Abstract
In this paper, we report extraction of cashew nut shell liquid (CNSL) from cashew nut shell waste (CNSW) and further use of residues for generation of activated carbon for removal of heavy metals and methylene blue (MB). Solvent extraction yielded 24.6 ± 0.4%, 38.2 ± 0.4% and 40.1 ± 0.9% for petroleum ether, hexane and ethanol respectively. Phytochemical screening showed presence of alkaloids, carbohydrates, saponins, phenols, tannins, flavonoids, amino acids, terpenoids, proteins, steroids, glycosides and carboxylic acids. The CNSL had a pH of 3.2, viscosity (104.6 ± 1.8 mPa s), moisture (6.5%), ash (1.6 ± 0.1%), refractive index (1.52 ± 0.001), specific density (0.9561 ± 0.0002 g/cm3), acid value (118.7 ± 9.2 mg KOH/g), free fatty acid value (60.1 ± 4.7%), saponification number (138.1 ± 3.2 mg KOH/g) and iodine value (188.1 ± 2.3 mgI 2/100 g). The average percentage removal of Cu (II), Pb (II), Cd (II) and Zn (II) was 99.4 ± 0.5, 95.4 ± 1.5, 99.5 ± 0.1, 98.4 ± 0.1%, and removal efficiency of MB at 50, 150, 250 and 350 mg/L was 99.63, 97.66, 96.48 and 94.81%, respectively. Equilibrium data were best described by the Freundlich isotherm model. The maximum monolayer adsorption capacity was 12.1 mg/g. The adsorption kinetics conformed to pseudo-second-order model. ∆G° was negative and a ∆H° of + 22.76 kJ/mol indicated that adsorption was endothermic. The ΔS° (+ 0.086 kJ/mol/K) showed that there was spontaneous interaction of the solution and adsorbate. These results show that CNSW is a potential bioresource for CNSL production for use in the paints, varnishes, surface coatings, agrochemicals and ethnomedicine industries. Residual shells can be exploited as fuels or converted to activated carbon for use as low-cost filters in water purification.
Similar content being viewed by others
Introduction
The cashew tree (Anarcardium occidentale) is a native of Brazil and the Lower Amazons. The major producing countries of cashew are Tanzania, India, Mozambique, Sri lanka, Kenya, Madagascar, Thailand, Malaysia, Indonesia, Nigeria, Senegal, Malawi and Angola1. In Zambia, Cashew trees were first introduced in 1940s by the Portuguese traders in Western Province (then, Barotseland), an area characterized by Kalahari sandy soils that are relatively poor for most conventional crops. In order to diversify the economic base of the poor households in the Western Province, the Government of the Republic of Zambia (GRZ) promoted the planting and processing of cashew trees in the 1980s. However, the growth of the cashew industry was very slow due to low production and lack of marketing and processing facilities2.
In 2015 Zambia acquired a loan from Africa Development Bank under the Cashew Infrastructure Development Project (CIDP) to boost cashew nut production2,3 as well as increasing production in the province that has largely remained poor for a long time4. In Zambia, cashew is grown mainly for its kernels while the shells are discarded in the environment as waste hence, contributing to the already existing waste management crisis in the country5. However, unlike other solid wastes, the cashew nut shell (CNS) harbour’s a dark brown viscous oil called cashew nut shell liquid (CNSL), located in the soft honeycomb structure found between the inner and outer shell6. Cashew nut shell liquid contributes approximately 30–35% to the total weight of shell and is by far the most important constitute of the shell7,8. It is a cheap, abundantly available, and renewable raw material with diverse industrial applications and biological activities9,10. It is composed of four naturally occurring substituted phenols that have great potential to replace synthetic phenols in many applications with equivalent or better results9. The extraction of CNSL from the CNS involves three major methods and depending on the method employed, CNSL can be classified into two types: technical and natural11. Natural CNSL is extracted by solvent extraction and mechanical pressing of the shell. It contains anacardic acid (70%), cardol (18%), cardanol (5%) and traces of methyl cardol8. It is best known for its diverse biological activities, with anacardic acid been linked to the observed physiological effects12. Among them being antimicrobial, fungicidal, insecticidal, termiticidal, antioxidant and enzymatic inhibition properties13,14,15,16,17,18. Technical CNSL is obtained by heating natural CNSL at temperatures above 180–200 °C. During the heat process, the thermolabile anacardic acid decarboxylate and converts to cardanol, leading to high content of cardanol (60–65%) in technical CNSL19. This form of CNSL has found wide industrial applications as raw material in friction linings, paints, varnishes, laminating, epoxy resins, foundry chemicals, plastic formulations and as antioxidant in biodiesel17,20,21. The innumerable industrial applications of CNSL are based on the fact that it leads itself to polymerization by various means. Simple phenols from petrochemicals have restrictions hence, the range of products obtained from them are few1. The current rise in the prices of petrochemical feedstocks as well as concerns of environmental pollution and depletion of natural reserves, puts CNSL at the center stage as the best sustainable alternative source of renewable energy9. Its advantages surpass those of other competing renewable bioresources such as vegetable and corn oils. Cashew nut shell liquid is non-edible hence, it does not put pressure on the food supply chain, and the fact that it is sourced from waste raw materials, it does not compete for production land9,22. Other parts of the Anarcardium occidentale plant have been exploited for their medicinal values23,24. The fruit juice and the nut shell oil are both said to be folk remedies for cancerous ulcers, elephantiasis and warts. The oily substance from pericarp is used for cracks on the feet25. Old leaves are applied to skin afflictions and burns. In Ghana, the bark and leaves are used for sore gums and toothache. Decoction of the astringent bark is suggested for severe diarrhea and thrush. In India, bark is used in herbal tea for asthma, cold and congestion and as an antidote for snake bites26. Other uses of cashew nut shell liquid derivatives include anticancer and cardiovascular activity14,,1827. In additional, residual shells after extraction of CNSL can be exploited as a source of fuel28, or as gasifier feed stocks29, or can be convert to bio-filters to remove heavy metals and organic pollutants from waste waters through adsorption processes30. The nature of CNS would make synthesis of bio-filters very cheap and accessible to the locals. The increase in chemical industries, agricultural activities and abuse of water resources has contributed greatly to water contamination by heavy metals and organic compounds such as paints, dyes, waste chemical effluents and agrochemical residues. A study by Ikenaka et al.31 showed that heavy metal pollution in many parts of Zambia includes high copper, zinc, cadmium and lead concentrations. Accumulation of these metals in the human body can cause carcinogenesis, neurotoxicity, cell damage and loss of cellular functions32. The maximum permissible limit of cadmium, zinc, copper and lead in drinking water by World Health Organization are 0.003 mg/L, 3 mg/L, 2 mg/L and 0.01 mg/L respectively33. Therefore, removal of these heavy metals from drinking water is a priority. In other studies34, pharmaceutical products have been shown to have low biodegradability and hence find themselves in wastewater and surface waters35. Other studies have shown potential of contamination of water and soil by agricultural chemicals such as pesticides36. The conventional techniques for removal of waste from water includes; ion exchange process, chemical precipitation, membrane separation, ultra-filtration, chemical oxidation, reverse osmosis process and many others. These techniques are costly and requires high energy input. On the other hand, adsorption has a greater advantage because it is simple, safe and less costly37. The main objective of this research was to extract CNSL, determine its phytochemical composition and physicochemical properties, as well as designing a low-cost adsorbent material from defatted shells which can be used to remove heavy metals and organic pollutants from water. Various literature cited gave this research room for adding baseline data for the Zambian grown cashew, as the family Anacardiaceae covers over 70 genera in which more than 600 species are distributed in tropical, sub-tropical and temperate regions in the world38 hence the plant cannot be easily generalized that Zambia has only one family or subspecies. Hence this paper seeks to evaluate the value of cashew nut shell waste for potential valorisation, wealth and employment creation under small scale enterprises.
Materials and methods
Materials
Concentrated sulfuric acid (H2SO4), hydrochloric acid (HCl) and nitric acid (HNO3) (purchased from Hi-Media) were a kind donation by Medical Stores Limited (Ministry of Health, Zambia). Analytical grade Mercuric chloride (HgCl2), sodium carbonate (Na2CO3), sodium citrate (NaC6H7O7), metallic magnesium (Mg), lead acetate (C4H12O7Pb), sodium hydroxide (NaOH) and methylene blue (C16H18CIN3S) (BDH) were all supplied by the Department of Chemistry, University of Zambia.
Cadmium nitrate tetra-hydrate (Cd (NO3)2·4H2O), Zinc nitrate hexa-hydrate (Zn (NO3)2·6H2O), Copper (II) sulphate (CuSO4·5H2O), lead (II) nitrate (Pb (NO3)2), hexane (C6H12), ethanol (C2H5OH), petroleum ether (40–60 °C), chloroform (CHCl3), acetic anhydride ((CH3CO)2O), methanol (CH3OH), acetic acid (CH3COOH), ninhydrin, potassium iodide (KI), potassium iodate (KIO3), Sodium thiosulphate (Na2S2O4), ferric chloride (FeCl3), potassium sodium tartrate ( KNaC4H4O6·4H2O), picric acid (C6H3N3O7), α-naphthol (C10H7OH), were purchased from Sigma Aldrich under Merck.
Methods
Sample preparation
Pre-processed (roasted) CNS were collected from small-scale cashew nut processors in Mongu District of the Western Province of Zambia. Upon their arrival in the laboratory, the shells were washed several times with tap water and twice with enough distilled water to remove all the dirty, contaminants and debris. After washing, the shells were air-dried under the shade for 7 days. Once dry, the shells were ground to homogeneity using a Thomas-Model-4-Wiley-Mill fitted with a 2 mm sieve, placed in airtight bags and stored in a refrigerator at 4 °C to avoid biological and chemical degradation of the constituents.
Extraction of cashew nut shell liquid
The extraction of CNSL from CNSW was carried out by using a soxhlet extractor system as described by6,39. Briefly, 20.00 g of ground CNSW was put into a clean 33 × 100 mm cellulose thimble (Whatman) and extracted with a particular solvent for 8 h. After several cycles of extraction, the soxhlet apparatus was disassembled and the remaining solvent in the extracting chamber was added to the other extract in round bottomed flask, and evaporated under mild conditions with a Buchi Rotavapor until a constant oily mass remained.
Phytochemical screening
Twenty grams of ground CNS were exhaustively extracted under cold conditions in 200 mL acetone, ethanol and hexane respectively for 72 h with interval shaking in the dark. The organic solvents were recovered under mild pressure with a Buchi Rotavapor. The effect of solvent on phytochemical is presented in Table 1. The water extract was warmed at 60 °C for 10 min and left to stand for a total of 24 h with interval shaking. Phytochemical analysis for alkaloids, flavonoids, glycosides, phenols, saponins, steroids, tannins and terpenoids was done according to Refs.40,41,42,43,44,45 and for amino acids, carbohydrates, carboxylic acids and proteins46 with minor modifications. All reagents used in this process were prepared fresh before use.
Physicochemical characterization of cashew nut shell liquid
Methods by Refs.1,11,47 with minor modifications were followed for characterization of the CNSL extracted from the roasted CNS. Moisture content was determined by heating 2.0 g of sample to a constant weight in a crucible placed in a Memmert oven (Memmert GmbH + Co. KG) maintained at 105 °C for 3.5 h. The crucible was cooled in the desiccator and reweighed, the mass change in the sample was recorded. Ash was determined by incinerating 1.0 g sample in a Carbolite muffle furnace (HTF ELP4, Bamford, Sheffield UK) maintained at 550 °C for 5 h1 using sintered glass crucibles. Specific gravity was determined using a standard pycnometer bottle with a stopper. The 25 mL bottle was filled with distilled water and the CNSL respectively and weighed independently. The acid and free fatty acid values were determined using the methods of Refs.48,49,50. The saponification number and iodine values were determined by the method of Ref.51. Refractive index at 20 °C was determined using Bellingham Stanley Abbe refractometer. Viscosity was determined by the Oswald viscometer using distilled water as a reference at 24 °C. The pH was determined with a calibrated pH meter (Crison base 20).
Preparation of activated carbon adsorbent
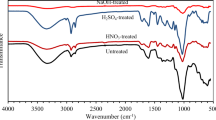
The defatted cashew nut shells were pre-heated at 110 °C for 2 h using a Carbolite AAF 11/7 Furnace at a heating rate of 10 °C/min. Chemical activation with 50 wt% sulphuric acid was carried out using an impregnation method. The impregnation ratio of sulphuric acid to the raw materials was 2: 1. Thus, 60 g of the pre-heated precursors were soaked in 86 mL of 50 wt% sulphuric acid for 24 h. After soaking, the precursor was dried in an oven at 110 °C. The dried precursors were carbonized in the same furnace as before at 400 °C for 3 h at a heating rate of 10 °C/min. The carbonized material was cooled to room temperature and washed severally with hot distilled water until the pH was neutral. The cooled activated carbon was then dried in an oven for 4 h, ground and sieved using a 0.5 mm sieve and stored in airtight bottles until use.
Analysis of heavy metals and methylene blue (MB)
Heavy metal concentrations were measured on a PerkinElmer Analyst 400 Atomic absorption spectrophotometer and a Shimadzu UV-2600 spectrometer was used to determine the concentration of Methylene blue standard before and after adsorption.
Adsorption experiments
Adsorption percentage (%) and the amount of adsorbate per unit mass of activated carbon (qe)52,53 was calculated using Eqs. (1) and (2).
where; Co is the initial concentration of adsorbate (mg/L), Ce is the final concentration of adsorbate after adsorption (mg/L), qe is the amount of adsorbate adsorbed at equilibrium (mg/g), m is the mass of activated carbon used (g) and V is the volume of adsorbate solution used (mL).
Adsorption isotherm models
Three commonly models used to fit adsorption experiment results are the Langmuir, Freundlich and Temkin adsorption isotherm models53,54.
The Langmuir isotherm model
The isotherm assumes that the monolayer adsorption process happens between the adsorbate and homogenous surface of the adsorbent55. The binding sites have the same affinity for adsorption. The linear equation is given below;
where; qe is the metal ions adsorbed (mg/g) at equilibrium, Ce is the equilibrium concentration (mg/L), qmax is the monolayer adsorption capacity (mg/g) and KL is the Langmuir adsorption constant which is related to the energy of adsorption and is a measure of the metal ions affinity to the adsorption sites. If the magnitude of KL is large, the interaction of the adsorbent with the adsorbate molecules will be more while a smaller value indicates a weak interaction. The Langmuir parameters qmax and KL were calculated from the slope (1/qmax) and intercept (1/qmaxKL) of the plot of Ce/qe versus Ce. An important characteristic of the Langmuir isotherm can be expressed in terms of the dimensionless equilibrium parameter or the separation factor, RL, which is defined as;
where; KL is the Langmuir adsorption constant and Co is the initial metal ion concentration. The value of the separation factor gives an indication of the shape of the isotherm and the nature of the adsorption process. The values of the RL between 0 and 1 indicates favourable adsorption, unfavourable adsorption occurs when RL is greater than 1 and adsorption is linear when RL is equal to 156.
The Freundlich isotherm model
The Freundlich isotherm model is an empirical model that explains that adsorption occurs on an unevenly distributed or heterogeneous surface of the adsorbent. The adsorbent surface has different affinity and energy for adsorption. Stronger binding sites are occupied first and then the binding strength decreases with the rise in the degree of site occupation. It is represented by the equation below;
where; qe is the metal ions adsorbed at equilibrium (mg/g), Ce is the equilibrium concentration (mg/L), and KF is the Freundlich constant and n is the adsorption intensity. The value of n indicates the degree of non-linearity between metal ions concentration and its adsorption in the following manner; if n is equal to 1 (n = 1) then adsorption is linear, adsorption becomes a favourable physical process when n is greater than 1 (n > 1) and when n is less than 1 (n < 1) then adsorption is a chemical process52,54. From the slope (1/n) and intercept (log KF) of the plot of log qe versus log Ce, the constant KF and n can be calculated.
Temkin isotherm model
The Temkin isotherm model considers the effect of indirect adsorbate-adsorbent interaction on the adsorption process. It is based on the assumption that the heat of adsorption of all the molecules in a layer decreases linearly due to increase in surface coverage of the adsorbent. The decrease in heat of adsorption is linear rather than logarithmic, as implied in the Freundlich isotherm. Further, the adsorption is characterized by uniform distribution of binding energies, up to a maximum binding energy. The Temkin isotherm model is represented by the following equation53:
where; KT is the equilibrium binding constant (L/mol) corresponding to the maximum binding energy, b is related to the adsorption heat, R is the universal gas constant (8.314 J/K/mol) and T is the temperature at 298 K. The constants KT and b can be calculated from the slope (RT/b) and intercept (RTIn KT/b) of the plot of qe versus ln (Ce)53.
Ethics approval and consent to participate
Ethical approval and waiver was obtained from the Natural and Applied Sciences Research Ethics Committee (NASREC) of the University of Zambia (UNZA) under the project REF. NO. NASREC: 2019-AUG-009.
Results and discussion
Cashew nut shell liquid was extracted from CNSW with different organic solvents using a soxhlet extractor system. The percent yields are represented in Fig. 1. Extraction methods involving organic solvents under mild conditions have been reported to preserve the natural composition of CNSL6. The quality and percent yields of extracted CNSL varied among the organic solvents. The hight yield was recorded from ethanol 40.1 ± 0.9% followed by hexane 38.2 ± 0.4% and petroleum ether 24.6 ± 0.4% respectively. Although, ethanol recorded the highest yield, the quality of CNSL it extracted was poor, as it extracted more of undesirable polar coloured compounds from the shell57. Even during the carbonization process, ethanol defatted shells produced toxic fumes, which led to a conclusion that it did not completely remove CNSL from the shell58. Phytochemical analysis of the aqueous, ethanol, acetone, and hexane extracts of CNSW (Table 1) revealed the presence of phenols, tannins, flavonoids, saponins, steroids, terpenoids, glycosides, carboxylic acids, carbohydrates, proteins and amino acids. Various extracts from CNSW have been reported to have antimicrobial, antifungal59, insecticidal (Acero, 2018) and antioxidant properties60. Phyto-compounds such as saponins have been reported to have anticancer and anticholesterol activity61. Flavonoids and other polyphenolic acids show antioxidant, anti-inflammatory, antidiabetic and anticarcinogenic activities62,63. Alkaloids are natural anticancer and analgesic agents64,65. Steroids and terpenoids have anti-tumor, neuroprotection, antihypertensive, antimicrobial and insecticidal properties66,67. Glycosides are better known for their physiological effect on the cardiovascular system, with cardiac glycosides being the drug of choice for treating congestive heart failure68. Thus, the presence of these essential phyto-compounds in CNSW extracts signifies the importance of this wasted raw material.
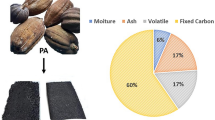
The physico-chemical properties of CNSL are presented in Table 2, and were all determined using hexane extracted CNSL, because the quality and yield were better than the ethanolic and petroleum ether extracted-CNSLs respectively. The extracted CNSL was a reddish-brown viscous oil with a pH value of 3.2, probably due to a high concentration of anacardic acid and other phenolic compounds8. The moisture and ash content of the CNSW biomass expressed in percentages were 6.5% and 1.6 ± 0.1% respectively. These values were in line with 6.7% and 1.3% reported by Ref.1. The specific gravity, and refractive index values were 0.9561 ± 0.0002 (g/cm3) and 1.52 ± 0.001 respectively. These values were higher than 0.9118 (g/cm3) and 1.4325 reported by Ref.39, but lower than 1.686 and 0.9999 (g/cm3) reported by Refs.1,69 respectively.
The viscosity of CNSL in this work was 104.6 ± 1.8 mPa s. This value was lower than 160 mPa s and 410 mPa s reported by (Mohammed69) and (Rodrigues et al.11). The standard viscosity range for CNSL at 25 °C is 150–600 mPa s72. The reason for low viscosity in this work could be that the shells were roasted under uncontrolled conditions by the local cashew nut farmers during processing. However, CNSL with low viscosity presents an advantage, as it can be blended with diesel to form biodiesel for heavy engines73. Biodiesels with high viscosity values are characterised with poor fuel atomization, larger droplet size and spray jet penetration, leading to inefficient mixing of fuel and air in the combustion chambers74. The acid and free fatty acid values were 118.7 ± 9.2 (mg KOH/g) and 60.1 ± 4.7% respectively. The acid value in this work was higher than most literature values 12.1, 15.5, and 112 (mg KOH/g) reported by Ref.1,71,75, but lower than 141 mg KOH/g reported by Ref.70. A high acid value suggests that CNSL cannot be consumed by humans or directly applied on acid sensitive surfaces to avoid corrosion. Ingestion of oils with high acid value leads to human gastrointestinal discomfort, diarrhea and liver damage76,77. Cashew nut shell liquid with high acid value is first neutralized with alkaline bases before it is applied in paints, vanishes and others surface coating agents70. The saponification number and iodine values for this work were 138.1 ± 3.2 (mg KOH/g) and 188.1 ± 2.3 (gI2/100 g) respectively. The obtained saponification number was lower than 161 mg KOH/g reported by7. Saponification number depend on the amount of fatty acids present in a fat or oil sample. The higher the number, the higher the amount of fatty acids and vice versa. It is also used to determine the average molecular weight of fatty acid chains in fats/or oils74. Fats/ or oils with long fatty acids have low saponification values because they have fewer carboxylic group per unit mass of fat/oil, as compared to short chain fatty acids. Fatty acids with longer chains make good surfactants. Their surfactants have excellent detergent properties and they do not irritate the skin78. Iodine value indicates the unsaturation of fats/or oils74. The value 188.1 ± 2.3 (mgI2/100 g) obtained in this work was in line with and 177.7 (mg I2/100 g) reported by Ref.7. The higher the iodine value, the more unsaturated the fat/or oil sample is. Highly unsaturated oils or fats are good for paints and surface coating materials, as they dry faster and their conjugated double bonds help to slowdown the oxidation process of painted objects1. The difference in the composition and physicochemical properties of CNSL in this work and other literature sources may be due to variation in the species, climate and geography where cashew was grown as well as the operating conditions employed during analysis79.
Batch adsorption of heavy metals (copper, lead, cadmium and zinc) onto CNS-AC
The cashew nut shell activated carbon (CNS-AC) was used to remove heavy metals (lead, cadmium, copper and zinc) from synthetic aqueous solutions. The batch adsorption was carried out at conditions of 1 g adsorbent dosage, 0.002 to 3 mg/L initial metal concentration, 30 mL of adsorbate solution, pH of 6.98, 30 min contact time and agitation speed of 250 rpm. The average percentage removal of Cu (II), Pb (II), Cd (II) and Zn (II) is shown in Table 3.
Batch adsorption of MB onto CNS-AC
Some of the factors that affects adsorption such as pH, temperature, contact time and concentration were considered in this study.
Effect of solution pH on MB adsorption onto CNS-AC
The influence of initial pH value of the solution on the adsorption process of MB onto CNS-AC was carried out at 50 mg/L initial MB concentration, 298 K temperature and contact time of 160 min. The adsorption efficiency increases from 94.8 to 99.1% for an increase in pH from 2 to 11 (Fig. 2). The near sigmoidal adsorption pattern under different pH units agrees with other studies done on adsorbates for methylene blue removal from aqueous solutions 80,81. The adsorption of MB was highly favoured under basic compared to acidic conditions with the highest removal percentage of 99.1 at pH 10. Uptake of MB by CNS-AC was constant at pH 10 and 11 as shown in Fig. 2. The low adsorption efficiency of MB in acidic media could be attributed to high competition for adsorption sites between the excess hydrogen ions (H+ ions) in the solution and the cation groups on MB80.
Adsorption efficiency of MB on CNS-AC at various pH values ranging from 2 to 11. The adsorbent dosage (S/L) was = 33.33 g/L, initial concentration of dye was 50 mg/L, total time was 160 min at 25 °C (298 K). Plots were generated in Graphpad Prism 9.1.0. Values were expressed as means ± SEM. (https://www.graphpad.com/).
Effect of initial dye concentration and contact time on adsorption of MB onto CNS-AC
The relationship between adsorption of MB and contact time was investigated to establish the rate of MB removal. Figure 3 shows the plot of removal percentage versus contact time for different MB concentrations ranging from 50 to 350 mg/L. The adsorption of MB increased with the increase in contact time until equilibrium was reached in about 120, 150, 210 and 250 min for an MB concentration of 50, 150, 250 and 350 mg/L respectively. Also, the percentage removal decreased from 99 to 93.74% for an increase in initial MB concentration from 50 to 350 mg/L. The reason for this behaviour can be attributed to the fact that, there are more active adsorption sites on the surface of the adsorbent compared to the total MB molecules in solution at lower concentrations, thus, more molecules interacts with the adsorbent and are removed from the solution54,80.
MB concentration ranged from 50 to 350 mg/L at an adsorbent dosage (S/L) of 33.33 g/L, pH 10 and contact time from 30 to 280 min at room temperature and pressure. Plots were generated in Graphpad Prism 9.1.0 version Values were expressed as means ± SEM (https://www.graphpad.com/).
Adsorption isotherms
Analysis of isotherm models is significant in modelling and designing of the adsorption process as they show the distribution of the adsorbate molecules between the liquid phase and the solid phase when an equilibrium state is reached. In this study, Langmuir, Freundlich and Temkin isotherm models were considered. The isotherm constants and regression coefficients (R2) calculated from adsorption experiments are given in Table 4. The Freundlich isotherm model was suitable since regression coefficient (R2) was higher than that of the Langmuir and Temkin isotherm models as shown in Fig. 4. Thus, adsorption of MB onto CNS-AC fitted best the Freundlich isotherm model.
Adsorption kinetic models
The adsorption kinetic models are important in evaluating the rate and kinetic behaviour of the adsorption process. The kinetic parameters provide substantial information in designing and modelling of the adsorption process. The kinetic of methylene blue (MB) adsorption onto CNS-AC was analysed using pseudo-first-order and pseudo-second-order kinetic models.
A pseudo-first-order kinetic equation is given as;
where; \(q_{e}\) and \(q_{t}\) (mg/g) are the amounts of methylene blue (MB) adsorbed at equilibrium and at time t (min), \(K_{1}\)(min−1) is the adsorption rate constant. The parameters \(q_{e}\) and \(K_{1}\) were determined from the intercept and slope of a plots of \(\log \left( {q_{e} - q_{t} } \right)\) versus t as shown in Fig. 5. The parameters of pseudo-first-order kinetic are tabulated in Table 4.
Pseudo-second-order kinetic model is expressed as;
where; \(K_{2}\) (g/mg/min) is second order adsorption rate constant, h (mg/g/min) is the initial adsorption rate. The parameters \(q_{e}\) and \(K_{2}\) were calculated from the slope and intercept of the plots of \(\frac{t}{{q_{t} }}\) versus t as shown in Fig. 6.
Pseudo-first-order and pseudo-second-order kinetic parameters for different initial concentrations of methylene blue are tabulated in Table 5. The value of the correlation coefficient (R2) for pseudo-second order model is higher than the value of pseudo-first-order adsorption model. Furthermore, pseudo-second-order model has values of \(q_{e}\), cal which are close to \(q_{e}\), exp. It can be concluded that the adsorption of methylene blue onto CNS-AC follows pseudo-second-order kinetic model. This implies that, the rate-limiting step is the surface adsorption that involves chemisorption. Thus, chemical adsorption likely occurs through the formation of a covalent bond.
Effect of temperature and thermodynamic parameters
The influence of temperature on adsorption of MB using CNS-AC was investigated at different temperatures (298, 308, 318 and 328 K) and MB concentrations of 50, 150, 250 and 350 mg/L. Increasing the temperature from 298 to 328 K as shown in Fig. 7 increased the adsorption efficiency of MB for all the concentrations from 94.81 (350 mg/L) to 99.63 (50 mg/L). Thus, increase in temperature increases the thermal motion, chemical potential and solubility of MB molecules82, thereby, enhancing the interaction of MB molecules with the adsorbent. The maximum removal efficiency of MB by CNS-AC at 50, 150, 250 and 350 mg/L was found to be 99.63, 97.66, 96.48 and 94.81, respectively.
Effect of temperature on percentage MB removal by CNS-AC (adsorbent dosage S/L = 33.33 g/L, pH 10 and contact time = 120 min). Plots were generated in Graphpad Prism 9.1.0 version. Values were expressed as means ± SEM (https://www.graphpad.com/).
Thermodynamic study of adsorption process of MB onto CNS-AC to estimate the feasibility of the adsorption process was investigated. The Gibbs free energy change (ΔG°) values are useful in determining whether the process is spontaneous or not. A positive value of ΔG° means that the adsorption process is non-spontaneous and a negative value shows that the process is spontaneous. The enthalpy change (ΔH°) differentiates a physical adsorption process from a chemical adsorption process and provides information about the exothermic nature or endothermic nature of the adsorption process83. The entropy change (ΔSo) indicates the disorder of the solid/solution interface during the adsorption process.
The change in Gibbs free energy (ΔG°) was calculated using the following equations54,84;
The enthalpy change (ΔH°) and entropy change (ΔSo) change was determined from the equation below82;
where; T is the absolute temperature (K), R is the universal gas constant (8.314 J/mol/K), ∆G° (kJ/mol) is the Gibbs free energy change, ∆H° (kJ/mol) is the enthalpy change and ∆S° (kJ/mol/K) is the entropy change. The values of ∆S° and ∆H° (Table 6) are obtained from the slope and intercept of the plot of lnK versus 1/T (K−1) and are shown in Fig. 8.
The negative values obtained for Gibbs free energy (∆G°) indicates the feasibility and spontaneity of the adsorption process. The positive value (22.76 kJ/mol) of enthalpy change (∆H°) indicates that the adsorption of MB by CNS-AC at different temperatures was endothermic. The value of the entropy change (ΔS°) was 0.086 kJ/mol/K implying that the randomness of solid/solution interface during adsorption process increased. These results were consistent with studies done by Ref.54.
Conclusion
In this paper, we analyzed the potential use of cashew nut shell regarded as a waste in Zambia and indeed in many countries growing cashew. Solvent extraction and synthesis of activated carbon experiments were done to evaluate the potential value of cashew nut as a source of both chemical feedstock and activated carbon for use as a low cost filtration system matrix. Best yields of CNSL were achieved by hexane (38.2 ± 0.4%). Physicochemical results showed that CNSL has high potential as an intermediate in the synthesis of paints, varnishes, dyeing-stuff, binders, lubricants, nanotechnology73 and the presence of bioactive compounds such as alkaloids, steroids, terpernoids, polyphenols, saponins and glycosides indicates that CNSW can be a cheap source for pharmaceutical compounds59,85. The iodine ( 188.1 ± 2.3 gI2/100 g) and saponification (138.1 ± 3.2 mg KOH/g) values indicated that CNSL was a drying oil making it suitable for resins, surface coating materials17,86 and soap making respectively. The adsorption of heavy metals and MB onto CNS-AC has been studied. The average percentage removal of Cu (II), Pb (II), Cd (II) and Zn (II) was 99.4, 95.4, 99.5, 98.4%, and the removal efficiency of MB at 50, 150, 250 and 350 mg/L was 99.63, 97.66, 96.48 and 94.81, respectively. The study showed that increasing the initial pH, temperature and contact time increased the adsorption of MB onto CNS-AC and a decrease in initial MB concentration increased percentage removal of MB. Equilibrium data were fitted to Langmuir, Freundlich and Temkin isotherms models and the equilibrium data were best described by the Freundlich isotherm model. The maximum monolayer adsorption capacity was 12.1 mg/g. The kinetics of the adsorption process conformed to pseudo-second-order model and the negative value of the Gibbs free energy (∆G°) and positive value of enthalpy change (∆H°) indicates that the adsorption process was endothermic and spontaneous. This paper therefore provided useful information thatcashew nut shell can be be used as a source for CNS-AC, a suitable adsorbent for removal of heavy metals and organic soluble matter from water as modeled by Cd, Cu, Pb, Zn and methylene blue dye30,80,82 removal respectively. The waste shells on one hand are also as a source of cardanol, cardol and other phenolic compounds useful in the chemical industry73,87,88.
Abbreviations
- AC:
-
Activated carbon
- CIDP:
-
Cashew Infrastructure Development Project
- CNS:
-
Cashew nut shell
- CNS-AC:
-
Cashew nut shell-activated carbon
- CNSL:
-
Cashew nut shell liquid
- CNSW:
-
Cashew nut shell waste
- DRGS:
-
Directorate of Research and Graduate Studies
- FFA:
-
Free fatty acid
- GRZ:
-
Government of the Republic of Zambia
- MB:
-
Methylene Blue
- MIN:
-
Minutes
- NASREC:
-
Natural and applied sciences research ethics committee
- RPM:
-
Revolutions per minute
- SEM:
-
Standard error of the mean
- UNZA:
-
University of Zambia
References
Akinhanmi, T. F., Atasie, V. N. & Akintokun, P. O. Chemical composition and physicochemical properties of cashew nut (Anacardium occidentale) oil and cashew nut shell liquid. J. Agric. Food Environ. Sci. 2, 1–10 (2008).
Africa Development Bank. Cashew Infrastructure Development Project (Cidp) (Africa Development Bank, 2015).
Lusaka-Times. Government Reaches 80 Percent of Cashew-Nut Seedling Distribution in Lukulu District (2018). https://www.lusakatimes.com/2018/02/21/government-reaches-80-cashew-nut-seedling-distribution-lukulu-district/.
Lusaka-Times. The Cashew Infrastructure Development Project: Taking Western Province to Another Level (2016). https://www.lusakatimes.com/2016/11/13/cashew-infrastructure-development-project-taking-western-province-another-level/.
Siame, G. Waste Management Status Programmes Undertakings and Innovations in Lusaka (The University of Zambia, Department of Geography & Environmental Studies, Centre for Urban Research and Planning, 2018).
Gandhi, T. S., Dholakiya, B. Z. & Patel, M. R. Extraction protocol for isolation of Cnsl by using protic and aprotic solvents from cashew nut and study of their physico-chemical parameter. Polish J. Chem. Technol. 15, 24–27 (2013).
Idah, P. A., Simeon, M. I. & Mohammed, M. A. Extraction and characterization of cashew nut (Anacardium occidentale) oil and cashew shell liquid oil. Acad. Res. Int. 5, 50 (2014).
Senthil Kumar, P., Arun Kumar, N., Sivakumar, R. & Kaushik, C. Experimentation on solvent extraction of polyphenols from natural waste. J. Mater. Sci. 44, 5894–5899 (2009).
Coline, V. et al. Functionalizatio of cardanol towards biobased polymers and additives. Polym. Chem. 5, 3142–3162 (2014).
Stasiuk, M. & Kozubek, A. Biological activity of phenolic lipids. Cel. Mol. Life Sci. 67, 841–860 (2010).
Rodrigues, F. H., França, F. C., Souza, J. R., Ricardo, N. M. & Feitosa, J. Comparison between physico-chemical properties of the technical cashew nut shell liquid (Cnsl) and those natural extracted from solvent and pressing. Polímeros 21, 156–160 (2011).
Shobha, S. V. & Ravindranath, B. Supercritical carbon dioxide and solvent extraction of the phenolic lipids of cashew nut (Anacardium occidentale) shells. J. Agric. Food Chem. 39, 2214–2217 (1991).
Acero, L. H. Cashew (Anacardium occidentale) nut shell, ethanol extract in the control of cockroach (Periplaneta americana). Sci. Int. 30, 427–429 (2018).
Ha, T. J. & Kubo, I. Lipoxygenase inhibitory activity of anacardic acids. J. of Agric. Food Chem. 53, 4350–4354 (2005).
Himejima, M. & Kubo, I. Antibacterial agents from the cashew Anacardium occidentale (Anacardiaceae) nut shell oil. J. Agric. Food Chem. 39, 418–421 (1991).
Kubo, I., Komatsu, S. & Ochi, M. Molluscicides from the cashew anacardium occidentale and their large-scale isolation. J. Agric. Food Chem. 34, 970–973 (1986).
Lubi, M. C. & Thachil, E. T. Cashew nut shell liquid (Cnsl)—A versatile monomer for polymer synthesis. Des. Monom. Polym. 3, 123–153 (2000).
Seong, Y., Shin, P.-G. & Kim, G.-D. Anacardic acid induces mitochondrial-mediated apoptosis in the A549 human lung adenocarcinoma cells. Int. J. Oncol. 42, 1045–1051 (2013).
Rodrigues, F. H., Feitosa, J., Ricardo, N. M., França, F. C. & Carioca, J. O. Antioxidant activity of cashew nut shell liquid (Cnsl) derivatives on the thermal oxidation of synthetic cis-1, 4-polyisoprene. J. Braz. Chem. Soc. 17, 265–271 (2006).
Bastos, F. A. & Tubino, M. The use of the liquid from cashew nut shells as an antioxidant in biodiesel. J. Braz. Chem. Soc. 28, 747–755 (2017).
Swain, S. K. et al. Polymers from renewable resources. V. Synthesis and characterization of thermosetting resins derived from cashew nut shell liquid (Cnsl)–furfural-substituted aromatic compounds. J. Appl. Polym. Sci. 54, 1413–1421 (1994).
Bragoni, V., Rit, R. K., Kirchmann, R., Trita, A. S. & Gooßen, L. J. Synthesis of bio-based surfactants from cashew nutshell liquid in water. Green Chem. 20, 3210–3213 (2018).
Chinsembu, K. C. & Cheikhyoussef, A. Indigenous Knowledge of Namibia (University of Namibia Press, 2016).
Hemshekhar, M., Santhosh, M. S., Kemparaju, K. & Girish, K. S. Emerging roles of anacardic acid and its derivatives: A pharmacological overview. Basic Clin. Pharmacol. Toxicol. 110, 122–132 (2012).
Tyman, J. H. P., Johnson, R. A., Muir, M. & Rokhgar, R. The Extraction of natural cashew nut-shell liquid from the cashew nut (Anacardium occidentale). Journal of the American Oil Chemists’ Society 66, 553–557 (1989).
Kumar Phani, P., Paramashivappa, R., Vithayathil, P. J., Rao Subba, P. V. & Rao Srinivasa, A. Process for Isolation of cardanol from technical cashew (Anacardium occidentale L.) nut shell liquid. J. Agric. Food Chem. 50, 4705–4708 (2002).
Paramashivappa, R., Phani Kumar, P., Subba Rao, P. V. & Srinivasa Rao, A. Synthesis of sildenafil analogues from anacardic acid and their phosphodiesterase-5 inhibition. J. Agric. Food Chem. 50, 7709–7713 (2002).
Tsamba, A. Multiple Uses of Cashew Nut Shells as Solid and Liquid Biofuel, Vol. 183, 435–440 (2009).
Singh, R. N., Jena, U., Patel, J. B. & Sharma, A. M. Feasibility study of cashew nut shells as an open core gasifier feedstock. Renew. Energy 31, 481–487 (2006).
Kumar, P. S. et al. Removal of cadmium (Ii) from aqueous solution by agricultural waste cashew nut shell. Korean J. Chem. Eng. 29, 756–768 (2012).
Ikenaka, Y. et al. Heavy metal contamination of soil and sediment in Zambia. Afr. J. Environ. Sci. Technol. 4, 729–739 (2010).
Engwa, G. A., Ferdinand, P. U., Nwalo, F. N. & Unachukwu, M. N. Mechanism and health effects of heavy metal toxicity in humans. In Poisoning in the Modern World-New Tricks for an Old Dog? (eds Karcioglu, O. & Arslan, B.) (IntechOpen, 2019).
W.H.O. Guidelines for drinking-water quality. WHO Chronicle 38, 104–108 (2011).
Nyirenda, J., Mwanza, A. & Lengwe, C. Assessing the Biodegradability of Common Pharmaceutical Products (Pps) on the Zambian Market. Heliyon 6, e05286 (2020).
Ngumba, E., Gachanja, A., Nyirenda, J., Maldonado, J. & Tuhkanen, T. Occurrence of antibiotics and antiretroviral drugs in source-separated urine, groundwater, surface water and wastewater in the peri-urban area of chunga in Lusaka, Zambia. Water SA 46, 278–284 (2020).
Malambo, M. J., Mukanga, M., Nyirenda, J., Kabamba, B. & Salati, R. K. Knowledge and practice of pesticides use among small holder farmers in Zambia. Int. J. Hortic. Agric. Food Sci. 3, 184 (2019).
Tripathi, A. & Ranjan, M. R. Heavy metal removal from wastewater using low cost adsorbents. J. Bioremed. Biodegr. 6, 315 (2015).
de Sousa Leite, A. et al. Pharmacological properties of cashew (Anacardium occidentale). Afr. J. Biotechnol. 15, 1855–1863 (2016).
Gandhi, T., Patel, M. & Dholakiya, B. K. Studies on effect of various solvents on extraction of cashew nut shell liquid (Cnsl) and isolation of major phenolic constituents from extracted Cnsl. Nat. Prod. Plant Resour. 2, 135–142 (2012).
Banda, D., Nyirenda, J. & Sijumbila, G. Aphrodisiac properties of mutimba vula and mwana apeluke herbs sold in Lusaka, Zambia. Med. J. Zambia 44, 133–139 (2017).
Banda, M., Nyirenda, J., Muzandu, K., Sijumbila, G. & Mudenda, S. Antihyperglycemic and antihyperlipidemic effects of aqueous extracts of Lannea edulis in alloxan-induced diabetic rats. Front. Pharmacol. 9, 1099 (2018).
Evans, W. C. & Trease, G. E. Pharmacognosy Vol. 121, 247–249 (H arcourt Publishers limited, 2002).
Jaradat, N., Hussen, F. & Ali, A. A. Preliminary phytochemical screening, quantitative estimation of total flavonoids, total phenols and antioxidant activity of ephedra alata decne. J. Mater. Environ. Sci. 6, 1771–1778 (2015).
Sofowora, A. Phytochemical Screening of Medicinal Plants and Traditional Medicine in Africa 150–156 (Spectrum Books Limited, 1993).
Trease, G. E. & Evans, W. C. Pharmacognosy, Saunders Vol. 36, 51 (Elsevier, 2002).
Tiwari, P., Kumar, B., Kaur, M., Kaur, G. & Kaur, H. Phytochemical screening and extraction: A review. Int. Pharm. Sci. 1, 98–106 (2011).
Aremu, M. O. & Akinwumi, O. D. Extraction, compositional and physicochemical characteristics of cashew Anarcadium occidentale. Nuts reject oil. Asian J. Appl. Sci. Eng. 3, 33–40 (2014).
Suzanne Nielsen, S. Food Analysis Laboratory Manual 2nd edn. (Springer, 2010).
Tawa, D., Ebun, O. & Ishiaka, A. Effect of roasting on some physicochemical and antimicrobial properties of cashew nut (Anacardium occidentale) oil. Int. J. Sci. Technol. 4, 555–559 (2015).
Zhang, Q., Wu, J., Ma, P., Cai, J. & Zhang, Y. Acid Value determination and pre-esterification of crude Euphorbia lathyris L. oil. World J. Eng. Technol. 3, 70 (2015).
FSSAI. Manual of Methods of Analysis of Foods: Oils and Fats (Food Safety and Standard Authority of India, 2015).
Senthil Kumar, P. et al. Lead(Ii) adsorption onto sulphuric acid treated cashew nut shell. Sep. Sci. Technol. 46, 2436–2449 (2011).
Piccin, J. S., Dotto, G. L. & Pinto, L. A. A. Adsorption isotherms and thermochemical data of Fd&C red N 40 binding by chitosan. Braz. J. Chem. Eng. 28, 295–304 (2011).
Üner, O., Geçgel, Ü. & Bayrak, Y. Adsorption of Methylene blue by an efficient activated carbon prepared from Citrullus lanatus Rind: Kinetic, isotherm, thermodynamic, and mechanism analysis. Water Air Soil Pollut. 227, 247 (2016).
Abdelbassit, M. S. A., Alhooshani, K. R. & Saleh, T. A. Silica nanoparticles loaded on activated carbon for simultaneous removal of dichloromethane, trichloromethane, and carbon tetrachloride. Adv. Powder Technol. 27, 1719–1729 (2016).
Meroufel, B., Benali, O., Benyahia, M., Benmoussa, Y. & Zenasni, M. A. Adsorptive removal of anionic dye from aqueous solutions by algerian kaolin: Characteristics, isotherm, kinetic and thermodynamic studies. J. Mater. Environ. Sci. 4, 482–491 (2013).
Tyman, J. H. P. Synthetic and Natural Phenols (Elsevier, 1996).
Mohod, A. G., Sanger, S. H., Khandetode, Y. P., Shrirame, H. Y. & Deshmukh, A. S. Study of carbonization for cashew nut shell. Res. J. Chem. Sci. 1, 43–55 (2011).
Dorathy, S., Jebapritha, S. & Karpagam, I. Phytochemical content and antimicrobial activity of cashew nut shell oil. IOSR J. Pharm. Biol. Sci. 12, 61–64 (2017).
Prakash, A. et al. Evaluation of Antioxidant and antimicrobial properties of solvent extracts of agro-food by-products (cashew nut shell, coconut shell and groundnut hull). Agric. Nat. Resour. 52, 451–459 (2018).
Güçlü-Üstündağ, Ö. & Mazza, G. Saponins: Properties, applications and processing. Crit. Rev. Food Sci. Nutr. 47, 231–258 (2007).
Ghasemzadeh, A. & Ghasemzadeh, N. Flavonoids and phenolic acids: Role and biochemical activity in plants and human. J. Med. Plants Res. 5, 6697–6703 (2011).
Gutiérrez-Grijalva, E. P. et al. ’Flavonoids and phenolic acids from oregano: Occurrence, biological activity and health benefits. Plants 7, 2 (2018).
Ma, Q., Jiang, J.-G., Yuan, X., Qiu, K. & Zhu, W. Comparative antitumor and anti-inflammatory effects of flavonoids, saponins, polysaccharides, essential oil, coumarin and alkaloids from cirsium japonicum Dc. Food Chem. Toxicol. 125, 422–429 (2019).
Praful, T. Anti cancer approach with natural products. Int. J. Pharm. Sci. Res. 2, 2514–2520 (2011).
Dembitsky, V. M., Savidov, N., Poroikov, V. V. & Gloriozova, T. A. Naturally occurring aromatic steroids and their biological activities. Appl. Microbiol. Biotechnol. 102, 4663–4674 (2018).
Martin-Smith, M. & Sneader, W. E. Biological activity of the terpenoids and their derivatives. In Progress in Drug Research/Fortschritte der Arzneimittelforschung/Progrès des Recherches Pharmaceutiques Vol. 13 (eds Martin-Smith, M. et al.) 11 (Birkhäuser Basel, 2013).
Kelly, R. A. Cardiac glycosides and congestive heart failure. Am. J. Cardiol. 65, E10–E16 (1990).
Mohammed, A. N. Development of high performance cashew nut shell liquid (Cnsl) based surface coatings cross linked with styrene and methylmethacrylate (2015).
Achi, S. S. & Myina, O. M. Preliminary investigation of Kaduna-Grown cashew nutshell liquid as a natural precursor for dyestuffs, pigments and binders for leather finishing. Niger. J. Chem. Res. 16, 9–14 (2011).
Mahanwar, P. A. & Kale, D. D. Effect of cashew nut shell liquid (Cnsl) on properties of phenolic resins. J. Appl. Polym. Sci. 61, 2107–2111 (1996).
Silva, M. C. D., Conceição, M. M., Fernandes, V. J., dos Santos, I. M. G. & Souza, A. G. Avaliação do efeito antioxidante do líquido da castanha de Caju (Lcc) em óleo e biodiesel de Mamona. In Congresso da Rede Brasileira de Tecnologia de Biodiesel; Anais; MCT/ABIPTI: Brasília, Brazil 192–95 (2006).
Mubofu, E. B. & Mgaya, J. E. Chemical valorization of cashew nut shell waste. Top. Curr. Chem. 376, 8 (2018).
Toscano, G., Riva, G., Pedretti, E. F. & Duca, D. Vegetable oil and fat viscosity forecast models based on iodine number and saponification number. Biomass Bioenergy 46, 511–516 (2012).
Mahanwar, P. A. & Kale, D. D. Effect of Processing Parameters on Refining of Cnsl (1996).
Hammond, E. G., Johnson, L. A., Su, C., Wang, T. & White, P. J. Bailey’s Industrial Oil and Fat Products 592–614 (Wiley, 2005).
Thomas, A., Matthäus, B. & Fiebig, H.-J. Fats and fatty oils. In Ullmann’s Encyclopedia of Industrial Chemistry (eds Thomas, A. et al.) 1–84 (Wiley, 2000).
Habib, S. K. A., Sorowar, M. S., Karmoker, J., Khatun, M. K. & Al-Reza, S. M. Study on the physicochemical properties of some commercial soaps available in Bangladeshi Market. Int. J. Adv. Res. Chem. Sci. 3, 9–12 (2016).
de Sousa Rios, M. A., Nascimento, T. L., Santiago, S. N. & Mazzetto, S. E. Cashew nut shell liquid: A versatile raw material utilized for syntheses of phosphorus compounds. Energ. fuel. 23(11), 5432–5437 (2009).
Kumar, P. S., Ramalingam, S. & Sathishkumar, K. Removal of methylene blue dye from aqueous solution by activated carbon prepared from cashew nut shell as a new low-cost adsorbent. Korean J. Chem. Eng. 28, 149–155 (2011).
Mouni, L. et al. Removal of methylene blue from aqueous solutions by adsorption on kaolin: Kinetic and equilibrium studies. Appl. Clay Sci. 153, 38–45 (2018).
Kuang, Y., Zhang, X. & Zhou, S. Adsorption of methylene blue in water onto activated carbon by surfactant modification. Water 12, 587 (2020).
Rincón-Silva, N. G., Moreno-Piraján, J. C. & Giraldo, L. G. Thermodynamic study of adsorption of phenol, 4-chlorophenol, and 4-nitrophenol on activated carbon obtained from eucalyptus seed. J. Chem. 2015, 1–12 (2015).
Al-Hakeim, H. K., Al-Dahan, I. M., Al-Hillawi, Z. H. & Bustan, R. S. Interaction of prolactin hormone with the surfaces of two new azo compounds. Int. J. Pharm. Pharm. Sci. 6, 383–387 (2014).
Leite, A. et al. Pharmacological properties of cashew (Anacardium occidentale). Afr. J. Biotechnol. 15, 1855 (2016).
Kanehashi, S. et al. Preparation and characterization of cardanol-based epoxy resin for coating at room temperature curing. J. Appl. Polym. Sci. 130, 2468–2478 (2013).
Mathew, O. E., Labake, F. & Rita, N. E. Extraction of polyphenols from cashew nut shell. Leonardo Electr. J. Pract. Technol. 9, 107–112 (2006).
Mgaya, J. et al. Cashew nut shell: A potential bio-resource for the production of bio-sourced chemicals, materials and fuels. Green Chem. 21, 1186–1201 (2019).
Acknowledgements
The authors wish to acknowledge the UNZA-DRGS for funding as well as waiving fees for ethical approval. We wish to thank the following technical staff; Mr. Adolf Lungu, Mr. Edward Mwendo, Mr. Edmond Bundala (School of Natural Sciences) and Mr. Derek Mwape (School of Agricultural Sciences). We also thank Dr. Onesmus Munyati, former Head of Chemistry and current Dean of School of Natural Sciences for access to the laboratories in Chemistry and Physics Departments respectively.
Funding
This work was supported by the funding given under the 2019 Seed Money Grant by the University of Zambia, Directorate of Research and Graduate Studies for the project entitled Extraction, Physicochemical Characterization of Cashew Nut Shell Liquid and Insoluble Residues of Cashew Nut Shell from Western Province: Value Chain Addition of Cashew Nut Shell Waste (REF. NO. NASREC: 2019-AUG-009) to help upcoming scientists.
Author information
Authors and Affiliations
Contributions
J.N., secured funding, designed and supervised the research. K.Z. and G.K., C.S. and I.M. carried out sample collection and physicochemical experiments. J.N., wrote the manuscript. J.N., K.Z. and G.K. analyzed the data, reviewed and edited the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nyirenda, J., Zombe, K., Kalaba, G. et al. Exhaustive valorization of cashew nut shell waste as a potential bioresource material. Sci Rep 11, 11986 (2021). https://doi.org/10.1038/s41598-021-91571-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-91571-y
This article is cited by
-
Characterization and use of activated carbon synthesized from sunflower seed shell in the removal of Pb(II), Cd(II), and Cr(III) ions from aqueous solution
Environmental Monitoring and Assessment (2024)
-
Magnetic pomegranate peels activated carbon (MG-PPAC) composite for Acid Orange 7 dye removal from wastewater
Applied Water Science (2024)
-
Orange peels magnetic activate carbon (MG-OPAC) composite formation for toxic chromium absorption from wastewater
Scientific Reports (2023)
-
Biorefinery of Cashew By-Products: Recovery of Value-Added Compounds
Food and Bioprocess Technology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.