, interference competitive strength, and host selection behavior. Anagyrus cachamai was the species that had the greatest ability to exploit the resource, while A. lapachosus was the strongest species in the interference competition. The functional response models highlighted a superior host exploitation ability for A. cachamai. On the other hand, the outcome of competition models indicated that asymmetric larval competition occurred between A. cachamai and A. lapachosus, with the latter outcompeting the former. Likewise, A. lapachosus females preferred parasitized mealybugs to unparasitized ones, while A. cachamai females avoided them. These behavioral differences played a key role in the wasp emergence patterns that were identified (Table 1).
The coexistence among species with different competitive strengths may be possible if the parasitoids possess differences in their competitive abilities36,37. In this context, it is expected that the weakest competitor presents the greatest ability to exploit the resource38,39. We found that A. cachamai, the less aggressive species, was the most efficient consumer since it had a shorter handling time. Although A. lapachosus females presented the highest change in the attack rate as a function of the number of nymphs offered, it was in the same order of magnitude as that observed in A. cachamai, while the difference observed in the handling time differed by an order of magnitude. Cusumano et al.40 found similar results for the interaction between Trissolcus basalis (Wollaston) (Hymenoptera: Scelionidae) and Ooencyrtus telenomicida (Vassiliev) (Hymenoptera: Encyrtidae), both egg parasitoids of Nezara viridula (L.) (Hemiptera: Pentatomidae). For this parasitoid-parasitoid interaction, T. basalis was the most efficient consumer, showing the shorter handling time, while O. telenomicida was better in larval competition. The authors suggested that T. basalis–O. telenomicida coexistence can be driven by a trade-off between host finding and competition.
Similar to many parasitoid species41,42,43, the order of arrival to the host affected the competitive strength of A. cachamai and A. lapachosus. Anagyrus cachamai females experienced a decrease in their competitive strength when the females arrived first to the host. The same pattern was observed in A. lapachosus females. However, the consequences for each parasitoid species were different. The second female species might have produced physical and chemical changes in the host’s environment to create conditions that favored its own larval survival in detriment to the parasitoid that arrived first24,44. For instance, the parasitoid female might inject viruses or toxins during oviposition45 or mechanically eliminate the immature competitor larva with its ovipositor23.
Regarding the behavior of attacking an already parasitized host, the strength of the second arriving species is expected to be higher than the one already inside the host46. Although the competitive strength of both Anagyrus species increased when they arrived second to the host, the proportion of times where A. cachamai arrived second to the host and won the competition against A. lapachosus was highly variable (0.7 (0.3–0.9)). Anagyrus cachamai females probably compensated their reduced competitive ability in the interference competition with a faster host manipulation and avoidance of parasitized hosts. As mentioned above, A. lapachosus females preferred parasitized nymphs to non-parasitized ones. The acceptance of hosts within the same trophic level is a mechanism to eliminate competitors, as well as a strategy to obtain high-protein or alternative hosts when the resource is scarce2,17. To our knowledge, our study is the first report of intraguild predation within the genus Anagyrus Howard. We hypothesized that A. lapachosus larvae behave either as predators of A. cachamai larvae during intrinsic competition, or, perhaps less likely, the former species could behave as a facultative hyperparasitoid. However, we have found no records of hyperparasitoid species in the genus Anagyrus, which is comprised entirely of primary parasitoids of various mealybugs.
Some of the selected models (44.4%) indicated that A. cachamai and A. lapachosus females did not attack a certain proportion of the hosts offered. Possibly, the hosts were not suitable for the development of their offspring. An alternative explanation may be that only 24–48 h old females were used, not considering the synovigenic behavior of the two parasitoid species28, where their egg load changed throughout the female’s lifespan. Transient egg limitation can make eggs more valuable than if the wasps never experienced a limitation, influencing females to be less likely to lay eggs in unsuitable hosts. Similarly, egg reabsorption often confers greater fitness than ovipositing in unsuitable hosts. To achieve a better understanding of the process or processes that affected host selection behavior of both Anagyrus species, it will be necessary to expand the interaction experiment design to include additional factors, for example, females of different ages.
Parasitoid impact on host population is underestimated when host mortality is not taken into account47. Both larval and adult parasitoids can induce host death following oviposition. When neither parasitoid nor host emerges, Abram et al.47 called it non-reproductive effect. However, host mortality cannot be easily measured, especially if it is not possible to detect the parasitoid via dissection or if the oviposition was interrupted and no egg was laid48. Currently, in biological control programs, the population consequences of non-reproductive mortality of hosts induced by their parasitoids and its effects in multiple-hosts systems are unknown48,49. Abram et al.47 proposed including the contribution of non-reproductive mortality both in models of host-parasitoid population dynamics, as in those that include multi-trophic interactions, in order to have a better understanding regarding its effect on the community interactions. With the methodological approach proposed in this study, we found that multi parasitism increased the death probability of the Hypogeococcus sp. nymphs. This result was also reported for other parasitoid species50,51. Host mortality increase can be caused by the physical damage produced by the increase in the number of stings with or without oviposited larvae per host, changes in its internal environment52, host rejection48, death of the egg or larva of the parasitoids that do not develop but end up killing the host53, and parasitized hosts being more susceptible to infections54.
Finally, to analyze the type of interactions that existed between A. cachamai and A. lapachosus, we used models both with host depletion (exponential decaying host density with consumption, according to Rogers55) or without it (the basic Holling’s models56). In our simulations, we found that one-third of the time, the Rogers models were selected, and another two-thirds, the Hollings were selected. These results suggest that indeed the host depletion is affecting the performance of the parasitoids, in an intermediate form between that proposed by Rogers and the original from Holling.
The role that intraguild predation played on the interaction between A. cachamai and A. lapachosus and its consequences for the control of Hypogeococcus sp. revealed two possible scenarios that depended on the order in which the Hypogeococcus sp. nymphs were exposed to these parasitoids. If the nymphs were first exposed to A. cachamai and then to A. lapachosus, given that A. lapachosus females preferred the parasitized nymphs, the degree of overlap between these two species would be high. As a consequence, control by the parasitoids would be lower than expected when the interaction is at random, as it is in the case when A. lapachosus females do not have a host selection behavior (Fig. 4). If the order of exposure of nymphs to parasitoids was reversed, the overlap between A. cachamai and A. lapachosus would decrease. The females of A. cachamai had a greater ability to exploit the resource than those of A. lapachosus, and the former species avoided the already parasitized nymphs, the total number of parasitized nymphs would increase, exceeding that expected by random (Fig. 5).
Expected functional response in the interaction between the parasitoids Anagyrus cachamai (A) and A. lapachosus (B), when the nymphs of Hypogeococcus sp. were first exposed to A. cachamai. Black line: number of nymphs offered is equal to the number of nymphs attacked by parasitoids. Dotted line: females of A. lapachosus do not have a host selection behavior; therefore, the number of nymphs attacked is at random.
Expected functional response in the interaction between the parasitoids Anagyrus cachamai (A) and A. lapachosus (B), when the nymphs of Hypogeococcus sp. were first exposed to A. lapachosus. Black line: number of nymphs offered is equal to the number of nymphs attacked by parasitoids. Dotted line: females of A. cachamai do not have a host selection behavior; therefore, the number of nymphs attacked is at random.
In conclusion, our models predicted that a multiple species release strategy would likely produce more control of the pest host than a single species release when A. lapachosus was released first. To obtain a more comprehensive knowledge of the interactions between these two parasitoids on the suppression of Hypogeococcus sp., investigations on continuous generations should be conducted. It is also necessary to identify and characterize the natural enemies present in the release area given that negative interactions with other parasitoids and/or predators could adversely affect pest control. Our next goal is to investigate the interactions between A. cachamai and A. lapachosus on continuous generations in field manipulative experiments.
References
Arim, M. & Marquet, P. A. Intraguild predation: A widespread interaction related to species biology. Ecol. Lett. 7, 557–564. https://doi.org/10.1111/j.1461-0248.2004.00613.x (2004).
Polis, G. A., Myers, C. A. & Holt, R. D. The ecology and evolution of intraguild predation: Potential competitors that eat each other. Annu. Rev. Ecol. Syst. 20, 297–330. https://doi.org/10.1146/annurev.es.20.110189.001501 (1989).
Rosenheim, J. A., Kaya, H. K., Ehler, L. E., Marois, J. J. & Jaffee, B. A. Intraguild predation among biological-control agents: Theory and evidence. Biol. Control 5, 303–335. https://doi.org/10.1006/bcon.1995.1038 (1995).
Rosenheim, J. A. & Harmon, J. P. The influence of intraguild predation on the suppression of a shared prey population: An empirical reassessment. In Trophic and Guild in Biological Interactions Control (eds Brodeur, J. & Boivin, G.) 1–20 (Springer, 2006) https://doi.org/10.1007/1-4020-4767-3_1.
Fonseca, M. M. et al. How to evaluate the potential occurrence of intraguild predation. Exp. Appl. Acarol. 72, 103–114. https://doi.org/10.1007/s10493-017-0142-x (2017).
Ferguson, K. I. & Stiling, P. Non-additive effects of multiple natural enemies on aphid populations. Oecologia 108, 375–379 (1996).
Hindayana, D., Meyhöfer, R., Scholz, D. & Poehling, H.-M. Intraguild predation among the hoverfly Episyrphus balteatus de Geer (Diptera: Syrphidae) and other aphidophagous predators. Biol. Control 20, 236–246 (2001).
Denoth, M., Frid, L. & Myers, J. H. Multiple agents in biological control: Improving the odds?. Biol. Control 24, 20–30. https://doi.org/10.1016/S1049-9644(02)00002-6 (2002).
Muştu, M., Kilinçer, N., Ülgentürk, S. & Kaydan, M. B. Feeding behavior of Cryptolaemus montrouzieri on mealybugs parasitized by Anagyrus pseudococci. Phytoparasitica 36, 360–367 (2008).
Lucas, E. Intraguild predation among aphidophagous predators. Eur. J. Entomol. 102, 351–364 (2005).
Muştu, M. & Kilinçer, N. Intraguild predation of Planococcus ficus parasitoids Anagyrus pseudococci and Leptomastix dactylopii by Nephus kreissli. Biocontrol Sci. Technol. 24, 257–269. https://doi.org/10.1080/09583157.2013.856866 (2014).
Diehl, S. & Feißel, M. Effects of enrichment on three-level food chains with omnivory. Am. Nat. 155, 200–218 (2000).
Holt, R. D. & Polis, G. A. A theoretical framework for intraguild predation. Am. Nat. 149, 745–764 (1997).
Kuijper, L. D. J., Kooi, B. W., Zonneveld, C. & Kooijman, S. A. L. M. Omnivory and food web dynamics. Ecol. Modell. 163, 19–32 (2003).
Morin, P. Productivity, intraguild predation, and population dynamics in experimental food webs. Ecology 80, 752–760 (1999).
Mylius, S. D., Klumpers, K., de Roos, A. M. & Persson, L. Impact of intraguild predation and stage structure on simple communities along a productivity gradient. Am. Nat. 158, 259–276 (2001).
Polis, G. A. & Holt, R. D. Intraguild predation: The dynamics of complex trophic interactions. Trends Ecol. Evol. 7, 151–154 (1992).
Janssen, A. et al. Intraguild predation usually does not disrupt biological control. In Trophic and Guild in Biological Interactions Control (eds Brodeur, J. & Boivin, G.) 21–44 (Springer, 2006) https://doi.org/10.1007/1-4020-4767-3_2.
Skalski, G. T. & Gilliam, J. F. Functional responses with predator interference: Viable alternatives to the Holling type II model. Ecology 82, 3083–3092 (2001).
de Villemereuil, P. B. & López-Sepulcre, A. Consumer functional responses under intra- and inter-specific interference competition. Ecol. Modell. 222, 419–426. https://doi.org/10.1016/j.ecolmodel.2010.10.011 (2011).
Sutherland, W. J. From Individual Behaviour to Population Ecology (Oxford Series in Ecology and Evolution, 1996).
Pedersen, B. S. & Mills, N. J. Single vs. multiple introduction in biological control: The roles of parasitoid efficiency, antagonism and niche overlap. J. Appl. Ecol. 41, 973–984 (2004).
Godfray, H. C. J. & Godfray, H. C. J. Parasitoids: Behavioral and Evolutionary Ecology Vol. 67 (Princeton University Press, 1994).
Harvey, J. A., Poelman, E. H. & Tanaka, T. Intrinsic inter-and intraspecific competition in parasitoid wasps. Annu. Rev. Entomol. 58, 333–351 (2013).
Peri, E., Cusumano, A., Amodeo, V., Wajnberg, E. & Colazza, S. Intraguild interactions between two egg parasitoids of a true bug in semi-field and field conditions. PLoS ONE 9(6), e99876. https://doi.org/10.1371/journal.pone.0099876 (2014).
Bruzzone, O. A., Logarzo, G. A., Aguirre, M. B. & Virla, E. G. Intra-host interspecific larval parasitoid competition solved using modelling and bayesian statistics. Ecol. Modell. 385, 114–123 (2018).
Triapitsyn, S. V. et al. Complex of primary and secondary parasitoids (Hymenoptera: Encyrtidae and Signiphoridae) of Hypogeococcus spp. mealybugs (Hemiptera: Pseudococcidae) in the New World. Florida Entomol. 101, 411–434. https://doi.org/10.1653/024.101.0320 (2018).
Aguirre, M. B. et al. Analysis of biological traits of Anagyrus cachamai and Anagyrus lapachosus to assess their potential as biological control candidate agents against Harrisia cactus mealybug pest in Puerto Rico. Biocontrol 64, 539–551. https://doi.org/10.1007/s10526-019-09956-y (2019).
Poveda-Martínez, D. et al. Species complex diversification by host plant use in an herbivorous insect: The source of Puerto Rican cactus mealybug pest and implications for biological control. Ecol. Evol. 10, 10463–10480. https://doi.org/10.1002/ece3.6702 (2020).
Poveda-Martínez, D. et al. Untangling the Hypogeococcus pungens species complex (Hemiptera: Pseudococcidae) for Argentina, Australia, and Puerto Rico based on host plant associations and genetic evidence. PLoS ONE 14(7), e0220366. https://doi.org/10.1371/journal.pone.0220366 (2019).
Thurstone, L. L. A law of comparative judgment. Psychol. Rev. 34, 273 (1927).
Bradley, R. A. & Terry, M. E. Rank analysis of incomplete block designs: I. The method of paired comparisons. Biometrika 39, 324–345. https://doi.org/10.2307/2334029 (1952).
Gelman, A., Carlin, J. B., Stern, H. S. & Rubin, D. B. Bayesian data analysis. In Texts Stat. Sci. 2nd ed, 661 (CRC Press, 2003).
Stevens, S. S. On the Theory of Scales of Measurement, vol. 103, 677–680 (1946).
Patil, A., Huard, D. & Fonnesbeck, C. J. PyMC: Bayesian stochastic modelling in Python. J. Stat. Softw. 35, 1 (2010).
Zwölfer, H. The structure and effect of parasite complexes attacking phytophagous host insects. In Proc. Adv. Study Inst. Dyn. Numbers Popul. 405–418 (1971).
Zwölfer, H. Strategies and counterstrategies in insect population systems competing for space and food in flower headsand plant galls. Fortschr. Zool. 25(2/3), 331–353 (1979).
Vance, R. R. The stable coexistence of two competitors for one resource. Am. Nat. 126, 72–86 (1985).
Fellers, J. H. Interference and exploitation in a guild of woodland ants. Ecology 68, 1466–1478 (1987).
Cusumano, A., Peri, E., Vinson, S. B. & Colazza, S. Intraguild interactions between two egg parasitoids exploring host patches. Biocontrol 56, 173–184 (2011).
Mizutani, N. Interspecific larval competition among three egg parasitoid species on the host, Riptortus clavatus (Thunberg) (Heteroptera: Alydidae). Proc. Assoc. Plant Prot. Kyushu 40, 106–110 (1994).
Weber, C. A., Smilanick, J. M., Ehler, L. E. & Zalom, F. G. Ovipositional behavior and host discrimination in three scelionid egg parasitoids of stink bugs. Biol. Control 6, 245–252 (1996).
Alim, M. A. & Lim, U. T. Interspecific larval competition between two egg parasitoids in refrigerated host eggs of Riptortus pedestris (Hemiptera: Alydidae). Biocontrol Sci. Technol. 21, 395–407 (2011).
De Moraes, C. M. & Lewis, W. J. Analyses of two parasitoids with convergent foraging strategies. J. Insect Behav. 12, 571–583 (1999).
Mackauer, M. Host discrimination and larval competition in solitary endoparasitoids. Crit. Issues Biol. Control, Intercept, Andover, Hants, UK. xvii + 330 pp (1990).
Strand, M. R. & Godfray, H. C. J. Superparasitism and ovicide in parasitic Hymenoptera: Theory and a case study of the ectoparasitoid Bracon hebetor. Behav. Ecol. Sociobiol. 24, 421–432 (1989).
Abram, P. K., Brodeur, J., Urbaneja, A. & Tena, A. Nonreproductive effects of insect parasitoids on their hosts. Annu. Rev. Entomol. 64, 259–276 (2019).
Abram, P. K., Brodeur, J., Burte, V. & Boivin, G. Parasitoid-induced host egg abortion: An underappreciated component of biological control services provided by egg parasitoids. Biol. Control 98, 52–60 (2016).
Abram, P. K., Gariepy, T. D., Boivin, G. & Brodeur, J. An invasive stink bug as an evolutionary trap for an indigenous egg parasitoid. Biol. Invasions 16, 1387–1395 (2014).
Steiner, A. L. Stinging behaviour of solitary wasps. In Venoms of the Hymenoptera. Biochemical, Pharmacological and Behavioural Aspects (ed Piek, T.) 63–148 (Academic Press, London, 1986) https://doi.org/10.1016/b978-0-12-554770-3.50008-5.
Feng, Y., Wratten, S., Sandhu, H. & Keller, M. Interspecific competition between two generalist parasitoids that attack the leafroller Epiphyas postvittana (Lepidoptera: Tortricidae). Bull. Entomol. Res. 105, 426–433 (2015).
De Moraes, C. M. & Mescher, M. C. Intrinsic competition between larval parasitoids with different degrees of host specificity. Ecol. Entomol. 30, 564–570 (2005).
Desneux, N., Barta, R. J., Hoelmer, K. A., Hopper, K. R. & Heimpel, G. E. Multifaceted determinants of host specificity in an aphid parasitoid. Oecologia 160, 387–398 (2009).
Brodeur, J. & Boivin, G. Functional ecology of immature parasitoids. Annu. Rev. Entomol. 49, 27–49 (2004).
Rogers, D. Random search and insect population models. J. Anim. Ecol. 41, 369–383 (1972).
Holling, C. S. Some characteristics of simple types of predation and parasitism. Can. Entomol. 91, 385–398. https://doi.org/10.4039/Ent91385-7 (1959).
Acknowledgements
We thank Arabella Peard for reviewing the draft of the manuscript. María B. Aguirre is a posdoctoral fellow of CONICET (Consejo Nacional de Investigaciones Científicas y Técnicas, Argentina). Octavio Bruzzone is a member of CONICET Carrera del Investigador Científico. This study was funded by the USDA-APHIS Farm Bill 17-8130-0757-IA (2017) and 19-8130-0852-IA (2019), and USDA-APHIS Invasive Species Coordination Program from 2014 to 2016, APH-HQ-16-0181.
Author information
Authors and Affiliations
Contributions
M.B.A., O.A.B. and G.A.L. contributed to the study conception and design, investigation, data curation, and formal analysis. G.A.L. and O.A.B. supervised de project. O.A.B. designed mathematical models and with M.B.A. conducted statistical analysis. S.V.T. conducted data curation and editorial advice. H.D-S. and S.D.H. funded the research. All authors contributed to writing and revising the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aguirre, M.B., Bruzzone, O.A., Triapitsyn, S.V. et al. Influence of competition and intraguild predation between two candidate biocontrol parasitoids on their potential impact against Harrisia cactus mealybug, Hypogeococcus sp. (Hemiptera: Pseudococcidae). Sci Rep 11, 13377 (2021). https://doi.org/10.1038/s41598-021-92565-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-92565-6
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.