Abstract
The high-pressure phases of oxyhydroxides (δ-AlOOH, ε-FeOOH, and their solid solution), candidate components of subducted slabs, have wide stability fields, thus potentially influencing volatile circulation and dynamics in the Earth’s lower mantle. Here, we report the elastic wave velocities of δ-(Al,Fe)OOH (Fe/(Al + Fe) = 0.13, δ-Fe13) to 79 GPa, determined by nuclear resonant inelastic X-ray scattering. At pressures below 20 GPa, a softening of the phonon spectra is observed. With increasing pressure up to the Fe3+ spin crossover (~ 45 GPa), the Debye sound velocity (vD) increases. At higher pressures, the low spin δ-Fe13 is characterized by a pressure-invariant vD. Using the equation of state for the same sample, the shear-, compressional-, and bulk-velocities (vS, vP, and vΦ) are calculated and extrapolated to deep mantle conditions. The obtained velocity data show that δ-(Al,Fe)OOH may cause low-vΦ and low-vP anomalies in the shallow lower mantle. At deeper depths, we find that this hydrous phase reproduces the anti-correlation between vS and vΦ reported for the large low seismic velocity provinces, thus serving as a potential seismic signature of hydrous circulation in the lower mantle.
Similar content being viewed by others
Introduction
The circulation and distribution of “water” in the forms of hydrogen, hydroxyl, and molecular H2O in the Earth’s interior are important factors affecting the evolution and dynamics of the Earth’s interior1,2,3,4. Hydrous minerals store H2O in the order of 1–10 wt.% in their crystal structures, thus serving as large reservoirs of water even though their proportion in the mantle is relatively small.
An aluminum oxyhydroxide, δ-AlOOH, has a significantly wide stability field and therefore potentially plays a role as a hydrous reservoir in the Earth’s lower mantle. High pressure and high temperature experiments on δ-AlOOH have demonstrated that this phase is stable at 21–142 GPa and up to 2410 K5,6,7,8,9,10,11,12, comparable to the conditions from the lower regions of the mantle transition zone to the lowermost mantle. Experiments in natural-like multicomponent systems reveal the chemical composition, elasticity, and stability of δ-phase formed in the lower mantle environment, as discussed below13,14,15,16,17,18,19,20,21,22,23. In multicomponent systems, δ-AlOOH forms a solid solution with isostructural hydrous phases of ε-FeOOH and MgSiO4H2-Phase H13,14,15,16,17,18,19. However, the effect of incorporations of ε-phase (Fe) and Phase H (Mg, Si) on its stability appears to be limited because of strong partitioning of Al into the hydrous solid solution phase in lower mantle environments. In the MgO–Al2O3–Fe2O3–SiO2–H2O (60 mol% MgSiO3–30 mol% Al2O3–10 mol% Fe2O3 containing ~ 7 wt.% H2O) system, the hydrous solid solution (δ-phase) coexists with bridgmanite, its high-pressure polymorph (post-perovskite), or both at 104–126 GPa and 1750–2500 K18. The chemical analyses of the run products at 117 GPa and 2050 K in this system showed that the composition of the synthesized δ-phase was (Mg0.03(2)Si0.07(3)Al0.81(4)Fe0.09(3))OOH18. Such a Al-rich composition is similar to the hydrous phase (Mg0.11Al0.63Si0.2Fe0.03)OOH, formed in a natural basalt system at 25–26 GPa and 1273–1473 K17 and closer to AlOOH than FeOOH and MgSiO4H2. In contrast to the stability, the elasticity of δ-AlOOH might be altered largely due to a presence of modest amounts of Fe. δ-AlOOH, ε-FeOOH, and their solid solution (hereafter, δ-(Al,Fe)OOH) have a Pnnm structure with a symmetric hydrogen bond at lower mantle pressures20,21,22,23,24,25,26. Substitution of Fe into the Al site of the δ-phase causes an increase in density22. In addition, the high-spin–low-spin transition of Fe3+ in δ-(Al,Fe)OOH at 32–40 GPa, corresponding to shallow lower mantle depths, causes a softening of the isothermal bulk modulus, which consequently decreases vΦ22. Moreover, a theoretical study proposed a negative correlation of pressure (P)–shear modulus (μ) for low-spin ε-FeOOH, which causes a decrease of vS/vΦ with increasing pressure26. These studies point to the possibility that the solid solution may in part be responsible for regionally seismic heterogeneities observed in the lower mantle27,28.
As discussed above, δ-(Al,Fe)OOH is likely stable in subducted slab materials throughout the lower mantle18, yet the possible effects on the seismic wave velocities of a phase assemblage containing the δ-phase are not well constrained. In this study, we report the elastic wave velocities of the δ-(Al,Fe)OOH phases having an Fe/(Al + Fe) ratio of ~ 0.13 determined by nuclear resonant inelastic X-ray scattering (NRIXS). The Fe/(Al + Fe) ratio of our sample is within the rations of δ-phase formed in the MgO–Al2O3–Fe2O3–SiO2–H2O system that simplifies natural basaltic compositions (Fe/(Al + Fe) = 0.10–0.17)18. By combining our results from NRIXS with the equation of state22, we discuss the potential relationships between δ-(Al,Fe)OOH and seismic anomalies in the lower mantle.
Results
Determination of elastic wave velocities
NRIXS spectra of the two δ-(Al0.87357Fe0.127)OOH (δ-Fe13-r1) and δ-(Al0.86757Fe0.133)OOH (δ-Fe13-r2) samples were obtained up to 79 GPa (Fig. S1). The 57Fe-partial projected phonon density of state (pDOS) was subsequently extracted from each NRIXS spectrum using the PHOENIX software29 (Fig. 1). Generally, vibrational peak positions in the pDOS for minerals steadily shift to higher energies with compression30,31. However, the peak positions of pDOS of δ-Fe13 shift to lower energies with increasing pressure from ambient pressure to 10.8 GPa, above which they shift to higher energies (Fig. 1). This pressure at which the peak position of pDOS of δ-Fe13 reaches the lowest energy is very close to that of the structural transition from P21nm with asymmetric hydrogen bonds to Pnnm with symmetric hydrogen bonds inferred from XRD experiments on δ-(Al,Fe)OOH (Fe/(Al + Fe) = 0.047(10) (hereafter, δ-Fe5) and 0.123(2) (δ-Fe12))22. Static calculations for pure δ-AlOOH predicted that phonon-softening occurred as a result of this transition involving hydrogen bonding32. Through this transition, the lowest and highest A1 and B2 optic modes soften and the OH stretching frequency approaches nil32. The Raman spectra of polycrystalline δ-AlOOH show broadening or disappearance of B2 mode of P21nm and the appearance of Ag mode of Pnnm near 5.6 GPa20. Therefore, the shifting of the pDOS to lower energies below about 10 GPa and the trend observed in the Lamb-Mössbauer factor (fLM) of δ-Fe13 (Fig. 2) are likely related to this transition. Due to the likelihood that one or more optical branches cross into the accessible low-energy regime in this low-pressure range (~ 2 < E (meV) < ~ 15), the assumption that the low-energy region of the pDOS is occupied purely by acoustic modes breaks down. Thus, we restrict our determination of the Debye sound velocity (vD) to ambient pressure and pressures higher than 20 GPa.
(a) An example of the Debye sound velocity determination of δ-Fe13 from the pDOS determined at 67 GPa. Examples of two typical phonon dispersion models that are traditionally used for constraining the Debye sound velocity (vD; v(E) at E = 0) are plotted: the Debye-like model (horizontal black solid line) and the power law model (Eq. 1, black dotted line). The open circles are the pDOS data plotted according to Eq. (2). We note that the fit models plotted here are purely demonstrative and do not directly reflect our reported Debye velocities. Families of these models are considered, fit to the data, and plotted as probability distributions (ref.33) of the vD. (b) The energy ranges applied in the PDF analysis at 67 GPa fall within, but are not limited to, the Emin and Emax values given in Table S1. See text for a detailed description of our Debye velocity determination method. (c) The correlation of vD (solid circle) and Lamb–Mössbauer factor (fLM, open circle) of the δ-Fe13 samples; vD values account for the 57Fe enrichment of the samples.
A representative Debye sound velocity determination is shown in Fig. 2a. In this figure, two different models for phonon dispersion are plotted; one model is a ‘Debye-like’ model that plots as a constant value, and the other is an empirical power law model for phonon dispersion defined as Eq. 1:
where A1 and A2 are constants that are optimized by a standard least squares fitting process. In this power law model, vD is defined as v(0) in this profile. Note that ‘Debye-like’ dispersion is accommodated in Eq. (1).
However, low-count rate experiments such as these pose challenges to determining vD. For example, the values determined from these two dispersion models may be sensitive to the fitting energy range for pDOS and the phonon dispersion model, especially if there is scatter in the data. To place better constraints on vD without relying on the choice of an energy fit range, the analysis of a probability distribution function (PDF) of vD proposed by Morrison et al. (ref.33) was applied in this study and an example at 67 GPa is shown in Fig. 2. The PDF method accounts for hundreds of physically reasonable fit ranges and phonon dispersion behavior in the determination of vD (Eq. (1)), and therefore places a more reasonable constraint on vD and its uncertainty than would be calculated only from a single energy range33. The details of the PDFs, including the energy ranges considered and bin size are provided in Table S1. The reported vD values and their uncertainty are the peak positions and full width at half maximum of fitting an asymmetric function to the PDFs, respectively (Figs. 2b, S2; Tables S1 and S2). The 57Fe concentration of the sample was used in the determination of vD.
The elastic wave velocities, vP, vS, and vΦ, were calculated from vD using Eqs. (3) and (4) (see Methods). The isothermal bulk modulus (KT) and density (ρ) were calculated from the equation of state (EoS) parameters of δ-(Al0.87757Fe0.123)OOH (hereafter δ-Fe12, ref.22) using the MINUTI software version 2.1.029. The 2nd-order Birch-Murnaghan EoS parameters of δ-Fe12 with asymmetric (ordered) hydrogen bonds and high-spin state (KT0 = 147 ± 1 GPa, KT′ = 4, V0 = 57.85 ± 0.02 Å3)22 were applied to the calculation of vD at ambient conditions, and the spin crossover EoS parameters for δ-Fe12 with symmetric (disordered) hydrogen bonds (high-spin state, KT0 = 155 ± 22 GPa, KT′ = 8 ± 2, V0 = 57.5 ± 0.3 Å3; low-spin state, KT0 = 241 ± 14 GPa, KT′ = 4, V0 = 55.2 ± 0.4 Å3)22 were used to calculate the vD at pressures higher than 20 GPa. We first determined the Debye velocities and density with the experimental mass (i.e., 96.64% 57Fe-enriched molecular mass of δ-Fe13), and then converted these to values corresponding to natural isotopic enrichment by using Eqs. (8) and (9), which are used in the geophysical discussion. The KT obtained from the EoSs was converted to the adiabatic bulk modulus (KS) by using the Grüneisen parameter (γ0 = 0.64 ± 0.05), the exponent of its volume dependence (q = 1.8 ± 0.3) and Debye temperature (Θ0 = 1485 ± 300 K) of δ-AlOOH reported by ref.11.
Behavior of elastic wave velocities under compression
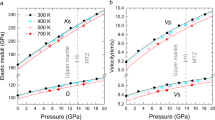
The vP, vS, and vΦ values of the two δ-Fe13 samples are summarized in Fig. 3 and Table S2. We make the following observations and compare with previous results. The concentration of iron in our sample is three times higher than the δ-(Al0.956Fe0.044)OOH polycrystalline sample (hereafter, δ-Fe4) measured using Brillouin light spectroscopy, thus when compared with the Al end-member values and the δ-Fe4 sample, our ambient pressure vP and vS values are consistent with this trend. The vS of δ-Fe13 increases up to the pressure conditions of the Fe3+ spin transition22, although the slope is shallower than that reported for δ-Fe423 and δ-AlOOH20 (Fig. 3). The lower vS values we report are reasonably explained by the difference in Fe concentration, as noted above. However, other factors such as preferred orientation could offer explanations. The NRIXS measurements used crushed single grains of a more iron-rich composition and the Brillouin scattering measurements used a potentially finer-grained polycrystalline iron-poor sample23. These differences might enhance the effect of orientation and/or intergranular stresses on measured data in the vicinity of phase transitions. Interestingly and unlike other materials undergoing a spin crossover, the polycrystalline Brillouin scattering results do not show any softening of vP near this transition23. At 32 and 37.1 GPa, both vP and vΦ decrease due to the reduction of K influenced by Fe3+ spin transition22 (Fig. 3a,c and Table S2). A decrease in K as a result of the spin transition is also observed in other low-Fe content minerals (e.g., ferropericlase34,35). The degree of reduction of vP and vΦ of δ-Fe13 due to the spin transition is significant. Specifically, the vP and vΦ values at 37.1 GPa are decreased by 13% and 23% compared to those at 27 GPa. After the completion of the Fe3+ spin transition (45 GPa and higher pressures), both vP and vΦ increase steadily with compression (Fig. 3a,c).
The elastic wave velocities of the δ-Fe13 samples computed using the natural abundance of Fe: (a), vP, (b), vS, (c) vΦ. Elastic wave velocities of other end-membered hydrous phases: δ-AlOOH20,21,26, δ-(Al0.956Fe0.044)OOH (δ-Fe4)23, and ε-FeOOH with low spin state of Fe3+26 are also shown. PREM is shown by gray lines36. The values indicated by solid and open symbols were obtained from room temperature experiments and calculations at 0 K, respectively. Abrupt reductions of vP and vΦ for the δ-Fe13 samples at 32 GPa and 37.1 GPa are due to the spin transition of Fe3+22. The numerical values for the data of δ-Fe13 samples in this figure are available in Table S2.
Above 45 GPa, the pressure–velocity profiles of δ-Fe13 do not show any abrupt changes (Fig. 3). Pressure dependencies of velocities, however, are significantly different among vP, vS, and vΦ. The vP and vΦ exhibit relatively large gradients with pressure, similar to the Preliminary Reference Earth Model (PREM)36. In contrast, the vS values are essentially invariant with respect to pressure, such that the values of vS determined in this study above 45 GPa are equal within the associated errors (6.38–6.55 ± 0.10–0.18 km/s). This leads to a weak pressure dependence of the shear modulus (μ). The μ increases from 190 to 206 GPa between 45 and 79 GPa, which is within the range of errors: ± 0.06 to ± 0.11 (Table S2).
Discussion
The spin transition of Fe3+ in the δ-Fe13 samples results in a high vS/vΦ (0.86 at 37.1 GPa) (Table S2), which is ~ 19% higher than PREM at this corresponding depth (~ 970 km). The spin transition of Fe3+ in the octahedral site is also observed in the Fe-bearing NAL phase37. However, pressure condition and width of spin crossover involving volume collapse are slightly lower and narrower in the δ-Fe5 and δ-Fe12 (32–40 GPa)22 than the (Na0.71Mg2.05Fe2+0.09Al4.62Fe3+0.17Si1.16O12) NAL phase (33–47 GPa)37. A computational study by ref.38 shows that the spin transition pressure of Fe3+ in the octahedral site of the NAL phase is ~ 40 GPa, and it remains mostly invariant to temperature and the width moderately increases with temperature. At 300 K, the isothermal bulk modulus of the Fe3+-bearing NAL phase (NaMg2(Al4.67SiFe0.33)O12) in the pressure range of spin crossover is up to ~ 25% lower than the bulk modulus-pressure trend expected without a spin transition38. The reduction of bulk modulus is limited to ~ 8% at 1200 K38, the estimated temperature of a slab penetrated into the top of the lower mantle39,40, resulting in the ~ 4% reduction of vΦ. At 300 K, the isothermal bulk modulus of δ-Fe13 throughout the spin crossover is up to ~ 55% lower than the bulk modulus-pressure trend expected without a spin transition, resulting in the ~ 33% reduction of vΦ. This would imply that for δ-Fe13 at about 1200 K the pressure of the spin-crossover is likely to be unchanged from that measured at 300 K and the resultant softening of the bulk modulus remains appreciable compared to the Fe3+-bearing NAL phase37. This behavior is different than the Fe2+ spin crossover in ferropericlase, in that although ferropericlase exhibits elastic softening, the transition occurs at deeper depths34,35 and exhibits a stronger temperature dependence41 than that for the Fe3+-bearing phases discussed above. It is also different from that of Fe3+-bearing bridgmanite42 and stishovite43.
Seismic observations in this region just below the transition zone (< 1000 km in depth) suggest a range of anomalies44, inferred to be correlated with the presence or stagnation of slab debris45. Therefore, the high vS/vΦ in the spin crossover region of δ-Fe13 (and NAL) could be related to these seismic anomalies, especially in regions of relatively lower temperatures, such as those calculated for subducted slabs. Further studies on the temperature dependence of these properties across the spin transition would help quantify this effect.
Above 45 GPa, the gradients of vP and vΦ (dvP/dP and dvΦ/dP) of δ-Fe13 are close to those of PREM (Fig. 3a,c). By contrast, that of vS (dvS/dP) is practically constant above 45 GPa (Fig. 3b). To discuss velocity relations between δ-Fe13 and PREM at deep-lower mantle pressure conditions, we calculated the elastic wave velocities for pressures higher than 80 GPa by using the following extrapolation procedure. First, we determined the elastic wave velocities at room temperature (Table S2). The vP values were extrapolated using a linear relationship (Birch’s law46) between the ρ calculated from the thermal EoS (see ‘Determination of elastic wave velocities’ section) and the measured vP of low-spin δ-Fe13 at 45 GPa, 59 GPa, 67 GPa, and 79 GPa (Table S2). The vΦ was calculated from those thermal EoS. The vS was then calculated from the extrapolated vP and the calculated vΦ by using the Eqs. (5)–(7).
The vS of δ-Fe13 at room temperature is 5% slower than PREM at 1871 km in depth, and then becomes 9% slower than PREM at 2771 km in depth, 120 km above the core–mantle boundary. The difference of vS between δ-Fe13 and PREM becomes larger with depth because of the very week pressure dependence of shear modulus of δ-Fe13 (Fig. 4). On the other hand, vP and vΦ at room temperature are 1% and 6% faster than PREM at 1871–2771 km in depth, respectively (Fig. 4, Table S3). The difference of vS and vΦ between δ-Fe13 and PREM suggests that an incorporation of 17 vol.% δ-Fe13 into PREM (representing average mantle) can account for − 0.9 to − 1.5% anomaly for vS and + 1% anomaly for vΦ in this depth range if the temperature dependences of velocities of δ-Fe13 are not considered.
The depth–velocity profiles of δ-Fe13 and PREM36: (a) vS, (b) vΦ, and (c) (vS − vΦ)/vΦ. The plots of δ-Fe13 at room T (solid circle) and mantle T (open circle) indicate the velocities of δ-Fe13 at room temperature and along an average mantle geotherm48, respectively. The calculation procedure of vS and vΦ of δ-Fe13 under these conditions is explained in the main text. The velocities of δ-Fe13 shown in this figure are determined using the density of δ-Fe13-r2 with natural mass of Fe. The dotted gray lines indicating − 2% of vS and + 1% of vΦ relative to PREM (solid gray lines) are regarded as reasonable perturbations for each respective velocity within the LLSVPs, based on the SB10L18 model27. The degree of anti-correlation between vS and vΦ in δ-Fe13 is considered to be larger than the maximum anti-correlation observed in the LLSVPs. Estimated uncertainties for δ-Fe13 are shown by the vertical bars. The numerical values for the data shown in this figure are available in Table S3.
Such an anti-correlation between the vS and vΦ has been reported for the large low-shear-velocity provinces (LLSVPs) in the depth range of 2000 km down to the core-mantle boundary, beneath the Pacific Ocean and the region spanning underneath the Atlantic Ocean to the western and southern part of the African continent27,28. It has been suggested that the LLSVPs are surrounded by downwellings, driven by slab subduction47. Therefore, if slabs contain a hydrous δ-phase and these slabs are transported into the deep-lower mantle, then slab debris containing the δ-phase could accumulate in the LLSVPs over geologic time, although it is not clear what the exact thermal state of this assemblage would be. To compare the elastic wave velocities of δ-Fe13 and PREM along an average mantle geotherm48, we apply the temperature dependence of vS, which was reported for MgSiO3-bridgmanite at 100 GPa (i.e., dvS/dT = − 1 × 10–4 (km/s)/K) at 100 GPa49. Another study demonstrated that the dvS/dT of newly discovered FeO2Hx P-phase is also approximately − 1 × 10–4 (km/s)/K, at 133 GPa50. The vΦ along the mantle geotherm was calculated from the thermal EoS parameters used in the calculation of vΦ at room temperature, and vP was subsequently calculated from the extrapolated vS and vΦ along an average mantle geotherm by using the Eqs. (5)–(7). In this case, the vP and vS of δ-Fe13 along the mantle geotherm48 are 1% and 8 to 11% slower than PREM in the depth range of 1871–2771 km, respectively, whereas vΦ of δ-Fe13 is 4 to 5% faster than PREM (Fig. 4, Table S3). The anti-correlation between vS and vΦ results in ~ 30% lower (vS − vΦ)/vΦ ratio for δ-Fe13, compared with the PREM model (Fig. 4).
In this discussion, we used γ0 = 0.64 (with q = 1.8 and Θ0 = 1485 K reported for δ-AlOOH11). However, the effect of spin transition on the Grüneisen parameter of δ-Fe13 has not been investigated. To address the uncertainty of the Grüneisen parameter in our extrapolations, we tested a value of 1.28 as γ0 which is twice as large as the γ0 used in the present discussion (0.64). The doubled value of the Grüneisen parameter decreases the vΦ, the most sensitive parameter of all three velocities (vP, vS, and vΦ) to changes in the Grüneisen parameter, by \(\le\) 0.12 km/s (1.2% reduction) at the pressure and temperature conditions of 1871–2771 km depth, which are smaller than the errors of the extrapolated velocities (Fig. 4 and Table S3). Such a significant change in the Grüneisen parameter is unlikely for δ-Fe13. For example, a recent calculation for (Mg0.75Fe0.25)O ferropericlase showed that at 120 GPa the Grüneisen parameters of high- and low-spin states were 1.94 and 2.05 at 1400 K, respectively51. As discussed above, δ-Fe13 is primarily in the low-spin state at 80 GPa under elevated temperatures. If the difference in the Grüneisen parameter between high-spin and low-spin δ-Fe13 is also ~ 0.1, the velocity reductions are smaller than the example we tested above. To place a better constraint on the Grüneisen parameter of Fe-bearing δ-phase through the spin transition, high-temperature experiments and computational studies are required.
When assuming an incorporation of 7 vol.% of δ-Fe13 into the average mantle (PREM) at 1871–2771 km depth, − 0.6 to − 0.8 ± 0.2% anomaly of vS and + 0.3 ± 0.2% anomaly of vΦ would be generated, which are likely regarded as typical anomalies where the edge regions of LLSVPs have been sufficiently sampled. Although this discussion involves simplifications for the petrology and significant extrapolations, as well as noting that the magnitudes of anomalies in the seismic wave velocity and density depend on the seismic models52,53, our new data suggests that the presence of δ-(Al,Fe)OOH can produce seismic heterogeneities in the deep lower mantle. The total mass of LLSVPs is estimated to be 1.91 wt.% of the entire mantle54. On the other hand, the amount of recycled oceanic crust that entered and stayed in the lower mantle since 4 Gyr is estimated as ~ 3–5 wt.% of the present mantle55,56, which is at least 1.5 times larger than the mass of LLSVPs. It has been suggested that about one ocean mass of water (0.037 wt.% of the mantle) has been transported via slabs to the deep mantle over the age of Earth57. If this amount of H2O is completely stored in LLSVPs, a hypothetical and unlikely end-member scenario, via a dense oxyhydroxide like the delta phase, the H2O content of LLSVPs is calculated to be 1.9 wt.%. This scenario suggests that a hydrous mafic system, such as the system examined in ref.17 where the Al-rich Phase H (i.e., the Mg, Si, Fe-bearing δ-phase) could accumulate in LLSVPs. If this 1.9 wt.% H2O in the LLSVPs is completely stored in δ-Fe13, then this hydrous phase accounts for 12 vol.% of the LLSVP assemblage. This amount is larger than the 7 vol.% concentration of δ-Fe13 that can reproduce the typical anomalies at the edge regions of LLSVPs, as discussed above. The incorporation of 12 vol.% δ-Fe13 into “PREM” at 1871–2771 km depth can cause − 1.0 to − 1.4 ± 0.3% anomaly for vS and + 0.5 to + 0.6 ± 0.3% anomaly for vΦ, which are comparable to the anomalies reported in the central regions of LLSVPs27. If the temperature is higher than the phase stability, then the δ-phase will dissociate. If these conditions are met, then it is possible that released hydroxyl migrates upward and hydrates regions at lower temperatures58, although much work is still needed to understand the phase relations of such processes.
Our elastic wave velocity results shed a new light on lower mantle seismic observations. At shallow lower mantle depths (800–900 km), δ-(Al,Fe)OOH exhibits low-vΦ and vP due to the spin transition, which may explain some seismic anomalies in this depth range. At deeper depths, the elastic behavior of δ-(Al,Fe)OOH is unlike typical lower mantle phases, and we discuss its relevance to LLSVP observations. Compositional differences between the LLSVPs and the surrounding mantle have been proposed as an origin of LLSVPs’ seismic structure47, 59,60,61. Our study proposes that if δ-(Al,Fe)OOH is formed by the reaction between oceanic crust and water17,18, and accumulates in deep local regions such as the edge regions of LLSVPs, those regions can reproduce the seismically observed negative anomaly of vS and the positive anomaly of vΦ.
Methods
Single crystals of Fe-bearing δ-phase were synthesized by the high-pressure hydrothermal method using a 1000-ton multi anvil apparatus (Hymag installed at Bayerisches Geoinstitut, University of Bayreuth), as previously reported by ref.62. The starting material of synthesis was a mixture of reagent-grade Al(OH)3 (Rare Metallic Co., Ltd.) and Fe2O3 (96.64% 57Fe, ISOFLEX) powders with Fe/(Al + Fe) = 0.15 in molar. This powder mixture packed into a welded Au80Pd20 capsule was hold at 21 GPa and 1470 K for 4 h, followed by rapid quenching. From the aggregates of synthesized crystals, two grains of δ-(Al0.873(3)57Fe0.127(3))OOH with dimensions of ~ 50 × 60 × 10 µm (Run 1, δ-Fe13-r1) and δ-(Al0.867(3)57Fe0.133(3))OOH with dimensions of ~ 40 × 50 × 20 µm (Run 2, δ-Fe13-r2) were selected for this study. The chemical compositions of the two samples were determined using an electron microprobe operating in wavelength-dispersive mode (JEOL, JXA-8800 installed at Tohoku University) operated at 15 kV and 10 nA. The numbers in parentheses in the chemical compositions indicate one standard deviation of ten measurement data for each sample. The H2O contents were calculated from oxide deficit in total mass in the microprobe analyses, as reported in ref.62. The weight deficit analyses indicated that δ-Fe13-r1 and δ-Fe13-r2 contain 2.3 wt.% and 2.9 wt.% excess H2O, respectively, which are included in calculation of the density of the two samples.
High-pressure NRIXS experiments were performed using panoramic diamond anvil cells (pDACs). Beryllium disks are used as gaskets. The disks were pre-indented to thicknesses of 40–50 μm, and then 210 μm or 165 μm (in diameter) holes were drilled in the center of gaskets. The former and latter gaskets were used for the pDACs with 400 μm-culet anvils (Run 1, δ-Fe13-r1) and 300 μm-culet anvils (Run 2, δ-Fe13-r2), respectively. A mixture of 10–20 μm thick boron epoxy (amorphous boron powder:epoxy = 4:1 by weight) is attached to the gasket holes, which can stabilize the sample room at high pressures. We put the sample in the center of gasket hole and two ruby spheres beside the sample as pressure markers, and then loaded a compressed neon gas as a pressure-transmitting medium into the sample chamber of pDACs. The gas-loading was conducted at the Seismological Laboratory, California Institute of Technology. The cell design for the pDAC using 300 μm-culet anvils is same as that used in the previous synchrotron Mössbauer spectroscopy experiments by Ref.22.
We conducted NRIXS measurements at room temperature and high pressures up to 79 GPa at Sector 3-ID-B at the Advanced Photon Source. During the measurements, the storage ring was operated in 24-bunch top-up mode. Each bunch was separated by 153 ns. Incoherent inelastic X-ray scattering was detected with three avalanche photodiode detectors (APDs) positioned radially around the pDACs. In addition to the three APDs, another APD located at a downstream parallel to X-ray path was used for collecting forward elastic scattering, which helps constrain the resolution function. The energy of incident X-ray was tuned around 14.4125 keV, the energy of nuclear resonance of 57Fe, by using a high-resolution monochrometer63 with a typical energy resolution (full width at half maximum at zero energy transfer) measured to be about 1.1 meV. The details of the setup of NRIXS experiments at Sector 3-ID-B are presented elsewhere33,63,64. Here, we note a brief summary of conditions of the NRIXS experiments. Energy scans were performed over the ranges of − 100 to + 150 meV (ambient conditions in air), − 80 to + 110 meV (at 5.6 and 10.8 GPa), − 80 to + 120 meV (18.1, 21.1, 27, 32 and 37.1 GPa) and − 80 to + 150 meV (45, 59, 67 and 79 GPa), with an energy step-size of 0.25 meV. The pressure at each compression point was determined using the ruby fluorescence method65. The ruby fluorescence spectra were obtained before and after collecting NRIXS spectra at each pressure point using an off-line Raman spectrometer at the Sector 3-ID-B. Pressure errors arise from the standard deviation between the four ruby measurements obtained before and after NRIXS measurements for two ruby spheres, and the error of ruby scale66. The raw NRIXS data were analyzed by using the version 3.0.0 PHOENIX software package (www.NRIXS.com) to obtain the partial phonon density of states (pDOS), vD, and the Lamb-Mössbauer factor33,67,68. From the low-energy region of pDOS, vD is calculated from the following equation:
where ρ is the density of sample, m is the mass of the nuclear resonant isotope, and D(E) indicates the pDOS in the low energy region. v(E) is equal to vD in the limit when energy (E) approaches zero. The pDOS shown in Fig. 2a are scaled by this Eq. (2). For a single fit range, a vD value is determined from the fitting using an empirical power law model (Eq. (1)) of the phonon dispersion curve, noting that ‘Debye-like’ dispersion is accommodated in this form. To determine a PDF for vD, binning of hundreds of possible fit ranges was used (Table S1). The reported vD and its uncertainty is defined as the peak position and FWHM of the fitting by asymmetric function in the probability distributions, respectively (Table S1). The details of this PDF method used in determination of vD was discussed in ref.68. The elastic wave velocities (vP, vS, and vΦ) are then calculated using the K and ρ determined with XRD measurements22 and the vD value, via the following equations.
Here, vP, vS, and vΦ are defined as in the following equations:
The isothermal bulk modulus (KT) obtained from the EoSs was converted to the adiabatic bulk modulus (KS) by using the Grüneisen parameter (γ0 = 0.64 ± 0.05), the exponent of its volume-independent (q = 1.8 ± 0.3) and Debye temperature (Θ0 = 1485 ± 300 K) of δ-AlOOH reported by ref.11.
To calculate the velocities and density of the δ-Fe13 samples having natural isotopic enrichment of iron, we applied the following equations to the velocities and density determined with the 57Fe-enriched experimental mass:
where Mnat amd Menr are the molecular mass of the samples having natural isotopic enrichment and 57Fe-enrichment, respectively.
Data availability
The data that support the findings of this study are available from the corresponding author(s) upon reasonable request.
References
Karato, S.-I. Water distribution across the mantle transition zone and its implications for global material circulation. Earth Planet. Sci. Lett. 301, 413–423 (2011).
Sandu, C., Lenardic, A. & McGovern, P. The effects of deep water cycling on planetary thermal evolution. J. Geophys. Res. 116, B12404 (2011).
Nakao, A., Iwamori, H. & Nakakuki, T. Effects of water transportation on subduction dynamics: Roles of viscosity and density reduction. Earth Planet. Sci. Lett. 454, 178–191 (2016).
Peslier, A. H., Schönbächler, M., Busemann, H. & Karato, S.-I. Water in the Earth’s interior: Distribution and origin. Space Sci. Rev. 212, 743–810 (2017).
Suzuki, A., Ohtani, E. & Kamada, T. A new hydrous phase δ-AlOOH synthesized at 21 GPa and 1000 °C. Phys. Chem. Miner. 27, 689–693 (2000).
Sano, A., Ohtani, E., Kubo, T. & Funakoshi, K.-I. In situ X-ray observation of decomposition of hydrous aluminum silicate AlSiO3OH and aluminum oxide hydroxide d-AlOOH at high pressure and temperature. J. Phys. Chem. Solids 65, 1547–1554 (2004).
Sano, A. et al. Aluminous hydrous mineral δ-AlOOH as a carrier of hydrogen into the core-mantle boundary. Geophys. Res. Lett. 35, L03303 (2008).
Pamato, M. G. et al. Lower-mantle water reservoir implied by the extreme stability of a hydrous aluminosilicate. Nat. Geosci. 8, 75–79 (2015).
Fukuyama, K., Ohtani, E., Shibazaki, Y., Kagi, H. & Suzuki, A. Stability field of phase Egg, AlSiO3OH at high pressure and high temperature: Possible water reservoir in mantle transition zone. J. Miner. Petrol. Sci. 112, 31–35 (2017).
Abe, R. et al. In situ X-ray diffraction studies of hydrous aluminosilicate at high pressure and temperature. J. Miner. Petrol. Sci. 113, 106–111 (2018).
Duan, Y. et al. Phase stability and thermal equation of state of δ-AlOOH: Implication for water transportation to the deep lower mantle. Earth Planet. Sci. Lett. 494, 92–98 (2018).
Piet, et al. Dehydration of δ-AlOOH in Earth’s deep lower mantle. Minerals 10, 384 (2020).
Nishi, M. et al. Stability of hydrous silicate at high pressures and water transport to the deep lower mantle. Nat. Geosci. 7, 224–227 (2014).
Ohira, I. et al. Stability of a hydrous δ-phase, AlOOH-MgSiO2(OH)2, and a mechanism for water transport into the base of lower mantle. Earth Planet. Sci. Lett. 401, 12–17 (2014).
Walter, M. J. et al. The stability of hydrous silicates in Earth’s lower mantle: Experimental constraints from the systems MgO–SiO2–H2O and MgO–Al2O3–SiO2–H2O. Chem. Geol. 418, 16–29 (2015).
Nishi, M., Irifune, T., Gréaux, S., Tange, Y. & Higo, Y. Phase transitions of serpentine in the lower mantle. Phys. Earth Planet. Inter. 245(106268), 52–58 (2015).
Liu, X., Matsukage, K. N., Nishihara, Y., Suzuki, T. & Takahashi, E. Stability of the hydrous phases of Al-rich phase D and Al-rich phase H in deep subducted oceanic crust. Am. Mineral. 104, 64–72 (2019).
Yuan, H. et al. Stability of Fe-bearing hydrous phases and element partitioning in the system MgO–Al2O3–Fe2O3–SiO2–H2O in Earth’s lowermost mantle. Earth Planet. Sci. Lett. 524, 115714 (2019).
Nishi, M. et al. Solid solution and compression behavior of hydroxides in the lower mantle. J. Geophys. Res. Solid Earth 124, 10231–10239 (2019).
Mashino, I., Murakami, M. & Ohtani, E. Sound velocities of δ-AlOOH up to core-mantle boundary pressures with implications for the seismic anomalies in the deep mantle. J. Geophys. Res. 121, 595–609 (2016).
Tsuchiya, J. & Tsuchiya, T. Elastic properties of δ-AlOOH under pressure: First principles investigation. Phys. Earth Planet. Inter. 174, 122–127 (2009).
Ohira, I. et al. Compressional behavior and spin state of δ-(Al, Fe)OOH at high pressures. Am. Miner. 104, 1273–1284 (2019).
Su, X. et al. The effect of iron on the sound velocities of δ-AlOOH up to 135 GPa. Geosci. Front. 12, 937–946 (2021).
Tsuchiya, J., Tsuchiya, T., Tsuneyuki, S. & Yamanaka, T. First principles calculation of a high-pressure hydrous phase, δ-AlOOH. Geophys. Res. Lett. 29, 1909 (2002).
Sano-Furukawa, A. et al. Direct observation of symmetrization of hydrogen bond in δ-AlOOH under mantle conditions using neutron diffraction. Sci. Rep. 8, 15520 (2018).
Thompson, E. C., Campbell, A. J. & Tsuchiya, J. Elasticity of ε-FeOOH: Seismic implications for Earth’s lower mantle. J. Geophys. Res. 122, 5038–5047 (2017).
Masters, G., Laske, G., Bolton, H., & Dziewonski, A. The relative behavior of shear velocity, bulk sound speed, and compressional velocity in the mantle: implications for chemical and thermal structure. in S. I. Karato, A. Forte, R. Liebermann, G. Masters, & L. Stixrude (Eds.), Earth's Deep Interior: Mineral Physics and Tomography From the Atomic to the Global Scale. American geophysical Union Monograph. 117, 63–87 (2000).
Trampert, J., Deschamps, F., Resovsky, J. & Yuen, D. Probabilistic tomography maps chemical heterogeneities throughout the lower mantle. Science 306, 853–856 (2004).
Sturhahn, W. PHOENIX (PHOnon Excitation by Nuclear Inelastic X-ray scattering) and MINUTI (MINeral physics UTIlities) open source software. Online report, https://www.nrixs.com (2020).
Zhang, D. et al. Elasticity and lattice dynamics of enstatite at high pressure. J. Geophys. Res. 118, 4071–4082 (2013).
Wicks, J. K., Jackson, J. M., Sturhahn, W. & Zhang, D. Sound velocity and density of magnesiowüstites: Implications for ultralow-velocity zone topography. Geophys. Res. Lett. 44, 2148–2158 (2017).
Tsuchiya, J., Tsuchiya, T. & Wentzcovitch, R. M. Vibrational properties of δ-AlOOH under pressure. Am. Miner. 93, 477–482 (2008).
Morrison, R. A., Jackson, J. M., Sturhahn, W., Zhao, J. & Toellner, T. S. High pressure thermoelasticity and sound velocities of Fe-Ni-Si alloys. Phys. Earth Planet. Inter. 294, 106268 (2019).
Marquardt, H., Speziale, S., Reichmann, H. J., Frost, D. J. & Schilling, F. R. Single-crystal elasticity of (Mg0.9Fe0.1)O to 81 GPa. Earth Planet. Sci. Lett. 287, 345–352 (2009).
Yang, J., Tong, X., Lin, J.-F., Okuchi, T. & Tomioka, N. Elasticity of ferropericlase across the spin crossover in the Earth’s lower mantle. Sci. Rep. 5, 17188 (2015).
Dziewonski, A. M. & Anderson, D. L. Preliminary reference Earth model. Phys. Earth Planet. Inter. 25, 297–356 (1981).
Wu, Y. et al. Spin transition of ferric iron in the NAL phase: Implications for the seismic heterogeneities of subducted slabs in the lower mantle. Earth Planet. Sci. Lett. 434, 91–100 (2016).
Hsu, H. First-principles study of iron spin crossover in the new hexagonal aluminous phase. Phys. Rev. B 95, 020406(R) (2017).
Ricard, Y., Mattern, E., & Matas, J. Synthetic Tomographic Images of Slabs from Mineral Physics. in R. D. van der Hilst, J. D. Bass, J. Matas, & J. Trampert (Eds.) Earth's Deep Mantle: Structure, Composition, and Evolution. American geophysical Union Monograph 160, 283–300 (2005).
Kirby, S. H., Stein, S., Okal, E. A. & Rubie, D. C. Metastable mantle phase transformations and deep earthquakes in subducting oceanic lithosphere. Rev. Geophys. 34, 261–306 (1996).
Sturhahn, W., Jackson, J. M. & Lin, J.-F. The spin state of iron in minerals of Earth’s lower mantle. Geophys. Res. Lett. 32, L12307 (2005).
Fu, S. et al. Abnormal elasticity of Fe-bearing bridgmanite in the Earth’s lower mantle. Geophys. Res. Lett. 45, 4725–4732 (2018).
Buchen, J. et al. Equation of state of polycrystalline stishovite across the tetragonal-orthorhombic phase transition. J. Geophys. Res. Solid Earth 123, 7347–7360 (2018).
Deuss, A., Andrews, J., & Day, E. Seismic observations of mantle discontinuities and their mineralogical and dynamical interpretation. In Physics and Chemistry of the Deep Earth (Ed. Karato, S.-i.) pp. 297–323 (Wiley, 2013).
Fukao, Y. & Obayashi, M. Subducted slabs stagnant above, penetrating through, and trapped below the 660 km discontinuity. J. Geophys. Res. Solid Earth 118, 5920–5938 (2013).
Birch, F. Composition of the Earth’s mantle. Geophys. J. Int. 4, 295–311 (1961).
Garnero, E. J., McNamara, A. K. & Shim, S.-H. Continent-sized anomalous zones with low seismic velocity at the base of Earth’s mantle. Nat. Geosci. 9, 481–489 (2016).
Brown, J. M. & Shankland, T. J. Thermodynamic parameters in the Earth as determined from seismic profiles. Geophys. J. R. astr. Soc. 66, 579–596 (1981).
Wentzcovitch, R. M., Wu, Z. & Carrier, P. First principles quasiharmonic thermoelasticity of mantle minerals. Rev. Miner. Geochem. 71, 99–128 (2010).
Liu, J. et al. Hydrogen-bearing iron peroxide and the origin of ultralow-velocity zones. Nature 551, 494–497 (2017).
Song, Y. et al. Effects of iron spin transition on the electronic structure, thermal expansivity and lattice thermal conductivity of ferropericlase: A first principles study. Sci. Rep. 9, 4172 (2019).
Koelemeijer, P., Deuss, A. & Ritsema, J. Density structure of Earth’s lowermost mantle from Stoneley mode splitting observations. Nat. Commun. 8, 15241 (2017).
Lau, H. C. P. et al. Tidal tomography constrains Earth’s deep-mantle buoyancy. Nature 551, 321–326 (2017).
Burke, K., Steinberger, B., Torsvik, T. H. & Smethurst, M. A. Plume generation zones at the margins of large low shear velocity provinces on the core–mantle boundary. Earth Planet. Sci. Lett. 265, 49–60 (2008).
Niu, Y. Origin of the LLSVPs at the base of the mantle is a consequence of plate tectonics: A petrological and geochemical perspective. Geosci. Front. 9, 1265–1278 (2018).
Helffrich, G. R. & Wood, B. J. The Earth’s mantle. Nature 412, 501–507 (2001).
van Keken, P. E., Hacker, B. R., Syracuse, E. M. & Abers, G. A. Subduction factory: 4. Depth-dependent flux of H2O from subducting slabs worldwide. J. Geophys. Res. 116, B01401 (2011).
Ohtani, E. The role of water in Earth’s mantle. Natl. Sci. Rev. 7, 224–232 (2020).
Tan, E. & Gurnis, M. Metastable superplumes and mantle compressibility. Geophys. Res. Lett. 32, L20307 (2005).
Wolf, A. S., Jackson, J. M., Dera, P. & Prakapenka, V. B. The thermal equation of state of (Mg, Fe)SiO3 bridgmanite (perovskite) and implications for lower mantle structures. J. Geophys. Res. Solid Earth 120, 7460–7489 (2015).
Thomson, A. R. et al. Seismic velocities of CaSiO3 perovskite can explain LLSVPs in Earth’s lower mantle. Nature 572, 643–647 (2019).
Kawazoe, T. et al. Single crystal synthesis of δ-(Al, Fe)OOH. Am. Miner. 102, 1953–1956 (2017).
Toellner, T. S. Monochromatization of synchrotron radiation for nuclear resonant scattering experiments. Hyperfine Interact. 125, 3–28 (2000).
Sturhahn, W., & Jackson, J. M. Geophysical applications of nuclear resonant spectroscopy. in E. Ohtani (Ed.) Advances in High-Pressure Mineralogy, Geological Society of America Special Paper, 421, 157–174 (2007).
Dewaele, A., Torrent, M., Loubeyre, P. & Mezouar, M. Compression curves of transition metals in the Mbar range: Experiments and projector augmented-wave calculations. Phys. Rev. B 78, 104102 (2008).
Chijioke, A. D., Nellis, W. J., Soldatov, A. & Silvera, I. F. The ruby pressure standard to 150 GPa. J. Appl. Phys. 98, 114905 (2005).
Sturhahn, W. CONUSS and PHOENIX: Evaluation of nuclear resonant scattering data. Hyperfine Interact. 125, 149–172 (2000).
Sturhahn, W. Nuclear resonant spectroscopy. J. Phys. Condens. Matter 16, S497–S530 (2004).
Acknowledgements
We warmly thank Y. Ito for his help with polishing and performing the EPMA-analysis of the crystals used in this work, and Natalia V. Solomatova, Rachel A. Morrison, and Vasilije Dobrosavljevic for help during the experiments. This work was supported by JSPS KAKENHI Grant Numbers JP16J04690, JP20K22369 to I.O., JP15H05828, JP19H01985, JP19K21890 to A.S., JP15H05748, JP20H00187 to E.O., the International Research and Training Group “Deep Earth Volatile Cycles” funded by the German Science Foundation (Grant Number: GRK 2156/1), the JSPS Japanese-German Graduate Externship, the International Joint Graduate Program in Earth and Environmental Science, Tohoku University (GP-EES), and by Grants awarded to J.M.J by the National Science Foundation (NSF-CSEDI-EAR-1600956 and 2009935) and the W.M. Keck Institute for Space Studies. The NRIXS experiments were conducted at 3-ID-B, Advanced Photon Source, the United States, which is partially supported by COMPRES. This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
Author information
Authors and Affiliations
Contributions
I.O., E.O. and J.M.J. designed and planned the project of this research. T.K. and I.O. synthesized the experimental samples. I.O., J.M.J, W.S., G.J.F., and T.S.T. conducted the NRIXS experiments at 3-ID-B, APS. I.O. and J.M.J analyzed the NRIXS spectra using the software developed and maintained by W.S. I.O., J.M.J., A.S., and E.O. discussed the interpretation of the experimental results. All authors contributed to the preparation the manuscript and have approved the submitted version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ohira, I., Jackson, J.M., Sturhahn, W. et al. The influence of δ-(Al,Fe)OOH on seismic heterogeneities in Earth’s lower mantle. Sci Rep 11, 12036 (2021). https://doi.org/10.1038/s41598-021-91180-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-91180-9
This article is cited by
-
Lattice dynamics, sound velocities, and atomic environments of szomolnokite at high pressure
Physics and Chemistry of Minerals (2023)
-
The role of hydrogen bonds in hydrous minerals stable at lower mantle pressure conditions
Progress in Earth and Planetary Science (2022)
-
Constraining composition and temperature variations in the mantle transition zone
Nature Communications (2022)
-
Aluminous hydrous magnesium silicate as a lower-mantle hydrogen reservoir: a role as an agent for material transport
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.