Abstract
Our study aimed to identify the novel acaricidal compound in Xenorhabdus szentirmaii and X. nematophila using the easyPACId approach (easy Promoter Activated Compound Identification). We determined the (1) effects of cell-free supernatant (CFS) obtained from mutant strains against T. urticae females, (2) CFS of the acaricidal bioactive strain of X. nematophila (pCEP_kan_XNC1_1711) against different biological stages of T. urticae, and females of predatory mites, Phytoseiulus persimilis and Neoseiulus californicus, (3) effects of the extracted acaricidal compound on different biological stages of T. urticae, and (4) cytotoxicity of the active substance. The results showed that xenocoumacin produced by X. nematophila was the bioactive acaricidal compound, whereas the acaricidal compound in X. szentirmaii was not determined. The CFS of X. nematophila (pCEP_kan_XNC1_1711) caused 100, 100, 97.3, and 98.1% mortality on larvae, protonymph, deutonymph and adult female of T. urticae at 7 dpa in petri dish experiments; and significantly reduced T. urticae population in pot experiments. However, the same CFS caused less than 36% mortality on the predatory mites at 7dpa. The mortality rates of extracted acaricidal compound (xenocoumacin) on the larva, protonymph, deutonymph and adult female of T. urticae were 100, 100, 97, 96% at 7 dpa. Cytotoxicity assay showed that IC50 value of xenocoumacin extract was 17.71 μg/ml after 48 h. The data of this study showed that xenocoumacin could potentially be used as bio-acaricide in the control of T. urticae; however, its efficacy in field experiments and its phytotoxicity need to be assessed in future.
Similar content being viewed by others
Introduction
Tetranychus urticae Koch is one of the most important and widespread pest species of plant-feeding mites. It is found in tropical and temperate zones of the world especially in greenhouses. T. urticae uses its piercing and sucking mouthparts to aspirate contents of plant cells from a broad range of plant host species (> 1100 species). They attack several fruit and vegetables grown in greenhouses, ornamentals and field-grown crops like maize, cotton, etc.1. Feeding of both adult and immature stages on the lower surfaces of plant causes leaf chlorosis which eventually leads to fruit deformation, plant growth inhibition and even plant death2.
Generally, chemical pesticides have been used to control this mite pest, but T. urticae has a short life cycle and a high generative potential which has enabled it to develop resistance to various insecticides and acaricides3. In addition, these pesticides also kill natural enemies of T. urticae such as predatory mites which are biocontrol option used to suppress T. urticae populations4. The most commonly used predatory mites of T. urticae are Phytoseiulus persimilis and Neoseiulus californicus (Acari: Phytoseiidae)5,6. Because of environmental concerns and developing resistance of T. urticae to conventional pesticides, it is necessary to search for new acaricidal pesticides with great IPM value. Recently, several studies have investigated the effects of secondary metabolites produced by certain bacteria on insects and mites7,8. Recent studies have reported that some bacterial species in the genus Xenorhabdus produce secondary compounds with antibacterial, antifungal, nematicidal, and insecticidal properties9,10,11. These novel compounds have the potential to be developed into a new generation of pesticides including insecticides and acaricides.
Xenorhabdus spp. are motile, Gram-negative bacteria belonging to the family Morganellaceae12 and are symbiotically associated with entomopathogenic nematodes (EPNs) in the family Steinernematidae13. The nematode/bacterium complex has an intricate life cycle in which the bacteria are transported from one insect hemocoel to another by the steinernematid infective juveniles (IJs). The bacterial cells are sequestered in a special pouch in the intestine of the IJs. When the IJs enter their insect host through the mouth or anus and penetrate into the hemocoel or through the spiracles and penetrate directly into the hemocoel, they release the bacterial cells into the hemolymph. The highly virulent bacteria kill the insect host within 24–48 h; they also secrete enzymes, toxins and secondary metabolites with antimicrobial, insecticidal and cytotoxic activities, some of which protect the insect cadaver from saprophytic organisms14,15,16,17,18. Some of these Xenorhabdus compounds have potential applications in pest and disease control9,11. For example, numerous studies have evaluated the acaricidal activity of secondary metabolites produced by Xenorhabdus against several agriculturally important mite species such as Luciaphorus perniciosus19,20, Rhizoglyphus robini21, and T. urticae22,23. Moreover, Cevizci et al.23 reported that the egg and mobile stages of the predatory mites, N. californicus and P. persimilis, were not significantly affected by metabolites from X. nematophila and X. szentirmaii compared to T. urticae. They determined that the mode of entry of these bacterial metabolites into the mites was through the dorsal and ventral integument and that the predatory mites were less affected because of their longer legs which resulted in their less contact with the acaricide-treated surfaces.
Although the abovementioned studies have established that secondary metabolites from some Xenorhabdus are effective in killing mites, none was identified as to the actual compound(s) responsible for the acaricidal effects. Therefore, our study is aimed at isolating and identifying the acaricidal compound(s) using easyPACId approach (easy Promoter Activated Compound Identification)24. This biotechnology enables us to investigate and compare the effects of a natural products (NPs) produced by a specific gene using mutants in which the expression of the gene can be regulated25,24. In this approach, biological gene clusters synthesized by non-ribosomal peptide synthetases (NRPS) or polyketide synthetases (PKS) enzymes and regulated by a single promoter in Xenorhabdus can be activated using inducible promotors24.
It is important to test the effects of the new potential acaricide on the pest, T. urticae and its natural enemies (in this case predatory mites), to determine whether any side effects on non-target or beneficial organisms are likely to occur. Thus, the objectives of our study were to determine the (1) active acaricidal compound(s) using promoter exchange mutant strains, (2) cell-free supernatant (CFS) of the acaricidal bioactive strain of X. nematophila (pCEP_kan_XNC1_1711) against different biological stages of T. urticae, and females of predatory mites, Phytoseiulus persimilis and Neoseiulus californicus, (3) effects of the extracted acaricidal compound on different biological stages of T. urticae, and (4) cytotoxicity of the active substance.
Materials and methods
Plants
Bean plants (Phaseolus vulgaris cv. Barbunia supplied by Migros supermarket, Aydin, Turkey) were grown for rearing T. urticae as well as used for our laboratory studies. Plants were grown in pots (15 × 15 cm) containing forest soil, peat and perlite at 25 ± 2 °C temperature, 60 ± 10% relative humidity and 16 h light conditions and maintained in a separate climate room (PG34 − 3 Digitech Ltd., Ankara, Turkey) dedicated for bean plant growth.
Rearing of mites
All mites used in the study are laboratory cultures previously identified based on morphological characteristics by Dr. Ibrahim Cakmak and used in previous studies22,23. Tetranychus urticae was obtained from strawberry plants in Aydin, Turkey. Bean plants which reached the 5–6 leaves were brought to the T. urticae rearing room and infested with different biological stages of the pest. The rearing of T. urticae was performed in another climate room with the same features as the plant growth room.
The predatory mites, P. persimilis and N. californicus, were obtained from bean plants in Hatay and strawberry plants in Aydin, respectively6,23. They were reared on detached bean leaves infested with all biological stages of T. urticae at 25 ± 1 °C temperature, 70 ± 10% relative humidity and 16 h light conditions in a third climate room. The detached bean leaves were placed on inverted pots in different size of two trays (45 × 32 × 8 cm; 78 × 56 × 18 cm). The trays were filled with water and covered with a plexiglass container to prevent the escape of the mites26,27. Three detached bean leaves infested with T. urticae were placed on each inverted pot three times a week to rear the predatory mites.
Identification of acaricidal bioactive compounds
Bacterial sources
In the study carried out by Eroglu et al.22, Xenorhabdus szentirmaii and X. nematophila were determined as the species with the highest acaricidal activity among the CFS obtained from many tested Xenorhabdus and Photorhabdus spp. To identify the bioactive compound(s), promoter exchange mutants of X. szentirmaii and X. nematophila were generated and bioactivity tests were performed.
Generation of deletion and promoter exchange mutants
The easyPACId approach (easy Promoter Activated Compound Identification) was used to identify the acaricidal compound(s) in X. szentirmaii and X. nematophila. The RNA chaperon, hfq, is directly associated with the production of natural products (NPs) as it controls the expression of biosynthetic gene clusters (BGCs) using the sRNA/mRNA interactions28. Therefore, ∆hfq mutants were generated in X. szentirmaii and X. nematophila to stop the biosynthesis of NPs. Subsequently activation of desired BGCs (Table 1) in a Δhfq background led to the nearly exclusive production of the corresponding NPs in Xenorhabdus strains following targeted BGC activation using the inducible promoter24. These methods can accelerate the identification of bioactive NPs by performing direct bioactivity tests without the need for purification of supernatants containing certain NPs24,25,29. Mutant strains of X. szentirmaii and X. nematophila with natural promoter regions replaced with inducible promoter regions were used in our study (Table 1). The generation of X. szentirmaii Δhfq and X. nematophila Δhfq as well as promotor exchange mutants shown in Table 1 were performed as described by Tobias et al.28,29 and Bode et al.24.
Preparation of bacterial supernatants of mutant strains
The 21-promoter exchange mutant strains (12 X. szentirmaii and 9 X. nematophila) listed in Table 1 were cultivated on LB agar, supplemented with a 50 μg/mL final concentration of kanamycin, and incubated for 48 h at 30°C30. A single colony was transferred into 10 ml LB medium, supplemented with a 50 μg/ml final concentration of kanamycin to obtain an overnight culture at 200 rpm and 30 °C. The optical densities of the overnight cultures (10 ml LB) were measured at 600 nm. The final OD of the cultures was adjusted to 0.1 after inoculation 100 ml Nutrient Broth (NB)30. For each strain, two flasks were prepared, and the cultures were incubated at 30 °C for 1 h. One of the flasks was induced with 0.2% L-arabinose (Carl Roth), and the other flask was not treated with L-arabinose (non-induced). All induced and non-induced cultures were incubated for 72 h at 200 rpm and 30 °C. The CFS was harvested by centrifugation at 10,000 rpm for 10 min, and the supernatant was filtered through a 0.22 μm millipore filter (Thermo scientific)10,31.
Determination of acaricidal compound/s using mutant strains
The effects of induced and non-induced CFS of mutant strains were tested on T. urticae adult females in Petri dishes. Experiments were carried out in a climate room (PG34 − 3 Digitech Ltd., Ankara, Turkey) at 25 ± 1 °C temperature, 70 ± 5% relative humidity and 16 h light conditions. Moistened cotton wool was placed on Petri dishes (15 cm in diameter) first, and then the bean leaf was placed with its bottom face up. The adult females of T. urticae were separately transferred with a fine brush in each Petri dish as 20 individuals. The CFS of mutant strains were sprayed on the leaves with a hand sprayer (2.5 ml/Petri dish). Sterile NB medium in which bacteria were grown was used as the control group. Mortality rates of mites were determined in 2, 5 and 7 days after the application (dpa). The experiments were carried out in 20 repetitions and repeated 4 times at different times.
The effect of the supernatant of induced mutant strain responsible from acaricidal activity against different biological stages of Tetranychus urticae
The acaricidal compound that causes high mortality on mites was determined and the gene region responsible for the production of the relevant bioactive compound in X. nematophila (pCEP_kan_XNC1_1711) was induced by L-arabinose, thus enabling the bacterium to produce an acaricidal bioactive compound only as a secondary metabolite. The effects of this CFS against the different biological stages of T. urticae were investigated in Petri dishes and pots.
Petri dish experiments
The effects of CFS of X. nematophila pCEP_kan_XNC1_1711 against different biological stages (egg, larva, protonymph, deutonymph, adult) of T. urticae were detected as previously described. Moistened cotton wool was placed on Petri dishes (15 cm in diameter) first, then the bean leaf was placed with its bottom face up. The egg, larva, protonymph, deutonymph and adult female of T. urticae were separately transferred with a fine brush in each Petri dish as 20 individuals. In order to obtain different biological stages of T. urticae at the same age to be used in the experiments, 25 gravid females of T. urticae were transferred on leaf discs. After 24 h, females were removed from the environment and the eggs remained. In this way, different biological stages (egg, larva, protonymph, deutonymph and adult female) of T. urticae were obtained at the same age. The CFS of mutant strain was sprayed on the leaves with a hand sprayer (2.5 ml/Petri dish). Sterile NB was used as the control group. Mortality rates of mites were determined in 2, 5 and 7 dpa. The experiments were carried out in 20 repetitions and repeated 4 times at different dates.
Pot experiments
As in Petri dish experiments, bean plants were used in pot experiments. Bean plants were grown in pots (7 × 5 cm) and used at the same age in the experiment. One leaf of the plants with two cotyledon leaves was cut, and only one leaf was left per each pot. A total of 60 individuals, including 10 individuals of each stage, egg, larva, protonymph, deutonymph, adult female and adult male, obtained from T. urticae culture were transferred to these plants with a fine brush. The CFS of X. nematophila pCEP_kan_XNC1_1711 mutant strain was sprayed on both the bottom and top surfaces of the leaves with a hand sprayer (5 ml/pot). The plants in the control group were sprayed with the same amount of sterile NB. The number of live and dead individuals was recorded 7 dpa. The experiments were carried out in 20 repetitions and repeated 4 times at different dates.
The toxicity of the supernatant of mutant strain on predatory mites
The potential toxic effect of the CFS of X. nematophila pCEP_kan_XNC1_1711 on the predatory mites, P. persimilis and N. californicus, was investigated in Petri dishes at 25 ± 1 °C, 70 ± 5% R.H. and 16 h L:D photoperiod in a climate room. Moistened cotton wool (10 cm diameter) was placed on the Petri dishes (15 cm in diameter), and the gap between the Petri dish and cotton was filled with tap water to prevent the escape of the predatory mites. Adult females of P. persimilis and N. californicus (20 individuals/Petri dish) obtained from the culture were separately transferred with a fine brush on the leaves in the Petri dishes. Bean leaves infested with different biological stages of T. urticae (~ 300 individuals) were brushed onto the leaves at two-day intervals to feed the predatory mites. The CFS of mutant strain was sprayed with a hand sprayer on the leaves, and sterile NB was used as control. The mortality rate of the predatory mites in each Petri dishes was recorded at 2, 5 and 7 dpa. The study was carried out in 20 repetitions and repeated 4 times at different times.
The effects of the acaricidal extract on different biological stages of Tetranychus urticae
Extraction of the acaricidal bioactive compound was performed as follows:
Induced X. nematophila pCEP_kan_XNC1_1711 mutant strain was cultured in LB (6L) with 2% XAD resin at 30 °C for 3 days. The resin was extracted exhaustively with methanol (3 × 2 L) at room temperature. The methanol extract was concentrated under reduced pressure to give extracted compound24. The obtained extracted compound was first dissolved in DMSO and prepared as a stock solution with distilled water at a concentration of 208 μg/ml. Different dilutions (100%, 50%, 25%, 12.5%, 6.25% and 3.125%) of this prepared stock solution to determine LC50 and LC90 value of extracted compound were applied to T. urticae females in Petri dishes. Then, the activity of the LC90 value of the acaricidal extracted compound on different biological stages (egg, larva, protonymph, deutonymph and adult female) of T. urticae was determined in Petri dishes. For these studies, moistened cotton wool was placed on Petri dishes (15 cm in diameter) first, then the bean leaf was placed with its bottom face up. 20 adult females of T. urticae were transferred to each petri dish with a fine brush. Different dilutions and the LC90 value of the extracted compound were sprayed on the leaves with a hand sprayer (2.5 ml / petri dish). Sterile distilled water with DMSO was used as the control group. Mortality rates of mites were determined in 2, 5 and 7 dpa. The experiments were carried out in 10 repetitions and repeated 2 times.
Cytotoxicity of extracted bioactive acaricidal compound
Cytotoxicity assay was conducted using MRC-5 normal human fetal lung fibroblast cell-line. MRC-5 cells were obtained from the cell culture bank of the Turkish Ministry of Agriculture and Forestry (MRC-5 An1, HÜKÜK no: 96101701). The cells were maintained in Eagle's Minimum Essential Medium (EMEM) supplemented with 10% fetal bovine serum (Sigma-Aldrich) and 5% penicillin–streptomycin solution. The cells were cultured in tissue culture flask and incubated at 37 °C, 5% carbon dioxide and 96% humidity. The culture medium was replenished in 2 day-intervals. The cytotoxic effects of the extracted acaricidal compound of X. nematophila pCEP_kan_XNC1_1711 were measured in MRC-5 cell line using the MTT method. MRC-5 cells were treated with various concentrations of extracted acaricidal compound for 48 h at 37 °C. The compound was first dissolved in Dimethyl sulfoxide (DMSO) and prepared as a stock solution with distilled water at a concentration of 208 µg/ml. Six different concentrations ranging from 1.04 to 72.8 µg/ml and 2.08 to 104 µg/ml were prepared in EMEM (Sigma-Aldrich) respectively. MRC-5 (1 × 104cells/well) were seeded in each well of 48-well microplates and incubated at 37 °C and 5% CO2 for 24 h. Then, extracted acaricidal compound were applied and the cells were incubated for 48 h. There were two control groups: one with culture medium and MRC-5 cells and the other had DMSO solvent. After 48 h, the MTT [3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide] solution (5000 µg/ml) was added to each well, and the cells were cultured for another 4 h at 37 °C in a 5% CO2 incubator32. A hundred microliters of DMSO was added to the cells to dissolve the formazan crystals that formed. After 15 min of mixing at room temperature, the level of colored formazan was determined by measuring optical density (OD) with Multiskan™ GO Microplate reader (Thermo Scientific™, Finland) at 570 nm (OD570-630 nm)33. The half-maximal inhibitory concentrations (IC50) values were measured after 48 h. The assays were performed in three independent experiments. The percentage viability was calculated as34: % Viability = (OD of treated cells/OD of control cells) × 100.
Statistical analyses
The data shown in Figs. 1, 3 and 6 were analyzed with the General Linear Model and the differences among the averages were grouped according to the Tukey’s Honestly Significant Difference (Tukey HSD) test at the level of P = 0.05. The data obtained in Figs. 3 and 6 were calculated by applying the Abbott formula35. The data in Figs. 4 and 5 were compared with Student’s t-test. Arcsine transformation was performed on mite mortality before statistical analyses36. The LC50 and LC90 values were determined in the POLO computer package program37.
Ethical statements
The authors declare that the use of bean plants in the present study complies with international, national and/or institutional guidelines. Bean plants, Phaseolus vulgaris cv. Barbunia were supplied by Migros supermarket, Aydin, Turkey.
Results
Determination of acaricidal compound/s using mutant strains
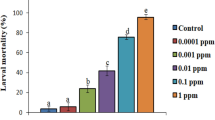
The experiments conducted with mutant strains showed that xenocoumacin induced strain of X. nematophila (pCEP_kan_XNC1_1711) exhibited the highest acaricidal effect on T. urticae (Fig. 1). When the gene region responsible for the production of xenocoumacin was induced, the mortality rate of mites at 7 dpa was 100%, while the mortality rate of the non-induced xenocoumacin gene was less than 40%. None of the other induced or non-induced mutant strains of X. nematophila caused more than 50% mortality at 7 dpa. There was a statistically significant difference between xenocoumacin and all of the other tested compounds and the control group (F = 16.695, df = 18, P < 0.001) (Fig. 1). On the other hand, induced or non-induced 12 mutant strains of X. szentirmaii displayed acaricidal activity less than 50% (Fig. 2).
The effect of the supernatant of induced mutant strain responsible from acaricidal activity against different biological stages of Tetranychus urticae
Petri dish experiments
The study showed that the CFS of X. nematophila (pCEP_kan_XNC1_1711) mutant strain had no effect on T. urticae eggs (ovicidal rate was 0%). The mortality rates on larva, protonymph, deutonymph and adult female of T. urticae were 100, 81, 44.9, and 43.1% at 2 dpa (Fig. 3). There was a statistical difference in mortality rates between different biological stages of T. urticae at 2 dpa, and the highest mortality rate was found in larvae (F = 187,580; P < 0.001). The highest mortality was detected in larvae and protonymphs at 5 and 7 dpa, and the lowest was in deutonymphs and adults (5 dpa F = 24.417, P < 0.001; 7 dpa F = 4.694, P < 0.05; Fig. 3). The mortality rate in all biological stages of T. urticae was over 85% at 5 dpa and over 97% at 7 dpa (Fig. 3).
Pot experiments
The number of live individuals at 7 days after the application of the CFS to the different biological stages of T. urticae is shown in Fig. 5. At 7 dpa, 57 and 485 eggs and 45 (42.4 larvae, 0.1 protonymph, 0.6 deutonymph, 1.9 adult) and 313 (130.6 larvae, 87 protonymph, 60.6 deutonymphs, 34.9 adults) mobile stages were obtained in CFS treated group and control, respectively. The number of both eggs and mobile stages was significantly different between the CFS and the control (eggs t = 42.988, P < 0.01; mobile stages t = 41,307, P < 0.01) (Fig. 4).
The toxicity of the supernatant of mutant strains on predatory mites
The mortality rates at 2, 5 and 7 dpa of the CFS of X. nematophila (pCEP_kan_XNC1_1711) mutant strain to the adult stages of the predatory mites P. persimilis and N. californicus are given in Fig. 5. The mortality rate of adult females of P. persimilis at 2, 5 and 7 dpa was 10.3, 23.5, 32.3% in the CFS treated group and 1, 3.8, 7.3% in the control, respectively. There was a statistically significant difference between the CFS and the control (2 dpa t = 5.54, P < 0.01; 5 dpa t = 8.454, P < 0.001; 7 dpa t = 9.499, P < 0.001) (Fig. 5). The mortality rate of adult females of N. californicus at 2, 5 and 7 dpa was 13.8, 28.8, 36.0% in the CFS treated group and 3, 7.3, 9.8% in the control, respectively. Statistically significant difference was observed between the CFS and the control groups (2 dpa t = 5.669, P < 0.05; 5 dpa t = 9.284, P < 0.01; 7 dpa t = 11.132, P < 0.01) (Fig. 5). However, no significant difference occurred between P. persimilis and N. californicus in terms of sensitivity to the CFS (2 dpa t = 1.520, P > 0.05; 5 dpa t = 1.752, P > 0.05; 7 dpa t = 1.193, P > 0.05).
The effects of the extracted acaricidal compound on different biological stages of Tetranychus urticae
The data revealed that xenocoumacin is an extremely effective acaricidal compound. Even a 25% concentration of xenocoumacin caused death by 93.8% of T. urticae adult females at 7 dpa. Because of DMSO, control mortalities were ranged between 1.3 (2 dpa) and 15.0% (7 dpa) (Table 2). The LC50 values of xenocoumacin for 2, 5 and 7 dpa were calculated as 60, 26, 21 µg/ml and the LC90 values as 301, 71, 55 µg/ml, respectively. When the LC90 value (71 µg/ml) in 5 days was applied to the different biological stages of T. urticae, the mortality rates of xenocoumacin on the larva, protonymph, deutonymph and adult female of T. urticae were 100, 92, 45, 44% at 2-dpa, 100, 100, 94, 92% at 5 dpa and 100, 100, 97, 96% at 7 dpa (Fig. 6). There was a statistically significant difference in the mortality rates of different biological stages of T. urticae at 2, 5 and 7 dpa (2 dpa F = 169.005; P < 0.001; 5 dpa F = 32.665 P < 0.001; F = 9.717; P < 0.001; Fig. 6). On the other hand, xenocoumacin had no effect on the egg stages of T. urticae (ovicidal rate 0%).
Cytotoxity of extracted acaricidal bioactive compound
Cytotoxicity of extracted acaricidal bioactive compound (xenocoumacin) increased with increasing concentration. The inhibitory concentration (IC50) value after 48-h incubation was 17.71 μg/ml.
Discussion
When entomopathogenic nematodes infect an insect host, their symbiotic bacteria produce a wide variety of biologically active compounds with a broad-spectrum activity to protect infected cadaver from opportunistic organisms and scavengers such as ants, crickets, cockroaches, mites etc.38,39. It has been reported that mites like Hypoaspis sp., Pergamasus nr. crassipes (Mesostigmata), Eugamasus sp. (Mesostigmata), Cosmolaelaps vacua, Ololaelaps veneta, Gamasellodes vermivorax, Antennoseius sp., Amblyseius setulus, Ascanesoica, Alycus roseus, Pilogalumna cozadensis (Oribatida), Alicorhagia fragilis (Endeostigmata), Tyrophagus putrescentiae (Astigmata) prey on EPN IJs40. Moreover, species like Sancassania polyphyllae (Astigmata) have been observed to feed on EPN-infected insect cadavers and on the developing EPN IJs herein41,42,43,44,45,46. To protect infected cadaver and developing nematodes from mites, nematode-bacteria complex has to produce bioactive acaricidal compound/s. Accordingly, numerous studies have shown that some species of Xenorhabdus bacteria have acaricidal activity19,20,21,22,23. However, none of these studies identified the bioactive acaricidal compound. Therefore, the aim of this study was to establish the acaricidal activities present in X. nematophila supernatant using the easyPACId biotechnological approach. This biotechnological approach allowed us to determine the bioactive compound by activating mutants with inducible promotors of encoding gene clusters and eliminating the background effect of genes of other compounds24,25. The experiments conducted with promoter exchange mutant strains showed that xenocoumacin induced strain of X. nematophila (pCEP_kan_XNC1_1711) exhibited the highest acaricidal effect on T. urticae.
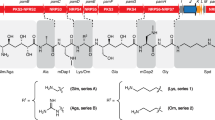
Xenocoumacins are benzopyran-1-one (isocoumarin) derivatives first identified by Mclnernery et al.47 in X. nematophila in two forms. Reimer et al.15 later discovered 4 additional derivatives of these natural products system from several Xenorhabdus strains and reported that they are synthesized by a hybrid polyketide synthase (PKS)‐nonribosomal polypeptide synthetase (NRPS). Both forms have many biological activities such as antifungal, antibacterial, anticancer and anti-ulcer however, xenocoumacin 1 is more biologically active11,48,49.
On the other hand, induced or non-induced 12 mutant strains of X. szentirmaii displayed acaricidal activity less than 50%. A large-scale genome and metabolome analysis of 25 Xenorhabdus strains by Tobias et al.29 revealed that X. szentirmaii DSM 16,338 and US strains do not produce xenocoumacin. So, the acaricidal compound must be a different compound than xenocoumacin. Our collection of mutant strains from X. szentirmaii in our study was limited. Further studies should be conducted with different promotor exchange mutants of X. szentirmaii.
We assessed the acaricidal effects of CFS of X. nematophila (pCEP_kan_XNC1_1711) against all biological stages of an important argonomic pest, T. urticae. First, we showed that the mobile stages of T. urticae were affected at different levels by the CFS of X. nematophila (pCEP_kan_XNC1_1711) mutant strain and xenocoumacin extract. Larval stages were more susceptible compared to adult female in Petri dish experiments, though the mortality rate in all biological stages of T. urticae was over 97% at 7 dpa. Similarly, Eroglu et al.22 found that female adults were relatively more tolerant to the supernatants of X. nematophila wildtype than larval and nymph stages as the supernatant exhibited 90% mortality on adult females and 98% mortality on the larvae of T. urticae at 7 dpa. The LC50 values of xenocoumacin extract against T. urticae adult females in our study for 2, 5 and 7 dpa were 60, 26, 21 µg/ml, respectively. Comparatively, Furuya et al.50 reported that a novel acaricidal compound, pyflubumide, had a LC50 value of 1.2 mg a.i./L against adult twospotted spider mites. The LC50 for cyflumetofen against T. urticae female adults as reported in Hayashi et al.51 was 1.1 mg/L.
Besides petri dish experiments, the results of our pot experiment showed that CFS of X. nematophila (pCEP_kan_XNC1_1711) mutant strains significantly reduced the T. urticae population. Likewise, Eroglu et al.22 showed that the supernatants from wildtypes of X. szentirmaii and X. nematophila, singularly and in combination, significantly reduced the T. urticae population in pot experiment.
We also tested the effects of CFS of X. nematophila (pCEP_kan_XNC1_1711) mutant strains against eggs of T. urticae. We found that xenocoumacin had no effect on T. urticae eggs (ovicidal rate was 0%). Generally, mite eggs have been observed to be resistant to acaricide52, supernatants of Xenorhabdus and Photorhabdus bacteria22,23 or infection from entomopathogenic fungi52.
Tetranychus urticae is the most resistant species among arthropod pests in the world as it has gained resistance to 96 currently available active ingredients53. Hence, predatory mites like P. persimilis and N. californicus are widely used as alternatives to control T. urticae populations. An ideal acaricidal compound should kill T. urticae and have minimum side effects on these predatory mites. Our study also evaluated the toxicity of CFS of X. nematophila (pCEP_kan_XNC1_1711) against the adult females of P. persimilis and N. californicus. Although, the CFS of X. nematophila (pCEP_kan_XNC1_1711) or xenocoumacin XAD extract caused over 90% mortality on the adult female of T. urticae, less than 40% mortality of both predatory mites were affected at 7dpa. Morphological differences between predatory mites and T. urticae have a key role in the different susceptibility of the mites to bacterial supernatants. For instance, T. urticae feeds on treated leaves and have shorter legs compared to predatory mites, their body parts are more in direct contact with applied compounds23. Besides this, predatory mites have a thicker cuticle than of T. urticae54.
Cytotoxicity assays revealed that xenocoumacin compound is not toxic on human cells when it is used at concentrations < 17.71 μg/ml. Bode et al.24 tested the effect of aqueous extract of xenocoumacin obtained from X. nematophila on the human microvascular endothelial cell line. Except for toxicity on cell proliferation, xenocoumacin extract displayed very low effect on the cell metabolic activity. Cytotoxicity of xenocoumacin was moderate and leucocyte adhesion to endothelial cell was low. We found that the LC50 values of xenocoumacin extract against T. urticae adult females in our study ranged between 21–60 µg/ml during the 7 days of assessment. However, this is higher than cytotoxicity against human cells. Future studies should assess the persistence of this compound on plant tissues.
In conclusion, the data of this study showed that xenocoumacins could potentially be used as bio-acaricides in the control of T. urticae at concentrations less than 17 μg/ml, however, the efficacy of xenocoumacin in the field experiment and its phytotoxicity need to be assessed in future.
References
Ilias, A., Vontas, J. & Tsagkarakou, A. Global distribution and origin of target site insecticide resistance mutations in Tetranychus urticae. Insect Biochem. Mol. Biol. 48, 17–28. https://doi.org/10.1016/j.ibmb.2014.02.006 (2014).
Jeppson, L. R., Keifer, H. H. & Baker, E. W. Mites Injurious to Economic Plants (University of California, 1975).
Cagatay, N. S., Riga, M., Vontas, J., Çevik, B. & Ay, R. Biochemical and molecular characterizations of cypermethrin resistance in laboratory-selected cypermethrin-resistant strains of Tetranychus urticae Koch. (Acari: Tetranychidae). Int. J. Acarol. 5, 1–7. https://doi.org/10.1080/01647954.2018.1500641 (2018).
Gatarayiha, C. M., Laing, M. D. & Miller, R. M. Effects of adjuvant and conidial concentration on the efficacy of Beauveria bassiana for the control of the two spotted spider mite Tetranychus urticae. Exp. Appl. Acarol. 50, 217–229. https://doi.org/10.1007/s10493-009-9307-6 (2010).
Cakmak, I., Baspinar, H. & Madanlar, N. Control of the carmine spider mite Tetranychus cinnabarinus Boisduval by the predatory mite Phytoseiulus persimilis (Athias-Henriot) in protected strawberries in Aydın Turkey. Turk. J. Agric. For. 29, 259–265 (2005).
Cakmak, I., Janssen, A., Sabelis, M. W. & Baspinar, H. Biological control of an acarine pest by single and multiple natural enemies. Biol. Control 50, 60–65. https://doi.org/10.1016/j.biocontrol.2009.02.006 (2009).
Seo, M. D., Won, H. S., Kim, J. H., Mishig-Ochir, T. & Lee, B. J. Antimicrobial peptides for therapeutic applications: a review. Molecules 17, 12276–12286. https://doi.org/10.3390/molecules171012276 (2012).
Dhanasekaran, D. & Thangaraj, R. Microbial secondary metabolites are an alternative approach against insect vector to prevent zoonotic diseases. Asian Pac. J. Trop. Dis. 4, 253–261. https://doi.org/10.1016/S2222-1808(14)60569-7 (2014).
Bode, H. B. Entomopathogenic bacteria as a source of secondary metabolites. Curr Opin Chem Biol 13, 224–230. https://doi.org/10.1016/j.cbpa.2009.02.037 (2009).
Hazir, S., Shapiro-Ilan, D. I., Bock, C. H., Hazir, C., Leite L. G. & Hotchkiss, M. W. Relative potency of culture supernatants of Xenorhabdus and Photorhabdus spp. on growth of some fungal phytopathogens. Eur. J. Plant Pathol. 146, 369–381. https://doi.org/10.1007/s10658-016-0923-9 (2016).
Dreyer, J., Malan, A. P. & Dicks, L. M. T. Bacteria of the genus Xenorhabdus, a novel source of bioactive compounds. Front. Microbiol. 9, 3177. https://doi.org/10.3389/fmicb.2018.03177 (2018).
Adeolu, M., Alnajar, S., Naushad, S. & Gupta, R. S. Genome-based phylogeny and taxonomy of the 'Enterobacteriales': proposal for Enterobacterales ord. nov. divided into the families Enterobacteriaceae, Erwiniaceae fam. nov., Pectobacteriaceae fam. nov., Yersiniaceae fam. nov., Hafniaceae fam. nov., Morganellaceae fam. nov., and Budviciaceae fam. nov. Int. J. Syst. Evol. Microbiol. 66, 5575–5599. https://doi.org/10.1099/ijsem.0.001485 (2016).
Forst, S., Dowds, B., Boemare, N. E. & Stackebrandt, E. Xenorhabdus and Photorhabdus spp. Annu. Rev. Microbiol. 51, 47–72. https://doi.org/10.1146/annurev.micro.51.1.47 (1997).
Kaya, H. K. & Gaugler, R. Entomopathogenic nematodes. Ann. Rev. Entomol. 38, 181–206. https://doi.org/10.1146/annurev.en.38.010193.001145 (1993).
Reimer, D., Luxenburger, E., Brachmann, A. O. & Bode, H. B. A new type of pyrrolidine biosynthesis is involved in the late steps of xenocoumacin production in Xenorhabdus nematophila. ChemBioChem 13, 224–230. https://doi.org/10.1002/cbic.200900187 (2009).
Gualtieri, M., Aumelas, A. & Thaler, J. O. Identification of a new antimicrobial lysine-rich cyclolipopeptide family from Xenorhabdus nematophila. J. Antibiot. 62, 295–302. https://doi.org/10.1038/ja.2009.31 (2009).
Houard, J. et al. Cabanillasin, a new antifungal metabolite, produced by entomopathogenic Xenorhabdus cabanillasii JM26. J. Antibiot. 66, 617–620. https://doi.org/10.1038/ja.2013.58 (2013).
Fuchs, S. W., Grundmann, F., Kurz, M., Kaiser, M. & Bode, H. B. Fabclavines: bioactive peptide-polyketide-polyamino hybrids from Xenorhabdus. ChemBioChem 15, 512–516. https://doi.org/10.1002/cbic.201300802 (2014).
Bussaman, P., Sermswan, R. W. & Grewel, P. S. Toxicity of the entomopathogenic bacteria Photorhabdus and Xenorhabdus to the mushroom mite (Luciaphorus sp; Acari: Pygmephoridae). Biocontrol Sci. Technol. 16, 245–256. https://doi.org/10.1080/09583150500335822 (2006).
Bussaman, P., Sa-Uth, C., Rattanasena, P. & Chandrapatya, A. Acaricidal activities of whole cell suspension, cell-free supernatant, and crude cell extract of Xenorhabdus stokiae against mushroom mite (Luciaphorus sp.). J. Zhejiang Univ. Sci. B. 13, 261–266. https://doi.org/10.1631/jzus.B1100155 (2012).
Nermut, J., Zemek, R., Mracek, Z., Palevsky, E. & Puza, V. Entomopathogenic nematodes as natural enemies for control of Rhizoglyphus robini (Acari: Acaridae)?. Biol. Control 128, 102–110. https://doi.org/10.1016/j.biocontrol.2018.10.003 (2019).
Eroglu, C. et al. Acaricidal effect of cell-free supernatants from Xenorhabdus and Photorhabdus bacteria against Tetranychus urticae (Acari: Tetranychidae). J. Invertebr. Pathol. 160, 61–66. https://doi.org/10.1016/j.jip.2018.12.004 (2019).
Cevizci, D., Ulug, D., Cimen, H., Touray, M., Hazir, S. & Cakmak, I. Mode of entry of secondary metabolites of the bacteria Xenorhabdus szentirmaii and X. nematophila into Tetranychus urticae and their toxicity to the predatory mites Phytoseiulus persimilis and Neoseiulus californicus. J. Invertebr. Pathol. 174, 107418. https://doi.org/10.1016/j.jip.2020.107418 (2020).
Bode, E. et al. Promoter activation in Δhfq mutants as an efficient tool for specialized metabolite production enabling direct bioactivity testing. Angew. Chem. Int. 131, 19133–19139. https://doi.org/10.1002/anie.201910563 (2019).
Bode, E. et al. Simple “on-demand” production of bioactive natural products. ChemBioChem 16, 1115–1119. https://doi.org/10.1002/cbic.201500094 (2015).
Cakmak, I., Janssen, A. & Sabelis, M. W. Intraguild interactions between the predatory mites Neoseiulus californicus and Phytoseiulus persimilis. Exp. Appl. Acarol. 38, 33–46. https://doi.org/10.1007/s10493-005-6247-7 (2006).
Kustutan, O. & Cakmak, I. Development, fecundity, and prey consumption of Neoseiulus californicus (McGregor) fed Tetranychus cinnabarinus Boisduval. Turk. J. Agric. For. 33, 19–28. https://doi.org/10.3906/tar-0806-39 (2009).
Tobias, N. J. et al. Photorhabdus-nematode symbiosis is dependent on hfq-mediated regulation of secondary metabolites. Environ. Microbiol. 19, 119–129. https://doi.org/10.1111/1462-2920.13502 (2017).
Tobias, N. J. et al. Natural product diversity associated with the nematode symbionts Photorhabdus and Xenorhabdus). Nat. Microbiol. 2, 1676–1685. https://doi.org/10.1038/s41564-017-0039-9 (2017).
Wenski, S. et al. Fabclavine diversity in Xenorhabdus bacteria. Beilstein J. Org. Chem. 16, 956–965. https://doi.org/10.3762/bjoc.16.84 (2020).
Donmez-Ozkan, H. et al. Nematode-associated bacteria: Production of anti- microbial agent as a presumptive nominee for curing endodontic infections caused by Enterococcus faecalis. Front. Microbiol. 10, 2672. https://doi.org/10.3389/fmicb.2019.02672 (2019).
Freimoser, F. M., Jakob, C. A., Aebi, M. & Tuor, U. The MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay is a fast and reliable method for colorimetric determination of fungal cell densities. Appl. Environ. Microbiol. 65(8), 3727–3729. https://doi.org/10.1128/AEM.65.8.3727-3729.1999 (1999).
Abdi, G. N. et al. Estimation of radiation dose-reduction factor for cerium oxide nanoparticles in MRC-5 human lung fibroblastic cells and MCF-7 breast-cancer cells. Artif. Nanomed. Biotech. 46(3), S1215–S1225 (2018).
Örenlili Yaylagül, E. & Ulger, C. The effect of baicalein on Wnt/ß-catenin pathway and miR-25 expression in Saos-2 osteosarcoma cell line. Turk. J. Med. Sci. 50, 1168–1179. https://doi.org/10.3906/sag-2001-161 (2020).
Abbott, W. S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 18, 265–267. https://doi.org/10.1093/jee/18.2.265a (1925).
SPSS. SPSS v.20.0 for Mac. (SPSS Inc., Chicago, IL, USA, 2011).
LeOra Software POLO-PC: a user’s guide to probit or logit analysis. LeOra Software, Berkeley, CA (1994).
Gulcu, B., Hazir, S. & Kaya, H. K. Scavenger deterrent factor (SDF) from symbiotic bacteria of entomopathogenic nematodes. J. Invertebr. Pathol. 110, 326–333. https://doi.org/10.1016/j.jip.2012.03.014 (2012).
Ulug, D., Hazir, S., Kaya, H. K. & Lewis, E. Natural enemies of natural enemies: the potential top-down impact of predators on entomopathogenic nematode populations. Ecol. Entomol. 39, 462–469. https://doi.org/10.1111/een.12121 (2014).
Raja, R. K. et al. Antagonists and defence mechanisms of entomopathogenic nematodes and their mutualistic bacteria. Biol. Control 152, 104452. https://doi.org/10.1016/j.biocontrol.2020.104452 (2021).
Karagoz, M., Gulcu, B., Cakmak, I., Kaya, H. K. & Hazir, S. Predation of entomopathogenic nematodes by Sancassania sp. (Acari: Acaridae). Exp. Appl. Acarol. 43, 85–95. https://doi.org/10.1007/s10493-007-9105-y (2007)
Ekmen, Z. I. et al. Food preference of Sancassania polyphyllae (Acari: Acaridae): living entomopathogenic nematodes or insect tissues?. Biocontrol Sci. Technol. 20, 553–566. https://doi.org/10.1080/09583151003624696 (2010).
Ekmen, Z. I. et al. Potential negative effects on biological control by Sancassania polyphyllae (Acari: Acaridae) on an entomopathogenic nematode species. Biol. Control 54, 166–171. https://doi.org/10.1016/j.biocontrol.2010.05.004 (2010).
Cakmak, I., Ekmen, Z. I., Karagoz, M., Hazir, S. & Kaya, H. K. Development and reproduction of Sancassania polyphyllae (Acari: Acaridae) feeding on entomopathogenic nematodes and tissues of insect larvae. Pedobiologia 53, 235–240. https://doi.org/10.1016/j.pedobi.2009.11.002 (2010).
Cakmak, I., Karagoz, M., Ekmen, Z. I., Hazir, S. & Kaya, H. K. Life history of Sancassania polyphyllae (Acari: Acaridae) feeding on dissected tissues of its phoretic host, Polyphylla fullo (Coleoptera: Scarabaeidae): temperature effects. Exp. Appl. Acarol. 53, 41–49. https://doi.org/10.1007/s10493-010-9386-4 (2011).
Cakmak, I., Hazir, S., Ulug, D. & Karagoz, M. Olfactory response of Sancassania polyphyllae (Acari: Acaridae) to its phoretic host larva killed by the entomopathogenic nematode, Steinernema glaseri (Rhabditida: Steinernematidae). Biol. Control 65, 212–217. https://doi.org/10.1016/j.biocontrol.2013.02.006 (2013).
McInerney, B. V., Gregson, R. P., Lacey, M. et al. Biologically active metabolites from Xenorhabdus spp. Part 1. Dithiopyrrolone derivatives with antibiotics activity. J. Nat. Prod. 54, 774–784. https://doi.org/10.1021/np50075a005 (1991).
Webster, J. M., Chen, G., Hu, K. & Li, J. Bacterial metabolites. In Entomopathogenic Nematology (ed. Gaugler, R.) 99–114 (CABI International, 2002).
Park, D., Ciezki, K., Hoeven, R., Singh, S., Reimer, D., Bode, H. B. & Forst, S. Genetic analysis of xenocoumacin antibiotic production in the mutualistic bacterium Xenorhabdus nematophila. Mol. Microbiol. 73, 938–949. https://doi.org/10.1111/j.1365-2958.2009.06817.x (2009).
Furuya, T., Machiya, K., Fujioka, S., Nakano, M. & Inagaki, K. Development of a novel acaricide, pyflubumide. J. Pestic. Sci. 42(3), 132–136. https://doi.org/10.1584/jpestics.J17-02 (2017).
Hayashi, N., Sasama, Y., Takahashi, N. & Ikemi, N. Cyflumetofen, a novel acaricide - its mode of action and selectivity. Pest Manag. Sci. 69, 1080–1084. https://doi.org/10.1002/ps.3470 (2013).
Dogan, Y. O., Hazir, S., Yildiz, A., Butt, T. M. & Cakmak, I. Evaluation of entomopathogenic fungi for the control of Tetranychus urticae (Acari: Tetranychidae) and the effect of Metarhizium brunneum on the predatory mites (Acari: Phytoseiidae). Biol. Control 111, 6–12. https://doi.org/10.1016/j.biocontrol.2017.05.001 (2017).
Mota-Sanchez, D. & Wise J. C. The arthropod pesticide resistance database. Michigan State University. (2021) http://www.pesticideresistance.org (Accessed 08 January 2021).
Wu, S. et al. Comparison of mechanical properties for mite cuticles in understanding passive defense of phytoseiid mite against fungal infection. Mater. Des. 140, 241–248. https://doi.org/10.1016/j.matdes.2017.11.051 (2018).
Acknowledgements
This study was supported by the Scientific and Technical Research Council of Turkey (TUBITAK-Project Number: 1170172). Work in the Bode lab was supported by the BMBF (01DL17009) and the LOEWE Translational BiodiversityGenomics (TBG) research center.
Author information
Authors and Affiliations
Contributions
I.C. and S.H. conceived and designed the experiment; G.I., H.C., D.U. and E.O.Y. acquired the data; E.B. and H.B.B. obtained the mutant strains; I.C. performed statistical analyses; I.C., S.H. and M.T. wrote the manuscript, with input from all authors. All authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Incedayi, G., Cimen, H., Ulug, D. et al. Relative potency of a novel acaricidal compound from Xenorhabdus, a bacterial genus mutualistically associated with entomopathogenic nematodes. Sci Rep 11, 11253 (2021). https://doi.org/10.1038/s41598-021-90726-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-90726-1
This article is cited by
-
Effects of Xenorhabdus and Photorhabdus bacterial metabolites on the ovipositional activity of Aedes albopictus
Journal of Pest Science (2024)
-
Miticidal activity of Photorhabdus luminescens for controlling two spider mites, Tetranychus urticae and Tetranychus kanzawai, in Carica papaya
BioControl (2023)
-
Antiprotozoal activity of different Xenorhabdus and Photorhabdus bacterial secondary metabolites and identification of bioactive compounds using the easyPACId approach
Scientific Reports (2022)
-
Natural products from Photorhabdus and Xenorhabdus: mechanisms and impacts
Applied Microbiology and Biotechnology (2022)
-
Recombineering using RecET-like recombinases from Xenorhabdus and its application in mining of natural products
Applied Microbiology and Biotechnology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.