Abstract
Glomerular hyperfiltration alone or associated with albuminuria is a well-known feature of sickle cell associated nephropathy. Though, glomerular hyperfiltration is currently considered to be related to a high renal plasma flow and chronic hemolysis, cardiac output influence on measured glomerular filtration rate (mGFR) have not been investigated so far. Thirty seven homozygous sickle cell patients (SCA) from the RAND study investigated before and under angiotensin converting enzyme inhibitor (ACEI) were included. Both mGFR and cardiac index (CI) were high (> 110 ml/min/1.73 m2 and > 3.5 l/m2 in 81% and 97% of cases) with low systemic vascular resistance (SVR) (< 700 dynes/s/cm−5) in 38% of cases. mGFR association with CI and SVR were significant at baseline (respectively ρ: 0.44, p = 0.008 and ρ: − 0.37, p = 0.02) and under ACEI (p = 0.007 and 0.01 respectively), in accordance with previous data showing that hyperfiltration was linked to an increased glomerular perfusion and a glomerulomegaly rather than increased capillary hydrostatic pressure. Of notice, after adjustment on CI, mGFR remained associated with reticulocyte count and albuminuria under ACEI (p = 0.006 and 0.02 respectively). Our results suggest that hyperfiltration is tightly linked to an increased cardiac output which may account for an increased renal blood flow. Chronic hemolysis could be a relevant factor accounting for hyperfiltration potentially acting on glomerular enlargement which appears as a key factor. Our data suggest that cardiac output assessment is a relevant tool in the routine management and monitoring of SCA nephropathy.
Similar content being viewed by others
Introduction
Previous studies reported a very high prevalence of hyperfiltration in young sickle cell anemia (SCA) patients with up to 60–80% in children and 50–60% in young adults1,2,3. Sickle cell anemia nephropathy may also associate micro and macroalbuminuria and ultimately renal failure3,4,5,6,7,8,9, though the natural history of SCA nephropathy is poorly understood. Nevertheless, this early event, i.e. increased measured glomerular filtration rate (mGFR) was investigated in small but well designed studies3,10 suggesting that an unusual high renal plasma flow altogether with a low, but sometimes normal filtration fraction was at play10,11. Though cardiac output is increased in most SCA patients12 altogether with a low blood pressure13 suggesting low systemic vascular resistance (SVR), to our knowledge, cardiovascular parameters associated with mGFR have not been studied so far. We took advantage of our previous RAND trial14 showing a beneficial effect of a 6 months angiotensin converting enzyme inhibitor (ACEI) treatment on macroalbuminuria in adult SCA patients at the time of hyperfiltration to study mGFR correlates with hemodynamic and biological parameters before and under renin-angiotensin system blocker, i.e. presumably in the setting of a normal or low filtration fraction. Our results suggest that mGFR is tighly correlated to cardiac output suggesting an impairment of renal hemodynamic autoregulation.
Results
As shown Table 1, among 14 male and 23 female homozygous sickle cell patients, median age was 30.9 ± 7.4 years old with 5 (14%) overweight patients with a maximum value of 30.2 kg/m2. Thirty three percent of patients were under a long term hydroxycarbamide treatment and 17% with a history of blood transfusions. All patients were normotensive at baseline with a maximum value of 129 and 75 mmHg for systolic and diastolic blood pressure respectively. Pulse pressure (PP) and Pulse Wave Velocity (PWV) median values were 52.8 [49.0–59.0] mmHg and 8.0 [7.1–8.6]m/s respectively. Cardiac index (CI) was high, above 3.5 and 4.5 l/min/m2 in 97% and 51% of patients respectively, and SVR values below normal range (700 dynes/s/cm−5) in 14 cases (38%) with no patients with high SVR values (most below 1000 dynes/s/cm−5). Of notice, CI and SVR did not change under ACEI treatment (4.62 versus 4.66 ml/min/m2 (p = 0.36) and 759 versus 738 dynes/s/cm−5 (p = 0.28) respectively). TRV values were available in 26 patients and above 2.5 m/s in 46% of cases. Mean mGFR assessed by 51Cr EDTA renal clearance was 131 ml/min/1.73 m2 before initiation of ACEI (Table 2), with a mean serum creatinine of 46.6 µmol/l (Table 1). Thus hyperfiltration (assessed by mGFR > 110 ml/min/1.73 m2) was present in 81% of cases (n = 30) with no significant variation under ACEI treatment whereas DBP and MAP decreased significantly from 57.3 to 54.2 mmHg and 75.2 to 72.8 mmHg (p = 0.02 and p = 0.04 respectively). Before initiation of ACEI, microabuminuria and macroalbuminuria were detected in 48.6% and 43.2% of cases respectively with ACR < 5 mg/mmol only in two patients. Anemia was present in all patients with a median haemoglobin level of 8.3 [7.7–8.9]g/dl, with a high reticulocyte count, plasma LDH and bilirubin concentrations (Table 1).
As shown Table 3 and Fig. 1, mGFR was significantly associated with CI and SVR at baseline: high mGFR values were associated with high CI (rho = 0.44, p = 0.008) and low SVR (rho = − 0.37, p = 0.02). Of notice, under ACEI, mGFR association with CI and SVR was even stronger. Moreover, in this latter case, mGFR was also associated with some additional parameters such as TRV (rho = 0.40, p = 0.04) but also reticulocyte count (rho = 0.54, p = 0.002) and ACR (rho = 0.47, p = 0.005). However, as shown Table 4, ACR and reticulocyte count remained significant after adjustment either for CI or SVR.
Discussion
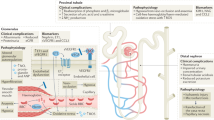
Our results show that in our SCA population with glomerular hyperfiltration, mGFR is strongly associated with cardiac output which is increased in most patients altogether with a low SVR in 38% of cases. Of notice, SVR was normal (but not high) in all other cases with no blood hypertension reported despite the presence of albuminuria, thus raising the issue of the pathophysiological process of glomerular hyperfiltration. It has been suggested that glomerular hyperfiltration is driven by increased glomerular perfusion and increased effective glomerular filtration surface area (glomerular enlargement) rather than by increased glomerular capillary hydrostatic pressure, which would separate this condition from early diabetic nephropathy15,16,17. Indeed, a high cardiac output and reduced peripheral vascular resistance was previously reported in six adult SCA patients with mean CI and SVR values in the same range than our population (5.0 l/min/m2 and 856 dynes/s/cm−5 respectively), however renal function was not reported18. Our findings that SVR are below 1000 dynes/s/cm−5 in all cases and low in 40% of patients suggest that renal vascular resistance may also be low. Indeed, an increased renal plasma flow was reported in several studies presumably related to an increased cardiac output though no data were available10,11,19. However, renal hemodynamic regulation is expected to increase afferent arteriolar tone in order to counterbalance an increased renal plasma flow20 and thus maintain a GFR within normal values. We may thus speculate that this regulation may be less efficient or possibly impaired in some individuals. Though we cannot fully address the issue of an impaired tubular glomerular feedback, we can rule out in most cases an increased glomerular capillary hydrostatic pressure responsible for hyperfiltration as (1) a low/normal filtration fraction was reported in previous studies10,11; (2) mGFR was similar before and under ACEI thus pointing out increased renal blood flow (rather than pressure) as a key determinant of hyperfiltration in our patients. We thus speculate that in most SCA patients with glomerular hyperfiltration, filtration occurs along the whole capillary surface due to increased renal plasma flow even in a normal hydrostatic pressure setting (i.e. no renal functional reserve). Thus according to this view, normalizing (under ACEI) hydrostatic pressure in a SCA patient would have no short term effect on GFR conversely to ACR which should decrease.
Alternatively, hemodynamic autoregulation may be preserved and increased effective glomerular filtration surface alone (in the setting of increased glomerular perfusion) may account for mGFR increase by 80% in some cases. Indeed, a glomerulomegaly responsible for an increased glomerular surface/volume and filtration coefficient (Kf) is reported to be a hallmark of SCA nephropathy10,11,21,22,23,24,25,26. This increased glomerular growth can be observed at a time of hyperfiltration as early as in infancy1,17. Altogether, glomerular hyperfiltration in SCA looks very similar to glomerular hyperfiltration in pregnancy where it is the consequence of increased cardiac output and decreased renal afferent and efferent arteriolar resistance responsible for an increased renal blood flow with glomerular enlargement and no increased filtration fraction16. However, the current view is that relaxin, a pregnancy hormone, which reduces myogenic reactivity of small renal arteries27 is responsible for glomerular hyperfiltration which is not the case in SCA patients, where the basis for renal hyperperfusion remains under debate. Indeed, such hyperperfusion cannot be readily ascribed to anaemia or increased plasma volume because correction of anaemia by HbA-containing RBC transfusions does not reverse hyperperfusion28 and chronic anaemia from causes other than SCA is not usually associated with renal hyperperfusion17,29. Among other causal factors, chronic hemolysis noteworthy through haem oxygenase–carbon monoxide (HO–CO) system is pointed out as induction of HO–CO might contribute to regional hyperperfusion of the kidney and other vascular beds17. Our previous findings showing that different markers of chronic hemolysis including reticulocyte count were independent risk factors for glomerular hyperfiltration in a SCA population2 are strengthened in this study showing that mGFR is associated with reticulocyte count even after adjustment for cardiac index or SVR in SCA patients under ACEI. Though there may be more than one mechanism by which greater rates of chronic hemolysis could contribute to hyperfiltration in the kidney, our data suggests that chronic hemolysis may be linked to glomerulomegaly dependent hyperfiltration process. According to this view, the reported beneficial effect of hydroxyurea treatment at decreasing hyperfiltration in children30 could be explained either by a sustained improvement of anemia decreasing cardiac output but also a beneficial effect on glomerular remodeling, i.e. glomerulomegaly. We speculate that a glomerular remodeling slowdown could then be an interesting therapeutical target.
In addition to hyperfiltration, glomerulomegaly accounts also for the reported increased glomerular permselectivity assessed by fractional clearances of dextran10, and the high prevalence of albuminuria in SCA patients2,3,4,5,6. Though an increased glomerular hydraulic pressure (ΔP) is likely to occur at least in macroalbuminuric patients, as a decreased albuminuria under ACEI treatment was detected in this subgroup14, our data showing that mGFR was associated with albuminuria only in patients under ACEI suggests that glomerular remodeling independently of ΔP may account for increased glomerular permeability to albuminuria, presumably through filtration slits stretching. According to this view, ACR values under ACEI would be an interesting bed-side relevant biomarker of glomerulomegaly, i.e. of renal structural remodelling in this population.
Limitation of the study: our data show that hyperfiltration is related to an increased cardiac output, thus leading presumably to a high renal plasma flow. The issue whether in our population, renal vascular resistance would be low as previously reported in SCA patients10 or normal11 is unfortunately unknown as renal plasma flow measurement was unavailable for this study. We speculate that alike systemic vascular resistance, low renal vascular resistance may occur in some patients accounting for unusual high mGFR values in the setting of a high cardiac output. Moreover, though we cannot exclude a further decrease renal plasma flow under ACEI, it seems unlikely as CI and SVR did not change. The complex pathological cascades of chronic hemolysis metabolites interplay with cardiovascular/ renal hemodynamics and the relevant determinants of glomerulomegaly responsible for hyperfiltration are obviously beyond the scope of the present study and require to be specifically addressed.
To conclude, our results suggest that hyperfiltration is tightly linked to an increased cardiac output and low systemic vascular resistance which may account for an increased blood flow both in kidneys and likely also in most other organs. These findings stress glomerular enlargement as a likely key step in the history of hyperfiltration and point out cardiac output assessment as a relevant tool in the routine management and monitoring of SCA nephropathy.
Material and methods
Ethics statement
All patients signed written informed consents before inclusion in the study. The Regression de l’Albuminurie dans la Nephropathie Drepanocytaire (RAND) study design was approved by the local ethics committee (Direction Générale pour la recherche et l'innovation. Comité Consultatif sur le traitement de l'information en matière de recherche dans le domaine de la santé, DGRI CCTIRS MG/CP09.503, 9 July 2009) and registered at ClinicalTrials.gov (NCT01195818). All methods were performed in accordance with the relevant guidelines and regulations.
Patients and study design
The RAND study is a prospective multicentric non-randomized trial that took place between September 2010 and September 2014 aimed at studying as a main objective the effect of ACEI treatment on albuminuria after a 6 month-trial (Supplementary information). Forty-two adult patients with sickle cell disease were enrolled and evaluated at month 6, as described in Fig. 1 and reported previously14. Thirty seven patients underwent a complete cardiac evaluation at baseline and under ACEI at 6 months and were thus included in the present study (Tricuspid regurgitation velocity (TRV) was available only in 26 patients). Eligible criteria were adult homozygous SS patients aged > 18 years, with a minimum urine albumin:creatinine ratio (ACR) > 10 mg/mmol creatinine on two different random urine spots performed at least 1 month after any acute SCA complication, and a recent estimated GFR (eGFR; calculated using the Modification of Diet in Renal Disease [MDRD] Study equation) > 130 ml/min/1.73 m2. Exclusion criteria were acute SCA complication within 1 month prior to study inclusion, patients receiving blood transfusions within a 3-month period or initiation of hydroxycarbamide within 3 months, ongoing pregnancy or treatment with nonsteroidal anti-inflammatory drugs, lithium or antihypertensive agents, human immunodeficiency virus or hepatitis C infection, known allergic reactions to ACEIs or angiotensin II receptor blockers, hereditary or idiopathic angio-oedema, galactosemia or lactase deficiency. Patients were included for 7 months: ramipril was started at 2.5 mg/day after baseline evaluation (Visit 1, V1), increased to 5 mg/day after Visit 2 (V2) 1 month later; treatment was stopped at month 6 following a complete evaluation on Visit 3 (V3). In case of side effects, ramipril was replaced by irbesartan (75 mg up to V2 and then 150 mg/day up to V3). Complete renal and cardiovascular evaluation including measured GFR (mGFR) and doppler echocardiography were performed at baseline and month 6th. ACR ≤ 3 mg/mmol creatinine was considered as normoalbuminuria, microalbuminuria was defined by an ACR of 3–30 mg/mmol creatinine and macroalbuminuria by an ACR > 30 mg/mmol creatinine. mGFR was assessed by 51Cr-EDTA renal clearance, as described previously3. Briefly, 1.8–3.5 MBq of 51Cr-EDTA (GE Healthcare, Velizy, France) was injected intravenously as a single bolus. Average urinary 51Cr-EDTA clearance, average plasmatic 51Cr EDTA and urinary creatinine clearance were determined during five to six consecutive 30-min clearance periods. GFR measurements were average and standardized for body surface area (1.73 m2). Hyperfiltration was defined above a threshold value of 110 ml/min/1.73 m2 for mGFR and renal insufficiency was defined as mGFR < 60 ml/ min/1.73 m2.
Cardiovascular evaluation
Patients were examined in a quiet, temperature-controlled room. Blood pressure was measured after 15 min of rest in a supine position with an oscillometric method and a cuff of appropriate size. The average of five consecutive measurements was calculated. Aortic stiffness was measured through the carotid to femoral pulsewave velocity (PWV) between the two sites by the foot-to-foot velocity method (Complior; ALAM Medical, Pantin, France), as described previously and validated31,32. Briefly, PWV was calculated from measurements of pulse transit time and the distance traveled by the pulse between two recording sites: pulse wave velocity distance (m)/transit time (seconds). It expresses the elastic properties of the descending and abdominal aorta and the iliofemoral segments. PWV was defined as the mean of four determinations. Doppler echocardiography examinations were performed by an experienced physician according to the guidelines33. All the examinations were analyzed off-line blinded to study time point, to clinical and biological data. From M mode, the following measurements were made at end diastole: left ventricular (LV) internal diameter, interventricular, septal and posterior wall thicknesses. LV mass was derived and indexed to body surface area33. From 2D mode, LV volumes and ejection fraction (LVEF) were derived from Simpson’s modified biplane method. Left atrial volume was also measured and indexed to body surface area. From pulsed wave Doppler mode, LV outflow tract time–velocity integral and early peak diastolic velocity of the mitral (E) inflow were measured. Cardiac output was calculated and indexed (CI) to body surface area as recommended34,35. From continuous wave Doppler, peak tricuspid regurgitation was recorded in multiple views and the highest level of velocity was selected. Elevated pulmonary systolic pressure was defined by a peak tricuspid regurgitation velocity (TRV) > 2.5 m/s36. Systemic Vascular Resistance (SVR) was calculated in units of dyn s cm−5 as: 80 × Mean Arterial Pressure/Cardiac Output (normal range 700–1600 dyn s cm−5).
Assays
As previously described assays were performed in our lab2,14. Serum and urinary creatinine levels were measured by the enzymatic method on a Konelab 20 analyzer from Thermo Fisher Scientific. Uric acid levels were measured with the Konelab analyzer. Total CO2 in blood, ionized calcium, sodium, and potassium levels were measured with an ABL 815 from Radiometer. Calcium and magnesium serum and urinary levels were measured with the PerkinElmer 3300 atomic absorption spectrometer. Plasma Aldosterone was measured by radioimmunoassay kits (Immunodiagnostics Systems Ltd.) Plasma asymmetric dimethyl arginine (ADMA), symmetric dimethyl arginine (SDMA) and arginin were measured by a high liquid performance chromatography coupled with fluorimeter detection. Other laboratory values were measured using standard hospital laboratory techniques.
Statistical analyses
Variables were expressed as percentages, means ± SD, or medians (IQR: interquartile range), as appropriate. Comparison of biological and hemodynamic data measured at different visits was tested with a non parametric Wilcoxon rank test for quantitative variables. Association between mGFR and variables of interest were tested separately using Spearman correlation test and a multilinear regression analysis when adjustment on CI or SVR was performed. A two-sided P-value < 0.05 indicated statistical significance. Statistical analyses were performed with Statview 5.0.
References
Ware, R. E. et al. BABY HUG Investigators: Renal function in infants with sickle cell anemia: Baseline data from the BABY HUG trial. J. Pediatr. 156, 66–70 (2010).
Haymann, J. P. et al. Glomerular hyperfiltration in adult sickle cell anemia: A frequent hemolysis associated feature. Clin. J. Am. Soc. Nephrol. 5, 756–761 (2010).
Day, T. G., Drasar, E., Fulford, T., Sharpe, C. C. & Thein, S. L. Association between hemolysis and albuminuria in adults with sickle cell anemia. Haematologica 97, 201–205 (2012).
Dharnidharka, V. R., Dabbagh, S., Atiyeh, B., Simpson, P. & Sarnaik, S. Prevalence of microalbuminuria in children with sickle cell disease. Pediatr. Nephrol. 12, 475–478 (1998).
Guasch, A., Navarrete, J., Nass, K. & Zayas, C. F. Glomerular involvement in adults with sickle cell hemoglobinopathies. Prevalence and clinical correlates of progressive renal failure. J. Am. Soc. Nephrol. 17, 2228–2235 (2006).
Thompson, J., Reid, M., Hambleton, I. & Serjeant, G. R. Albuminuria and renal function in homozygous sickle cell disease observations from a cohort study. Arch. Intern. Med. 167, 701–708 (2007).
Nebor, D. et al. Alpha-thalassemia is associated with a decreased occurrence and a delayed age-at-onset of albuminuria in sickle cell anemia patients. Blood Cells Mol. Dis. 45, 154–158 (2010).
Asnani, M. R., Fraser, R. A. & Reid, M. E. Higher rates of hemolysis are not associated with albuminuria in Jamaicans with sickle cell disease. PLoS ONE 6, e18863 (2011).
Serjeant, G. R., Higgs, D. R. & Hambleton, I. R. Elderly survivors with homozygous sickle cell disease. N. Engl. J. Med. 356, 642–643 (2007).
Schmitt, F. et al. Early glomerular dysfunction in patients with sickle cell anemia. Am. J. Kidney Dis. 32, 208–214 (1998).
Guasch, A., Cua, M., You, W. & Mitch, W. E. Sickle cell anemia causes a distinct pattern of glomerular dysfunction. Kidney Int. 51(3), 826–833 (1997).
Hammoudi, N., Lionnet, F., Redheuil, A. & Montalescot, G. Cardiovascular manifestations of sickle cell disease. Eur. Heart J. https://doi.org/10.1093/eurheartj/ehz217 (2019) (Epub ahead of print).
Gordeuk, V. R. et al. Relative systemic hypertension in patients with sickle cell disease is associated with risk of pulmonary hypertension and renal insufficiency. Am. J. Hematol. 83, 15–18 (2007).
Haymann, J. P. et al. Renin-angiotensin system blockade promotes a cardio-renal protection in albuminuric homozygous sickle cell patients. Br. J. Haematol. 179(5), 820–828 (2017).
Hirschberg, R. Glomerular hyperfiltration in sickle cell disease. Clin. J. Am. Soc. Nephrol. 5, 748–749 (2010).
Helal, I., Fick-Brosnahan, G. M., Reed-Gitomer, B. & Schrier, R. W. Glomerular hyperfiltration: Definitions, mechanisms and clinical implications. Nat. Rev. Nephrol. 8, 293–300 (2012).
Nath, K. A. & Hebbe, R. P. Sickle cell disease: Renal manifestations and mechanisms. Nat. Rev. Nephrol. 11(3), 161–171 (2015).
Denenberg, B. S., Criner, G., Jones, R. & Spann, J. F. Cardiac function in sickle cell anemia. Am. J. Cardiol. 51, 1674–1678 (1983).
Yee, M. E. et al. Losartan therapy decreases albuminuria with stable glomerular filtration and permselectivity in sickle cell anemia. Blood Cells Mol. Dis. 69, 65–70 (2018).
Maddox, D. A. & Brenner, B. M. Glomerular ultrafiltration. In The Kidney (ed Brenner) 6th ed. 319–374 ISBN 0-7216-7998-6 (WB Saunders Company, 2000).
Bernstein, J. & Whitten, C. F. A histologic appraisal of the kidney in sickle cell anemia. Arch. Pathol. 70, 407–418 (1960).
Bhathena, D. B. & Sondheimer, J. H. The glomerulopathy of homozygous sickle hemoglobin (SS) disease: Morphology and pathogenesis. J. Am. Soc. Nephrol. 1, 1241–1252 (1991).
Pitcock, J. A., Muirhead, E. E., Hatch, F. E., Johnson, J. G. & Kelly, B. J. Early renal changes in sickle cell anemia. Arch. Pathol. 90, 403–410 (1970).
Buckalew, V. M. & Someren, A. Renal manifestations of sickle cell disease. Arch. Intern. Med. 133, 660–669 (1974).
Elfenbein, I. B., Patchefsky, A., Schwartz, W. & Weinstein, A. G. Pathology of the glomerulus in sickle cell anemia with and without nephrotic syndrome. Am. J. Pathol. 77, 357–374 (1974).
Falk, R. J. et al. Prevalence and pathologic features of sickle cell nephropathy and response to inhibition of angiotensin-converting enzyme. N. Engl. J. Med. 326, 910–915 (1992).
Conrad, K. P., Jeyabalan, A., Danielson, L. A., Kerchner, L. J. & Novak, J. Role of relaxin in maternal renal vasodilation of pregnancy. Ann. N. Y. Acad. Sci. 1041, 147–154 (2005).
Statius van Eps, L. W., Schouten, H., La Porte-Wijsman, L. W. & Struyker Boudier, A. M. The influence of red blood cell transfusions on the hyposthenuria and renal hemodynamics of sickle cell anemia. Clin. Chim. Acta. 17, 449–461 (1967).
Bradley, S. E. & Bradley, G. P. Renal function during chronic anemia in man. Blood 2, 192–202 (1947).
Aygun, B. et al. Hydroxyurea treatment decreases glomerular hyperfiltration in children with sickle cell anemia. Am. J. Hematol. 88, 116–119 (2013).
Asmar, R. et al. Assessment of arterial distensibility by automatic pulse wave velocity measurement. Validation and clinical application studies. Hypertension 26, 485–490 (1995).
Reference Values for Arterial Stiffness’ Collaboration. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: Establishing normal and reference values. Eur. Heart J. 31, 2338–2350 (2010).
Lang, R. M. et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 28, e14 (2015).
Quinones, M. A., Otto, C. M., Stoddard, M., Waggoner, A. & Zoghbi, W. A. Recommendations for quantification of Doppler echocardiography: A report from the Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 15, 167–184 (2002).
Nagueh, S. F. et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J. Am. Soc. Echocardiogr. 22, 107–133 (2009).
Klings, E. S. et al. An official American Thoracic Society clinical practice guideline: Diagnosis, risk stratification, and management of pulmonary hypertension of sickle cell disease. Am. J. Respir. Crit. Care Med. 189, 727–740 (2014).
Acknowledgements
The RAND study was funded by the French Ministry of Health, Delegation a la Recherche Clinique, Assistance Publique-Hopitaux de Paris (AP-HP), Programme Hospitalier de Recherche Clinique, Grant PHRC2009 AOR09063. We thank Dr Pauline Cavelier (DIRC Ile de France), Mrs Amal Abderrahim (URCEST), Mrs Christine Fauroux, URCEST and AGEPS for their logistical supply.
Author information
Authors and Affiliations
Contributions
J-P.H, M.C., F.L. and C.D. wrote the main manuscript text, Fig. 1 and Tables 1, 2, 3, 4. Design of study analyses: J-P.H. and F.L.; Data collection: J-P.H.; N.H., M.L., S.M., E.L., O.S., G.G., F.L. Interpretation of results: M.M., S.W., N.T., J-P.H., M.C. and F.L. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Haymann, JP., Hammoudi, N., Livrozet, M. et al. Hemodynamic and biological correlates of glomerular hyperfiltration in sickle cell patients before and under renin–angiotensin system blocker. Sci Rep 11, 11682 (2021). https://doi.org/10.1038/s41598-021-91161-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-91161-y
This article is cited by
-
Glomerular hyperfiltration: part 2—clinical significance in children
Pediatric Nephrology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.