Abstract
The oral microbiome plays an important role in the human microbial community and in maintaining the health of an individual. Imbalances in the oral microbiome may contribute to oral and systemic diseases. The progression of periodontal disease is closely related to the growth of bacteria, such as Porphyromonas gingivalis, in the oral cavity. However, the pathogen growth mechanism specific to periodontal disease remains unknown. This study aimed to identify bacteria associated with periodontal health by focusing on hemolytic bacteria. Unstimulated saliva samples were collected from ten periodontitis patients and five healthy subjects to detect and identify the presence of hemolytic bacteria. The saliva of healthy subjects contained a higher proportion of G. haemolysans than saliva samples from patients with periodontitis. Growth inhibition assays indicated that the protein components contained in the culture supernatant of G. haemolysans directly suppressed the growth of P. gingivalis. This study shows that the presence of G. haemolysans in saliva is associated with periodontal health and that it inhibits the growth of P. gingivalis in vitro.
Similar content being viewed by others
Introduction
Periodontal disease is one of the most common chronic diseases in humans, with a very high prevalence among adults across the world1,2,3,4,5. It has increased the disease burden and has become an important public health concern in the aging global population. Studies on pathogens and inflammation in periodontal disease have attracted attention in the fields of medicine and dentistry owing to the potential influence of periodontitis on the initiation and progression of a variety of systemic diseases. Several studies have provided evidence linking periodontal disease to various non-oral systemic diseases, including cancer, neurodegenerative disease, diabetes, respiratory tract infections, adverse pregnancy outcomes, and cardiovascular disease6,7,8,9,10.
The pathological process of periodontitis involves the destruction of the periodontal structures that support the teeth, which is a major cause of tooth loss11. The development and progression of this disease are associated with a multifactorial process that depends on the interaction between the host cells and microbes in the oral cavity12. Porphyromonas gingivalis is a Gram-negative anaerobic bacterium that colonizes dental plaque in the oral cavity and is one of the major pathogens directly responsible for the development of chronic periodontitis in humans13. Several virulence factors such as cysteine protease (Rgp and Kgp)14,15, fimbriae (FimA and Mfa1)16,17, lipopolysaccharide (LPS)18,19 and the capsule20,21 influence the activities of this bacterium. These surface components and secretory enzymes contribute to efficient growth, nutrient acquisition, colonization, and protection from host defense mechanisms. The high pathogenicity of P. gingivalis has been investigated not only in cell models in vitro but also in various animal models such as the monkey22, mouse23,24, rabbit25, rat26, and fly27. Recent studies have implicated P. gingivalis in the onset of different systemic pathologies, including rheumatoid arthritis28,29, cardiovascular pathologies30,31, and neurodegenerative pathologies32,33.
The oral cavity harbors one of the most diverse microbiomes in the human body with genetic signatures of more than seven hundred bacteria34,35. This microbiome is constructed by reciprocal interactions between different bacterial communities and between the bacteria and the host environment12. Any disruption of the equilibrium of the oral microbiome allows for the emergence of disease-promoting bacteria, which cause conditions such as gingival inflammation and periodontitis. Gemella is a genus of Gram-positive facultatively anaerobic bacteria that is indigenous in the oral cavity and has hemolytic activity36. Metagenomic analysis of subgingival plaque samples showed that levels of Gemella spp. or Gemella haemolysans were higher in healthy controls than patients with periodontitis37,38,39. It has been reported that G. haemolysans levels increased after periodontal therapy in subgingival plaques of periodontitis patients40. On the other hand, there was no change in the prevalence of this bacterium in the mesial sulcus of teeth of periodontal subjects as compared with healthy controls41. G. haemolysans has been described as an early colonizer of oral biofilms and is part of the symbiotic microbial flora42. Outside of the oral cavity, this species is associated with opportunistic infections in immunocompromised patients and causes infectious endocarditis43,44.
Patients with periodontal disease often have bleeding gums. However, it is unclear whether there is a relationship between periodontal disease and hemolytic bacteria. Therefore, the aim of this study was to identify the hemolytic bacteria associated with periodontal health. Several hemolytic bacteria were identified in the saliva of patients with periodontal disease and healthy subjects. Sequence analysis of the 16S ribosomal RNA (rRNA) gene showed that the genus Gemella accounted for a large proportion of the hemolytic bacteria in saliva. The three Gemella species detected in saliva were Gemella sanguinis, Gemella haemolysans, and Gemella morbillorum. G. haemolysans levels were elevated in the oral cavities of healthy subjects compared to those in periodontal disease patients. The proteins secreted by G. haemolysans directly suppressed the growth of P. gingivalis, suggesting that G. haemolysans is associated with a healthy oral environment by suppressing the growth of P. gingivalis.
Results
Distribution of volunteers
Saliva samples from 15 volunteers at the Matsumoto Dental University Hospital were obtained to examine the differences in salivary microbiomes between periodontal patients and healthy subjects. The subjects were divided into two groups based on the presence (periodontal pocket depth, ≥ 4 mm; n = 10) or absence (n = 5) of periodontal disease. The average age of the 10 periodontal disease patients was 57.5 years (range, 35–69 years) and that of the 5 healthy subjects was 55.6 years (range, 49–64 years). No statistically significant difference in the age was noted between the periodontal disease patients and healthy subjects. The demographic and clinical parameters of the subjects in this study are summarized in Table 1.
Hemolytic bacteria in the saliva of the healthy subjects and periodontal disease patients
A hemolytic activity assay of salivary bacteria was performed using horse blood agar plates to investigate the differences in the oral hemolytic bacteria between periodontal disease patients and healthy subjects. Some bacterial colonies in the saliva samples of the periodontitis patients and healthy subjects exhibited hemolytic activities (Fig. 1a,b). The average percentages of hemolytic colonies were 3.2% and 2.6% in the periodontitis patients and healthy subjects, respectively (Fig. 1c). Large variations in the proportions of hemolytic bacteria in the saliva were noted between individuals in the two groups (Fig. 1a,b); however, no significant differences were observed between the two groups of subjects (P = 0.59, Fig. 1c) or between males and females in this study (P = 0.67; Fig. 1d).
Measurement of hemolytic bacteria in human saliva. (a,b) Percentages of hemolytic bacterial cells in saliva from periodontal disease patients (a) or healthy subjects (b). Individual saliva samples (n = 3) were spread on agar plates containing horse blood and incubated at 37 °C under anaerobic conditions. The numbers of hemolytic and non-hemolytic bacterial colonies were determined using the plate count technique. Error bars show the mean ± standard deviation (SD) values. (c) No significant differences in the percentage of hemolytic bacteria were observed between healthy subjects (black circles) and periodontal disease patients (black squares). Mann–Whitney U-test. Error bars show the mean ± standard error of mean (SEM) values. (d) No significant differences in the percentage of hemolytic bacteria in saliva were observed between male and female subjects. Mann–Whitney U-test. Error bars show the mean ± SEM.
Sequence analysis of the 16S rRNA genes from hemolytic bacterial colonies was performed in two samples each from the periodontitis patients and healthy subjects. Highly hemolytic strains of G. sanguinis, G. haemolysans, and Streptococcus mitis were isolated from the saliva of the periodontal disease patients (Table 2). In addition to these bacteria, Haemophilus parainfluenzae and Streptococcus australis were isolated from healthy subjects. Furthermore, G. sanguinis and G. haemolysans accounted for 75% (15/20) of the isolated highly hemolytic bacteria, indicating that Gemella species represent a major proportion of the hemolytic bacteria present in the saliva of humans. Additionally, G. morbillorum and Streptococcus cristatus were identified as weakly hemolytic bacteria (Table 2).
Ratio of Gemella species in the saliva of periodontal patients
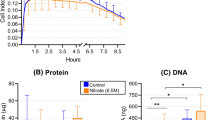
G. sanguinis, G. haemolysans, and G. morbillorum were detected in human saliva (Table 2). qPCR was performed using specific primers for the 16S rRNA of each Gemella species to investigate whether the abundance ratios of the three Gemella species were different between the periodontitis patients and healthy subjects. G. sanguinis and G. haemolysans were more predominant than G. morbillorum in the saliva of the healthy subjects, (Supplementary Fig. 1). G. haemolysans was more prevalent in the saliva of the healthy subjects (Fig. 2b); however, there was no difference based on gender (Supplementary Fig. 2). No significant differences in the abundance ratios of G. sanguinis and G. morbillorum were observed between the two groups of subjects (Fig. 2a,c). These data provide a preliminary indication that high levels of G. haemolysans in saliva are associated with periodontal health.
Levels of G. haemolysans in the saliva of healthy subjects and periodontitis patients. (a–c) Quantification of Gemella species in the saliva of healthy subjects and periodontal disease patients. The percentages of G. sanguinis (a), G. haemolysans (b), and G. morbillorum (c) among the total salivary bacteria were determined by quantitative PCR analysis using specific primers for the 16S rRNA gene. The results for samples from the periodontal disease patients and healthy subjects are shown as red circles and blue squares, respectively. All data were analyzed using a two-tailed Mann–Whitney U-test. ns, not significant. *P < 0.05. Error bars show the mean ± SEM.
Growth inhibition of P. gingivalis by G. haemolysans
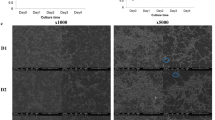
P. gingivalis is considered to be the main etiological agent in periodontal disease. Several studies have demonstrated the critical role of this bacterium in the pathogenesis of periodontal diseases45,46. To determine whether G. haemolysans is associated with the growth of periodontal pathogens, we investigated its effect on the growth of P. gingivalis using blood agar plates. The growth of P. gingivalis was inhibited in the region adjacent to colonies of G. haemolysans on agar plates (Fig. 3a). Furthermore, a growth inhibition zone for P. gingivalis was formed in regions close to the G. haemolysans colonies (Fig. 3b,c). On the other hand, G. sanguinis, G. morbillorum and Aggregatibacter actinomycetemcomitans did not affect the growth of P. gingivalis (Fig. 3d–g). These results indicate that G. haemolysans can directly suppress the growth of P. gingivalis.
Effect of Gemella species on the growth of P. gingivalis. (a) Effect of G. haemolysans on the growth of P. gingivalis. Cultures of G. haemolysans and P. gingivalis were spotted adjacent to each other on a blood agar plate and incubated at 37 °C under anaerobic conditions. The white and black colored colonies indicate G. haemolysans and P. gingivalis, respectively. (b–f) Cultures of G. haemolysans (b), G. sanguinis (d), G. morbillorum (e) and A. actinomycetemcomitans (f) were each spotted on an agar plate spread with P. gingivalis and plates were incubated at 37 °C under anaerobic conditions. (c) The zoom image of (b) from another angle. (g) Quantification of the growth inhibition zone of P. gingivalis caused by G. haemolysans (G. haem), G. sanguinis (G. sang), G. morbillorum (G. morb), A. actinomycetemcomitans (A. acti). Error bars show the mean ± SD. All results are representative of data generated in at least three independent experiments.
Inhibition of bacterial growth by G. haemolysans
A soft-agar overlay assay was performed to determine whether the inhibitory activity of G. haemolysans was specific for P. gingivalis. The ability of G. haemolysans to suppress the growth of several different bacterial strains was examined within the top agar on plates with actively growing G. haemolysans (Fig. 4a). Each top agar contained nutrients required for the growth of each target bacterial strain. The presence of G. haemolysans caused a growth inhibition zone for P. gingivalis in the top agar, indicating a strong growth-suppression activity (Fig. 4b). To examine whether differences in the cell wall structure of the target bacteria affected the inhibitory activity of G. haemolysans, similar inhibition experiments were performed using several Gram-positive and Gram-negative bacteria. No growth-suppressing effects were observed on other periodontal pathogens (Fusobacterium nucleatum and Treponema denticola: Gram-negative), oral streptococci species (S. mitis, S. mutans, S. sobrinus and S. gordonii: Gram-positive), and enteric bacteria (Escherichia coli: Gram-negative), whereas a slight effect was detected on Streptococcus anginosus (Gram-positive; Fig. 4c–k). These data indicate that the growth suppression activity of G. haemolysans was particularly effective against P. gingivalis.
Target specificity of the growth inhibition activity of G. haemolysans. (a) Schematic depiction of the soft-agar overlay technique used to investigate the growth inhibition activity of G. haemolysans. (b–j) The soft 0.7% agar in the top layer was inoculated with the target bacterium i.e., P. gingivalis (b), F. nucleatum (c), T. denticola (d), S. mutans (e), S. mitis (f), S. sobrinus (g), S. anginosus (h), S. gordonii (i) and E. coli (j), while the 1.5% agar bottom layer was inoculated with G. haemolysans. (k) Quantification of the growth inhibition zone between G. haemolysans and each bacterium. Error bars show the mean ± SD. All results are representative of data generated in at least two independent experiments.
Analysis of the culture supernatant of G. haemolysans
A blood agar medium impregnated with G. haemolysans culture supernatant was prepared to investigate whether the inhibitory agent was present in the culture supernatant. The growth of P. gingivalis was clearly inhibited on agar medium containing the culture supernatant of G. haemolysans (Fig. 5a). In addition, the inhibitory activity was dramatically reduced using heat-treated supernatant, suggesting that this inhibitor is a heat-labile molecule (Fig. 5a). Next, we focused on the protein components contained in the culture supernatant of G. haemolysans. Secreted proteins in the culture supernatant of G. haemolysans were obtained by ammonium sulfate precipitation. After dialysis, the secreted proteins were used in the growth inhibition assay. P. gingivalis growth was barely visible in liquid medium containing the G. haemolysans secreted proteins compared to that in the control medium without the secreted proteins (Fig. 5b,c). In addition, the growth inhibition of P. gingivalis was dramatically reduced following heat treatment of the secreted proteins (Fig. 5b,c). These results suggest that one or more proteins secreted by G. haemolysans directly inhibited the growth of P. gingivalis.
The cell culture supernatant of G. haemolysans exhibits the growth suppression ability against P. gingivalis. (a) P. gingivalis was cultured on blood agar containing sterile GAM medium (as a control, left panel), culture supernatant of G. haemolysans (middle panel), or heat-treated culture supernatant of G. haemolysans (right panel). (b) P. gingivalis was cultured in liquid medium containing PBS (as a control, left), culture supernatant of G. haemolysans (center), or heat-treated culture supernatant of G. haemolysans (right). (c) Quantification of the growth of P. gingivalis observed in (b). The optical density was measured at 600 nm to determine the concentration of P. gingivalis. Error bars show the mean ± SD. All results are representative of data generated in at least three independent experiments.
Discussion
In this study, we examined bacteria in the saliva of patients with periodontal disease and healthy controls. Saliva can be collected non-invasively, repeatedly and without trained personnel. Since the salivary microbiota are generally considered to reflect the overall periodontal condition in patients with periodontitis47, saliva is a promising diagnostic body fluid for clinical use in dentistry. In fact, research with oral plaque has shown that G. haemolysans levels increase after therapy for periodontal disease40, which is consistent with the high proportion of G. haemolysans in saliva samples from healthy subjects in our study.
The Human Oral Microbiome Database (https://www.homd.org) has registered four Gemella species with the scientific names G. haemolysans, G. morbillorum, G. sanguinis and G. bergeri. G. haemolysans was first described as Neisseria haemolysans48 because the bacterium was thought to be a Gram-negative aerobic diplococcus that causes hemolysis on rabbit blood agar. However, this organism did not possess the typical bacteriological characteristics of the genus Neisseria, such as catalase, cytochrome oxidase, and peroxidase activities49. For these reasons, a new genus was established. Likewise, G. morbillorum was previously identified as a Peptostreptococcus species. Analysis of the 16S rRNA gene sequence showed that G. haemolysans is more similar to G. sanguinis and considerably different from G. bergeri50,51.
Strong hemolytic activity is proposed as a leading characteristic of G. haemolysans. It distinguishes these bacteria from G. morbillorum52, which generally presents with no or weak hemolytic activity (α-hemolysis). In some cases, G. sanguinis53 and G. bergeri50 exhibit strong hemolytic activity depending on the blood and components of the animal species used in the medium. Furthermore, G. bergeri has not been detected in the microbiome of oral plaques using 16S rRNA gene sequencing, indicating the rarity of this species in the oral cavity54. In the current study, three Gemella species with hemolytic activity (G. sanguinis, G. haemolysans, and G. morbillorum) were detected in human saliva (Table 2). G. bergeri may have remained undetected as a hemolytic colony in the current study due to the aforementioned reasons.
The levels of G. haemolysans, but not G. sanguinis or G. morbillorum, were significantly elevated in the saliva of healthy subjects when compared to those in the periodontitis patients, suggesting that G. haemolysans plays a different role in the oral cavity than G. sanguinis and G. morbillorum (Fig. 2). A previous biochemical analysis showed that G. haemolysans could be distinguished from G. sanguinis and G. morbillorum due to its failure to produce acid from mannitol and sorbitol55; furthermore, G. sanguinis differs from G. morbillorum by producing alkaline phosphatase55. G. bergeri differs from G. haemolysans, G. sanguinis, and G. morbillorum by failing to produce acid from maltose and sucrose50. In an analysis of the zinc metalloproteinase (Zmp) protein family, G. haemolysans was found to possess all of the Zmp components (IgA, ZmpC, and ZmpD), except for ZmpB; in contrast, G. sanguinis and G. morbillorum carried only ZmpB, whereas G. bergeri did not possess any Zmp homolog56. These results highlight some of the unique characteristics of G. haemolysans in addition to the inhibitory activity against P. gingivalis that is not found in G. sanguinis and G. morbillorum (Fig. 3). However, further biochemical and genomic analyses need to be conducted to fully understand the role of the Gemella genus in the oral cavity.
The normal microbiome prevents the colonization of pathogens by competing for attachment sites or essential nutrients. Previous studies targeting P. gingivalis showed that the S. intermedius extracellular protein arginine deiminase induced the down-regulation of the fimA, and mfa1 genes, which encode the major subunits of fimbriae57,58. Thus, this protein abolished biofilm formation without affecting the growth rate of P. gingivalis. On the other hand, Bifidobacteria have been reported to inhibit the growth of P. gingivalis59; this effect was thought to be caused by a decrease in the pH due to acid production, nutrient competition, and the action of inhibitor molecules. G. haemolysans can produce acid, similar to Bifidobacterium60. In the current study, the effect of a decrease in pH in the P. gingivalis growth inhibition assay was resolved by changing the buffer using a dialysis membrane, and nutrient competition was ruled out by using an extracellular fraction without live bacteria (Fig. 5b). These results clearly showed that the components of G. haemolysans in the culture supernatant inhibited the growth of P. gingivalis. In addition, we presumed that the component(s) responsible for this activity would be a protein because it could be concentrated via ammonium sulfate precipitation and inactivated by heat treatment. However, the protein involved in inhibiting the growth of P. gingivalis has not been identified so far; additional studies are needed to determine this in the future.
Periodontal disease is one of the most common chronic inflammatory diseases1,2,3,4,5. Many studies have shown the association of several bacterial pathogens with this disease61. Conversely, little attention has been paid to the identification of health-associated and potentially beneficial bacterial species in the oral cavity. Probiotics technology is a groundbreaking approach to maintaining health by using beneficial bacteria that can support the natural defense system against pathogens62,63,64. Probiotics have emerged as an attractive oral medicine and the use of these agents is increasing65,66,67. In the present study, the symbiotic bacterium G. haemolysans suppressed the growth of P. gingivalis. This data presents G. haemolysans as a novel probiotic candidate for treating periodontal disease. Furthermore, secreted protein(s) from G. haemolysans that function similar to a bacteriocin might be used for the treatment of periodontal disease as a P. gingivalis–targeting drug. Nonetheless, it is essential to develop an understanding of the broad range of environmental changes that would be caused by the ingestion of G. haemolysans in the oral cavity and their long-term effects on oral diseases and health.
This study proposes a novel role for indigenous bacteria in controlling periodontal pathogens. However, the results are limited to the analysis of two bacterial strains using an artificial medium; hence the in vivo effect of G. haemolysans on the oral flora remains unclear. Additional studies are required to understand the relationship between G. haemolysans and periodontal disease in the oral cavity in more detail.
Conclusion
In summary, the findings of this study showed that the saliva of healthy subjects contained a higher proportion of G. haemolysans than that of patients with periodontitis. Growth inhibition assays indicated that the protein components contained in the culture supernatant of G. haemolysans directly suppressed the growth of P. gingivalis. These findings show that the presence of G. haemolysans in saliva is associated with oral health and that it inhibits the growth of P. gingivalis in vitro, which may prove useful during the development of therapies for this disease in the future.
Materials and methods
Ethics
The study was reviewed and approved by the research ethics committee of the Matsumoto Dental University (#0217) and all methods were carried out in accordance with relevant guidelines and regulations. All study subjects signed informed consent prior to participating.
Collection of saliva samples
Saliva samples were collected without stimulation from 15 volunteers at the Matsumoto Dental University Hospital in Japan. Data concerning the age, sex, and pocket depth, and location of the subjects were recorded during sample collection. Those with a periodontal pocket depth of ≥ 4 mm were categorized as patients with periodontal disease (n = 10) and the remaining 5 with depths of < 4 mm were grouped as healthy subjects.
Detection and identification of hemolytic bacteria in saliva
Saliva samples were dispersed by vortexing followed by a tenfold serial dilution with phosphate-buffered saline (PBS). Aliquots of 100 µl of each dilution were plated on TS blood agar (Trypticase Soy Broth; 5% horse blood and 1.5% agar) and incubated for 48 h at 37 °C in an anaerobic chamber (Anaerobox, Hirasawa, Tokyo, Japan) containing 85% N2, 10% H2, and 5% CO2. The colonies on the agar medium were clearly distinguished into hemolytic and non-hemolytic bacteria, and the numbers of each colony type were counted.
16S ribosomal RNA gene sequencing
Bacterial genomic DNA was extracted from each hemolytic colony using a heat extraction method68. The 16S rRNA genes of each hemolytic bacterium were amplified using KAPA HiFi HS ReadyMix (Kapa Biosystems), according to the manufacturer’s instructions. A polymerase chain reaction (PCR) amplification was performed using the following universal bacterial primers: 16S_27f (5′-AGA GTT TGA TCC TGG CTC AG-3′) and 16S_1492r (5′-GGT TAC CTT GTT ACG ACT T -3′)69. The PCR products were gel purified using the FastGene Gel/PCR Extraction Kit (Nippon Genetics, Japan) and the sequences were confirmed via a commercial sequencing service (Fasmac, Kanagawa, Japan). The sequences of the PCR products were compared with known 16S rRNA gene sequences in GenBank, and the hemolytic bacterial species were identified.
Quantification of each Gemella species in saliva by quantitative PCR
The extraction of bacterial DNA from saliva was performed using MORA-EXTRACT (Kyokuto Seiyaku, Tokyo, Japan) according to the manufacturer’s instructions. To compare the amount of Gemella species and total bacteria in saliva, quantitative real-time PCR (qRT-PCR) was performed using a Fast SYBR Green Master Mix (Applied Biosystems) according to the manufacturer’s instructions. The universal bacterial primers for the 16S rRNA gene in total bacteria (16S_27f; 5′-AGA GTT TGA TCC TGG CTC AG-3′, 16S_350r; 5′-CTG CTG CCT CCC GTA G -3′) and specific primers for the 16S rRNA genes in G. sanguinis (Gs153-176f; 5′-ATA ACA GCA TAA ATC GCA TGA TAT-3′, Gs455-478r; 5′-TGG TTA GGT ACC GTC TCT ACT GTA-3′), G. haemolysans (Gh170-189f; 5′-CAG CAT TAA CTG CAT GGT TG-3′, Gh467-489 5′-GGT TAG GTA CCG TCT CTA CTG TG-3′) and G. morbillorum (Gm165-188; 5′-ATA ACA GTA TTT CTC GCA TGA GAG-3′, Gm466-488;5′-GGT TAG GTA CCG TCT CTT ACA TG-3′) were used. The PCR cycling conditions were as follows: 95 °C for 30 s followed by 40 cycles of 95 °C for 5 s and 58 °C for 1 min (Takara Thermal Cycler Dice Real-Time System II). DNA melting curves were created to check for the presence of only one amplification fragment. The specificity of each primer set was confirmed by analyzing the PCR products by agarose gel electrophoresis (Supplementary Figs. 3 and 4). The relative amounts of total bacteria and Gemella species in saliva were calculated using the comparative CT method70.
Growth competition assay
To evaluate the ability of G. haemolysans to inhibit P. gingivalis growth, a growth competition assay was performed by culturing the two types of bacteria in close proximity to each other on blood agar plates. Strains of G. haemolysans (clinical isolates obtained in this study), G. sanguinis ATCC700632, G. morbillorum ATCC27824, P. gingivalis W83, and A. actinomycetemcomitans NK1651 were each cultured in Gifu anaerobic medium (GAM) containing hemin (5 µg/ml) and menadione (0.5 µg/ml) at 37 °C under anaerobic conditions containing 85% N2, 10% H2, and 5% CO2. After reaching the late stationary phase (approximately 48 h), 10 µl of G. haemolysans culture was spotted onto a blood agar plate and the P. gingivalis culture was spotted adjacent to it; the plate was incubated for 4 days at 37 °C under anaerobic conditions. In addition, the P. gingivalis culture was spread thinly on blood agar plates using a swab. After drying the plate, 10 µl of each culture of G. haemolysans, G. sanguinis, G. morbillorum and A. actinomycetemcomitans was spotted onto the blood agar plate, which was incubated for 4 days at 37 °C under anaerobic conditions. The inhibitory activity was detected by measuring the growth inhibition zone of P. gingivalis on the agar plates.
The growth inhibition assay for bacteria that grow in special media was performed using the soft-agar overlay technique71,72. G. haemolysans was spread onto half the area of a GAM agar (1.5%) plate and cultured for 2 days at 37 °C under anaerobic conditions. Next, a soft-agar (0.7%) medium (5 ml, 50 °C) containing each target bacterial strain (0.3 ml) was overlaid on the 1.5% agar plate and incubated for 48 h at 37 °C under anaerobic conditions. A modified GAM broth (Nissui) containing thiamin (2 µg/ml) and FBS (5%) was used as a soft-agar medium for T. denticola JCM8225, whereas GAM broth containing hemin (5 µg/ml) and menadione (0.5 µg/ml) was used for F. nucleatum JCM6328, S. anginosus NCTC10713, S. mitis ATCC9811, S. mutans MT8148, S. gordonii ATCC35105, S. sobrinus GTC278, and E. coli BL21. The growth inhibition activity of G. haemolysans for each bacterial strain was detected by measuring the zone of growth inhibition on the top agar.
Growth inhibition assay using bacterial culture supernatant
An assay was carried out using the bacterial culture supernatant of G. haemolysans to investigate the mechanism by which it inhibited the growth of P. gingivalis. The bacterial culture supernatant was obtained by culturing G. haemolysans in GAM medium for 48 h at 37 °C under anaerobic conditions and filtering it using a vacuum filtration system (rapid-Filtermax 0.2 µm, TPP, Switzerland). A P. gingivalis culture (10 µl) was spotted onto a blood agar plate soaked with the supernatant (2 ml) and incubated for 48 h at 37 °C under anaerobic conditions. The same process was performed for the heat-treated (at 90 °C for 5 min) supernatant and sterile GAM medium as a control. The growth rate of P. gingivalis on the blood agar was evaluated.
Furthermore, the inhibition activity was confirmed in liquid cultures. Proteins in the culture supernatant of G. haemolysans were obtained via ammonium sulfate precipitation. The 20-fold concentrated sample was dialyzed (VISKASE, MEMBRA-CEL dialysis membranes, MWCO: 14,000) in a buffer (20 mM Tris–HCl pH7.5, 150 mM NaCl) for 3 h, and a portion of the sample was incubated for 5 min at 90 °C. Each sample (20 µl) was filtered using MILLEX GV 0.22 µm filters (Millipore) and mixed with P. gingivalis (180 µl; OD at 600 nm, 0.04) diluted in GAM medium containing hemin (5 ug/ml) and menadione (0.5 µg/ml), and incubated in a 96 well culture plate (CELLSTAR, Greiner Bio-one, Australia) for 48 h at 37 °C under anaerobic conditions. The inhibition activity was evaluated by measuring the optical density at 600 nm (BioPhotometer-D30, Eppendorf, Germany).
References
Papapanou, P. N. Periodontal diseases: Epidemiology. Ann. Periodontol. 1, 1–36 (1996).
Albandar, J. M. & Rams, T. E. Global epidemiology of periodontal diseases: An overview. Periodontol. 2000(29), 7–10 (2002).
Albandar, J. M. Periodontal diseases in North America. Periodontol. 2000(29), 31–69 (2002).
Offenbacher, S. et al. Maternal periodontitis and prematurity. Part I: Obstetric outcome of prematurity and growth restriction. Ann. Periodontol. 6, 164–174 (2002).
Pihlstrom, B. L., Michalowicz, B. S. & Johnson, N. W. Periodontal diseases. Lancet 366, 1809–1820 (2005).
Kuo, L. C., Polson, A. M. & Kang, T. Associations between periodontal diseases and systemic diseases: A review of the inter-relationships and interactions with diabetes, respiratory diseases, cardiovascular diseases and osteoporosis. Public Health 122, 417–433 (2007).
Arigbede, A. O., Babatope, B. O. & Bamidele, M. K. Periodontitis and systemic diseases: A literature review. J. Indian Soc. Periodontol. 16, 487–491 (2013).
Nazir, M. A. Prevalence of periodontal disease, its association with systemic diseases and prevention. Int. J. Health Sci. (Qassim) 11, 72–80 (2007).
Li, X., Kolltveit, K. M., Tronstad, L. & Olsen, I. Systemic diseases caused by oral infection. Clin. Microbiol. Rev. 13, 547–558 (2000).
Kim, J. & Amar, S. Periodontal disease and systemic conditions: a bidirectional relationship. Odontology 94, 10–21 (2006).
Williams, R. C. Periodontal disease. N. Engl. J. Med. 322, 373–382 (1990).
Lamont, R. J., Koo, H. & Hajishengallis, G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Microbiol. 16, 745–759 (2018).
Mysak, J. et al. Porphyromonas gingivalis: major periodontopathic pathogen overview. J. Immunol. Res. 2014, 476068 (2014).
Travis, J., Pike, R., Imamura, T. & Potempa, J. Porphyromonas gingivalis proteinases as virulence factors in the development of periodontitis. J. Periodontal Res. 32, 120–125 (1997).
Kadowaki, T. et al. Porphyromonas gingivalis proteinases as virulence determinants in progression of periodontal diseases. J. Biochem. 128, 153–159 (2002).
Amano, A., Nakagawa, I., Okahashi, N. & Hamada, N. Variations of Porphyromonas gingivalis fimbriae in relation to microbial pathogenesis. J. Periodontal Res. 39, 136–142 (2004).
Weinberg, A., Belton, C. M., Park, Y. & Lamont, R. J. Role of fimbriae in Porphyromonas gingivalis invasion of gingival epithelial cells. Infect. Immun. 65, 313–316 (1997).
Hamada, S., Fujiwara, T. & Maihara, J. Bacterial endotoxic substances and their effects on host cells. in Molecular Pathogenesis of Periodontal Disease. (Genco, R., Hamada, S., Lehner, T., McGhee, J., Mergenhagen, S. eds.) 105–117 (ASM Press, 1994).
Bainbridge, B. W. & Darveau, R. P. Porphyromonas gingivalis lipopolysaccharide: An unusual pattern recognition receptor ligand for the innate host defense system. Acta. Odontol. Scand. 59, 131–138 (2001).
Brunner, J. et al. The capsule of Porphyromonas gingivalis reduces the immune response of human gingival fibroblasts. BMC Microbiol. 10, 5 (2010).
Singh, A. et al. The capsule of Porphyromonas gingivalis leads to a reduction in the host inflammatory response, evasion of phagocytosis, and increase in virulence. Infect. Immun. 79, 4533–4542 (2011).
Holt, S. C., Ebersole, J., Felton, J., Brunsvold, M. & Kornman, K. S. Implantation of Bacteroides gingivalis in nonhuman primates initiates progression of periodontitis. Science 239, 55–57 (1988).
Hanazawa, S. et al. Bacteroides (Porphyromonas) gingivalis fimbriae activate mouse peritoneal macrophages and induce gene expression and production of interleukin-1. Infect. Immun. 59, 1972–1977 (1991).
Kesavalu, L., Ebersole, J. L, Machen, R. L. & Holt, S. C. Porphyromonas gingivalis virulence in mice: induction of immunity to bacterial components. Infect. Immun. 60, 1455–1464 (1992).
Boggess, K. A., Madianos, P. N., Preisser, J. S., Moise, K. J. & Offenbacher, S. Chronic maternal and fetal Porphyromonas gingivalis exposure during pregnancy in rabbits. Am. J. Obstet. Gynecol. 192, 554–557 (2005).
Klausen, B. Microbiological and immunological aspects of experimental periodontal disease in rats: A review article. J. Periodontol. 62, 59–73 (1991).
Igboin, C. O., Tordoff, K. P., Moeschberger, M. L., Griffen, A. L. & Leys, E. J. Porphyromonas gingivalis-host interactions in a Drosophila melanogaster model. Infect. Immun. 79, 449–458 (2011).
Wegner, N. et al. Peptidylarginine deiminase from Porphyromonas gingivalis citrullinates human fibrinogen and α-enolase: implications for autoimmunity in rheumatoid arthritis. Arthritis Rheum. 62, 2662–2672 (2010).
Mikuls, T. R. et al. Antibody responses to Porphyromonas gingivalis (P. gingivalis) in subjects with rheumatoid arthritis and periodontitis. Int. Immunopharmacol. 9, 38–42 (2008).
Progulske-Fox, A. et al. Porphyromonas gingivalis virulence factors and invasion of cells of the cardiovascular system. J. Periodontal Res. 34, 393–399 (2000).
Deshpande, R. G., Khan, M. & Genco, C. A. Invasion strategies of the oral pathogen porphyromonas gingivalis: Implications for cardiovascular disease. Invasion Metastasis 18, 57–69 (1998–1999).
Singhrao, S. K., Harding, A., Poole, S., Kesavalu, L. & Crean, S. Porphyromonas gingivalis periodontal infection and its putative links with Alzheimer's disease. Mediators Inflamm. 2015, 137357 (2015).
Dominy, S. S. et al. Porphyromonas gingivalis in Alzheimer's disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Sci. Adv. 5, eaau3333 (2019).
Wade, W. G. The oral microbiome in health and disease. Pharmacol. Res. 69, 137–143 (2013).
Zaura, E., Keijser, B. J., Huse, S. M. & Crielaard, W. Defining the healthy “core microbiome” of oral microbial communities. BMC Microbiol. 9, 259 (2009).
Dewhirst, F. E. et al. The human oral microbiome. J. Bacteriol. 192, 5002–5017 (2010).
Chen, W. P. et al. Composition analysis and feature selection of the oral microbiota associated with periodontal disease. Biomed Res. Int. 2018, 3130607 (2018).
Arredondo, A., Blanc, V., Mor, C., Nart, J. & León, R. Tetracycline and multidrug resistance in the oral microbiota: differences between healthy subjects and patients with periodontitis in Spain. J. Oral Microbiol. 13, 1847431 (2020).
Kirst, M. E. et al. Dysbiosis and alterations in predicted functions of the subgingival microbiome in chronic periodontitis. Appl. Environ. Microbiol. 81, 783–793 (2015).
Colombo, A. P. et al. Impact of periodontal therapy on the subgingival microbiota of severe periodontitis: comparison between good responders and individuals with refractory periodontitis using the human oral microbe identification microarray. J. Periodontol. 83, 1279–1287 (2012).
Kumar, P. S. et al. New bacterial species associated with chronic periodontitis. J. Dent. Res. 82, 338–344 (2003).
Heller, D. et al. Microbial diversity in the early in vivo-formed dental biofilm. Appl. Environ. Microbiol. 82, 1881–1888 (2016).
Kaufhold, A., Franzen, D. & Lütticken, R. Endocarditis caused by Gemella haemolysans. Infection 17, 385–387 (1989).
La Scola, B. & Raoult, D. Molecular identification of Gemella species from three patients with endocarditis. J. Clin. Microbiol. 36, 866–871 (1998).
Haffajee, A. D. & Socransky, S. S. Microbial etiological agents of destructive periodontal diseases. Periodontol. 2000(5), 78–111 (1994).
Socransky, S. S., Haffajee, A. D., Ximenez-Fyvie, L. A., Feres, M. & Mager, D. Ecological considerations in the treatment of Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis periodontal infections. Periodontol. 2000(20), 341–362 (1999).
Kageyama, S. et al. Relative abundance of total subgingival plaque-specific bacteria in salivary microbiota reflects the overall periodontal condition in patients with periodontitis. PLoS One 12, e0174782 (2017).
Thjotta, Th. & Boe, J. Neisseria haemolysans. A hemolytic species of Neisseria Trevisan. Acta Pathol. Mierobiol. Scand. Suppl. 37, 527–531 (1938).
Berger, U. A proposed new genus of Gram-negative cocci: Gemella. Int. Bull. Bacteriol. Nomenclature Taxon. 11, 17–19 (1961).
Collins, M. D., Hutson, R. A., Falsen, E., Sjöden, B. & Facklam, R. R. Gemella bergeriae sp. nov., isolated from human clinical specimens. J. Clin. Microbiol. 36, 1290–1293 (1998).
Woo, P. C. et al. Gemella bacteraemia characterised by 16S ribosomal RNA gene sequencing. J. Clin. Pathol. 56, 690–693 (2003).
Berger, U. & Pervanidis, A. Differentiation of Gemella haemolysans (Thjøtta and Bøe 1938) Berger 1960, from Streptococcus morbillorum (Prevot 1933) Holdeman and Moore 1974. Zentralbl. Bakteriol. Mikrobiol. Hyg. A 261, 311–321 (1986).
Collins, M. D., Hutson, R. A., Falsen, E., Sjöden, B. & Facklam, R. R. Description of Gemella sanguinis sp. nov., isolated from human clinical specimens. J. Clin. Microbiol. 36, 3090–3093 (1998).
Kumar, P. S, Griffen, A. L., Moeschberger, M. L. & Leys, E. J. Identification of candidate periodontal pathogens and beneficial species by quantitative 16S clonal analysis. J. Clin. Microbiol. 43, 3944–3955 (2005).
Ulger-Toprak, N., Summanen, P. H., Liu, C., Rowlinson, M. C. & Finegold, S. M. Gemella asaccharolytica sp. nov., isolated from human clinical specimens. Int. J. Syst. Evol. Microbiol. 60, 1023–1026 (2010).
García López, E. & Martín-Galiano, A. J. The versatility of opportunistic infections caused by Gemella isolates is supported by the carriage of virulence factors from multiple origins. Front. Microbiol. 11, 524 (2020).
Christopher, A. B., Arndt, A., Cugini, C. & Davey, M. E. A streptococcal effector protein that inhibits Porphyromonas gingivalis biofilm development. Microbiology 156, 3469–3477 (2010).
Cugini, C., Stephens, D. N., Nguyen, D., Kantarci, A. & Davey, M. E. Arginine deiminase inhibits Porphyromonas gingivalis surface attachment. Microbiology 159, 275–285 (2013).
Jäsberg, H., Söderling, E., Endo, A., Beighton, D. & Haukioja, A. Bifidobacteria inhibit the growth of Porphyromonas gingivalis but not of Streptococcus mutans in an in vitro biofilm model. Eur. J. Oral. Sci. 124, 251–258 (2016).
Berger, U. Prevalence of Gemella haemolysans on the pharyngeal mucosa of man. Med. Microbiol. Immunol. 174, 267–274 (1985).
Socransky, S. S. & Haffajee, A. D. The bacterial etiology of destructive periodontal disease: Current concepts. J. Periodontol. 63, 322–331 (1992).
Salminen, S. et al. Demonstration of safety of probiotics – A review. Int. J. Food Microbiol. 44, 93–106 (1998).
Kopp-Hoolihan, L. Prophylactic and therapeutic uses of probiotics: A review. J. Am. Diet. Assoc. 101, 229–241 (2001).
Soccol, C. R. et al. The potential of probiotics: A review. Food Technol. Biotechnol. 48, 413–434 (2010).
Lin, T. H., Lin, C. H. & Pan, T. M. The implication of probiotics in the prevention of dental caries. Appl. Microbiol. Biotechnol. 102, 577–586 (2018).
Saraf, K., Shashikanth, M. C., Priy, T., Sultana, N. & Chaitanya, N. C. Probiotics–Do they have a role in medicine and dentistry?. J. Assoc. Phys. India 58, 488–492 (2010).
Anusha, R. L., Umar, D., Basheer, B. & Baroudi, K. The magic of magic bugs in oral cavity: Probiotics. J. Adv. Pharm. Technol. Res. 6, 43–47 (2015).
Dashti, A. A., Jadaon, M. M., Abdulsamad, A. M. & Dashti, H. M. Heat treatment of bacteria: A simple method of DNA extraction for molecular techniques. Kuwait Med. J. 41, 117–122 (2009).
Eden, P. A., Schmidt, T. M., Blakemore, R. P. & Pace, N. R. Phylogenetic analysis of Aquaspirillum magnetotacticum using polymerase chain reaction-amplified 16S rRNA-specific DNA. Int. J. Syst. Bacteriol. 41, 324–325 (1991).
Schmittgen, T. D. & Livak, K. J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 3, 1101–1108 (2008).
Hockett, K. L. & Baltrus, D. A. Use of the soft-agar overlay technique to screen for bacterially produced inhibitory compounds. J. Vis. Exp. 119, 55064 (2017).
Kropinski, A. M., Mazzocco, A., Waddell, T. E., Lingohr, E. & Johnson, R. P. Enumeration of bacteriophages by double agar overlay plaque assay. Methods Mol. Biol. 501, 69–76 (2008).
Acknowledgements
This work was supported by a Grant-in-Aid for Scientific Research (C) (19K06494 to T.M.) from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Contributions
T.M. and A.Y. conceived and designed the experiments, and wrote the manuscript; T.M., S.O., S.N, Y.U., H.U., R.K. and Y.M. performed experiments; N.Y. collected saliva samples. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Miyoshi, T., Oge, S., Nakata, S. et al. Gemella haemolysans inhibits the growth of the periodontal pathogen Porphyromonas gingivalis. Sci Rep 11, 11742 (2021). https://doi.org/10.1038/s41598-021-91267-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-91267-3
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.