Abstract
Resting state functional magnetic resonance imaging studies of nocturnal enuresis have focused primarily on regional metrics in the blood oxygen level dependent (BOLD) signal ranging from 0.01 to 0.08 Hz. However, it remains unclear how local metrics show in sub-frequency band. 129 children with nocturnal enuresis (NE) and 37 healthy controls were included in this study. The patients were diagnosed by the pediatricians in Shanghai Children’s Medical Center affiliated to Shanghai Jiao Tong University School of Medicine, according to the criteria from International Children's Continence Society (ICCS). Questionnaires were used to evaluate the symptoms of enuresis and completed by the participants. In this study, fALFF, ReHo and PerAF were calculated within five different frequency bands: typical band (0.01–0.08 Hz), slow-5 (0.01–0.027 Hz), slow-4 (0.027–0.073 Hz), slow-3 (0.073–0.198 Hz), and slow-2 (0.198–0.25 Hz). In the typical band, ReHo increased in the left insula and the right thalamus, while fALFF decreased in the right insula in children with NE. Besides, PerAF was increased in the right middle temporal gyrus in these children. The results regarding ReHo, fALFF and PerAF in the typical band was similar to those in slow-5 band, respectively. A correlation was found between the PerAF value of the right middle temporal gyrus and scores of the urinary intention-related wakefulness. Results in other bands were either negative or in white matter. NE children might have abnormal intrinsic neural oscillations mainly on slow-5 bands.
Similar content being viewed by others
Introduction
Nocturnal enuresis (NE) is defined as repeatedly urinating in clothes or bed that occurs only during sleep in an individual who has reached a developmental age when urinary continence is ordinarily expected at 5 years old1,2, with a prevalence of around 5% in children between 5 and 12 years old3. Children who suffer from enuresis often experience emotional distress and have low self-esteem4,5. Previous reports have shown that maturational delay of the central nervous system is an important factor in the pathogenesis of NE6. Thus, the characterization of alterations in brain function in NE may provide an insight into understanding enuresis pathophysiology.
Resting-state functional magnetic resonance imaging (rs-fMRI) is a non-invasive technique and has been widely employed to investigate brain function7,8. The RS-fMRI bold signal includes the magnetic field information resulted from the changes of oxygen and hypoxia in hemoglobin supplied to neuron blood9. Previous research findings suggested that bold signals are significantly correlated with field potentials reflecting electrical signals in the neural networks10. In recent years, a variety of metrics has been proposed in the field of rs-fMRI that allow us to understand the spontaneous neural activity of the subject's brain from various perspectives.
Amplitudes of low-frequency fluctuations (ALFF) and Regional homogeneity (ReHo) are two metrics particularly widely used for uncovering local spontaneous neural activity in the brain and have been used in thousands of articles11,12. ReHo is used to analyze spontaneous synchronization of local activity in the brain13, whereas ALFF is used to analyze the amplitude of single voxel low frequency oscillations (amplitude)14. However, ALFF was previously found susceptible to physiological noise such as respiratory and heartbeat. Therefore, an improved ALFF algorithm named fractional amplitude of low-frequency fluctuation (fALFF) was proposed and able to be used to suppress noise and to provide a better one-sample t-test patten than ALFF15. The signal power, fALFF, normalized by the power of the whole spectrum, effectively suppresses noise, though reduces the test–retest reliability15,16. PerAF, as a latest voxel-level amplitude metric, has the better retest reliability, both intra-machine and inter-machine than ALFF and fALFF16,17. Therefore, ReHo, fALFF and PerAF were both applied in this study.
In recent years, ReHo and fALFF have been widely used in the study of various types of brain disorders. The amplitudes of low frequency fluctuations and local synchronization in the 0.01–0.08 Hz frequency band in the patients with many neuropsychiatric disorders, e.g., depression18,19, schizophrenia20,21, Alzheimer's disease22,23 and Attention Deficit–Hyperactivity Disorder (ADHD)24,25 have been found distinguishable from those in the normal controls. As most patients with enuresis are children, it is relatively difficult to collect rs-fMRI data. To our acknowledge, only a few articles focused on local spontaneous activity in NE children6,26,27, suggesting abnormalities in left inferior parietal lobule, left inferior frontal gyrus and left medial orbital superior frontal gyrus in the 0.01–0.08 Hz range in NE children compared to healthy controls (HCs).
In recent years, increasing research interests has been on the experiments with non 0.01–0.08 Hz (non-traditional frequency bands)28,29. Zuo et al. firstly attempted to divide the rs-fMRI spontaneous signal into four sub-bands [slow-5 (0.01- 0.027 Hz), slow-4 (0.027–0.073 Hz), slow-3 (0.073–0.198 Hz), and slow-2 (0.198–0.25 Hz)]30. The signals of gray matter related oscillatory amplitudes were found in slow-5 and slow-4 ranges, while that of white matter signals and high-frequency physiological noises mainly in slow-3 and slow-2. Many studies have revealed frequency-dependent abnormalities in neurological and psychiatry diseases including autism spectrum disorder31, schizophrenia32, and depression29. Given limited findings in the studies related with the band 0.01–0.08 Hz, further information may be provided sub-bands (e.g. slow-4, slow-5) otherwise.
Based on previous study, we hypothesized that there were frequency-dependent abnormalities in children with NE. Therefore, to verify the results of previous studies and examine the frequency specificity of brain activity in children with enuresis, we compared the spontaneous activities between children and HCs in the typical frequency range and four different low-frequency bands.
Besides, many studies indicated that children with NE had increased arousal level than health controls, unable to wake in sleep even though the bladder was filled33. Abnormal urinary intention-related wakefulness played an important role in the occurrence of NE34. However, there were few studies exploring the relationship between the degree of urinary intention-related wakefulness and local brain abnormalities in children with NE. Therefore, correlation analysis was performed between index of spontaneous activity of local brain regions and scores on urinary intention-related wakefulness in different bands to detect frequency-dependent abnormalities in NE children.
Results
Compared with that of previous fMRI studies on nocturnal enuresis, the sample size of this research was the largest6,27,35,36,37. There were 129 NE children and 37 HCs enrolled in this study. Necessary exclusion was made due to various reasons. For example, 5 subjects with the head movement larger than 3.0 mm of translation or 3.0° of rotation were ruled out and 8 subjects due to poor registrations. More details could be seen in Methods.
There was no significant difference in age between NE and HCs (t = 1.118, P = 0.266). However, the gender difference between the two groups was statistically significant (χ2 = 7.807, P = 0.005), as displayed in Table 1. Covariance regression was conducted in our study as reduction in the difference caused by the group gender was widely reported in previous studies38,39. Thus, gender was considered as a covariate in the regression model.
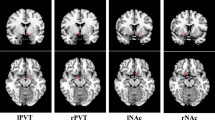
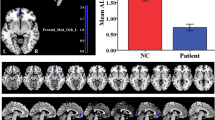
The results showed that in the typical band, ReHo increased in the left insula and the right thalamus in NE (voxel P < 0.05, cluster P < 0.05; Fig. 1A, Table 2). In addition, fALFF increased in the right insula in NE (voxel P < 0.05, cluster P < 0.05; Fig. 2A, Table 3). Moreover, PerAF increased in the right middle temporal gyrus in NE (voxel P < 0.05, cluster P < 0.05; Fig. 3A, Table 4). In slow5-band, ReHo increased in the thalamus in NE (voxel P < 0.05, cluster P < 0.05; Fig. 1B, Table 2), and fALFF increased in the superior cerebellum, superior temporal gyrus in NE (voxel P < 0.05, cluster P < 0.05; Fig. 2B, Table 3). The results in other bands were either negative or in white matter, where previously most BOLD signals were usually considered as noise and the white matter signal in the regression model. PerAF increased in left insula and temporal pole: superior temporal gyrus (voxel P < 0.05, cluster P < 0.05; Fig. 3B, Table 4). Results in other bands were either negative or in white matter (more details could be seen in supplementary materials). PerAF in the right middle temporal gyrus in typical band was positively associated with the scores of urinary intention-related wakefulness (r = 0.252; P = 0.0281; Fig. 4).
Brain regions with abnormal ReHo in typical low-frequency range and at slow-5 band in NE children. The results were corrected by GRF (voxel P < 0.05, cluster P < 0.05). More details of these regions were described in Table 2. (A) Left insula and right thalamus with increased ReHo in typical low-frequency range (0.01–0.08 Hz). B Left thalamus with increased ReHo in slow-5band (0.01-0.027 Hz).
Brain regions with abnormal fALFF in typical low-frequency range and at slow-5 band in NE children. The results were corrected by GRF (voxel P < 0.05, cluster P < 0.05). More details of these regions were described in Table 3. (A) Left insula with increased fALFF in typical low-frequency range (0.01–0.08 Hz). (B) Superior cerebellum, right rolandic operculum, superior temporal gyrus with increased fALFF in slow-5 band (0.01–0.027 Hz).
Brain regions with abnormal PerAF in typical low-frequency range and at slow-5 band in NE children. The results were corrected by GRF (voxel P < 0.05, cluster P < 0.05). More details of these regions were described in Table 4. (A) Right middle temporal gyrus with increased PerAF in typical low-frequency range (0.01–0.08 Hz). (B) Left insula, right temporal pole: middle temporal gyrus with increased PerAF in slow-5 band (0.01–0.027 Hz).
Discussion
In the present study, we analyzed the ReHo, fALFF and PerAF of children with enuresis in four sub-frequency bands and typical band, respectively. In typical band, ReHo increased in the left insula and the right thalamus in NE, and fALFF was increased in the right insula in NE. Moreover, PerAF was increased in the right middle temporal gyrus in NE. In slow-5 band, ReHo was increased in the thalamus in NE, and fALFF was increased in the superior cerebellum, superior temporal gyrus in NE. PerAF was increased in left insula and temporal pole: superior temporal gyrus. Results in other bands were either negative or in white matter, where previously most BOLD signals were usually considered to be noise and would regress to the white matter signal40,41.
The unusual fALFF and PerAF in NE existed in slow-5 band, while fALFF in slow-2 were mainly in white matter and appeared negative in other bands. Similarly, PerAF in NE children was negative in the other bands. Since the oscillations in different frequency bands reflect the characteristics of different oscillators30, the analysis in a specific sub-band was able to suggest the spontaneous activity more specifically after removing the interfering signals in other frequency bands42. Although oscillations in brain activity was mainly regarded as electrophysiological signals, it also has been used in fMRI study30,32. Based on the specificity of brain activity in NE children, frequency-specific alteration might contribute to classification of the diagnosis of NE, which has been used for many disease classification31,43.
In our study, increased fALFF was presented in the superior cerebellum of NE children in slow-5. Patients with cerebellar lesions had a hyperreflexia to urinate, possibly indicating that the cerebellum had an inhibitory effect on voiding44. However, there were few studies verifying this function in NE children. In other aspects, Yu et al. found the memory/caution factor correlated with the connectivity between cerebellum and middle frontal gyrus in children with enuresis45. In addition, structural differences in the cerebellum were also associated with attention/memory deficits in enuretic children through voxel-based morphometry (VBM) methodology46. The functional changes of cerebellum in NE children might be related to micturition function and congnitive function as well.
Both PerAF and fALFF increased in temporal lobe in NE in solw-5. Stasa D. et al. reported that temporal lobe was activated obviously during infusion of fluid in the bladder47. Lingna Zhao et al. also found that the functional connectivity between the temporal gyrus and the insula was enhanced when the bladder was full48. Detrusor overactivity was a feature of NE children49. The functional changes of the temporal lobe in NE children might be related to urinary storage dysfunctions. In the present study, PerAF value of the left insula was increased in children with NE in slow-5, suggesting that the spontaneous activity increased in insula after we calculated the percentage of BOLD fluctuations relative to the mean BOLD signal intensity for each time point and averaging across the whole time series. In previous study observed was that the insular activation increased with bladder filling so as to stir a desire to void50. The increased spontaneous overactivity and local synchronization of the insula might be an underlying mechanism for the decreased consciousness of controlling urine in NE children51. Insula was also found related with awareness, attention and motor response inhibition36,52. Consequently, functional changes in the insula might be a factor in abnormalities in the perception of urination and cognitive function in NE.
Furthermore, we also found that unusual ReHo in NE was linked with slow-5 band, while the results in slow-2 and slow-3 were mainly in white matter and negative in other bands . Oscillatory synchrony in specific frequencies were found to be related to particular cells and associated circuits and enabled the brain to be flexible so as to gear to behavioral demands53. Apparently, more studies were required to locate the particular oscillators in NE children’s brains. In the present study, higher ReHo values were presented in left thalamus among NE children, indicating that the left thalamus was a possible nidus with a possible increase in local potential54. Previous research on brains of NE children suggested not only structural but also functional changes in the thalamus. Moreover, Lei, D. et al. reported that enuretic children showed a decrease in fractional anisotropy (FA) while an increase in mean diffusivity (MD) in the thalamus, indicating developmental delay in thalamus55. In addition, the NE group showed remarkably decreased functional connectivity between thalamus and left medial superior frontal gyrus56. Furthermore, varying intra thalamic functional connectivity was found in many parts of the thalamus, during light NREM sleep57. Besides, Yu, B. et al. reported that longer light NREM sleep and higher awakening index were found related to the changed activity in thalamus57. Therefore, the developmental delay in the thalamus might lead to a compensatory increase in the functional connectivity between the voxels within it, which manifests itself macroscopically as abnormal functional connectivity in various parts of the interior thalamus55,57. The increased activity of the thalamus might block the conduction of signals in the brain network. As a result, abnormal connections to other brain areas occurred56 and eventually contributed to abnormal higher awakening index57.
In sum, despite the hypothetical brain regions for enuresis in this study, they were replicated through different metrics, e.g., fALFF and PerAF in different low-frequency band. To our best knowledge, this was the first time in children with NE and described brain abnormalities in terms of signal oscillations, whereas ReHo that did in terms of local synchrony. Functional connectivity3,57 described brain abnormalities in terms of loops and connections between brain regions. The importance of spontaneous activity within different frequency band in NE children was highlighted in our study, which might help explore more features of brain activities in NE children. Besides, we found that the right middle temporal gyrus was positively associated with degree of urinary intention-related wakefulness in typical band. Thus, the temporal lobe might be a significant brain area and call for further research.
Methods
Subjects
129 children with NE and 37 healthy controls were included in this investigation. The patients were diagnosed by pediatricians in Shanghai Children’s Medical Center affiliated to Shanghai Jiao Tong University School of Medicine, according to the criteria from International Children's Continence Society (ICCS). Nocturnal enuresis refers to intermittent incontinence while sleeping in children at least 5 years old. The exclusion criteria were: (1) any other neurological or psychiatric disorders (e.g., attention-deficit/hyperactivity disorder and autism); (2) metal implants; (3) claustrophobia; (4) IQ test scores lower than 75 (Wechsler Intelligence Scale for Children-Revised); (5) left-handedness; (6) images with inaccuracy registration; (7) head movement larger than 3.0 mm of translation or 3.0° of rotation were excluded. Healthy volunteers, recruited using advertisements, had not wet the bed since 5 years old.
There were 129 NE children from outpatient clinics and 37 healthy controls enrolled for this study from April 2016-August 2017. After ruling out those who met at least one of the exclusion criteria, eventually 76 children with NE and 30 normal children participated in this study. All of the exclusions were objective and indispensable, shown as follows:
-
1.
Five enuretic children with the head movement larger than 3.0 mm of translation or 3.0° of rotation were excluded, because their head motion had been shown to have systematic effects on fMRI data. According to previous studies, in order to mitigate the effect of head movement, head movement larger than 3.0 mm of translation or 3.0° of rotation had been routinely removed during fMRI.
-
2.
One subject in NE group did not meet the criteria, due to wetting bed once per year. According to the criteria from International Children's Continence Society (ICCS), nocturnal enuresis is defined as intermittent incontinence at least once a month and lasting for more than 3 months while sleeping, after the age of 5 years.
-
3.
One NE child was left-handed and thus excluded in this study. In fMRI studies, the identical brain regions were structurally different between left-handed and right-handed people58. In the task status, different brain regions were activated between the left-handed and right-handed59. Therefore, left-handedness and right-handedness needed to be considered in the studies regarding fMRI. Generally, the subjects in previous studies were right-handed, while the left-handed excluded, representing the majority of the populations.
-
4.
Three subjects were uncooperative with only structures scanned but no functional images obtained. Demographic data of forty NE children were not provided due to confidentiality.
-
5.
In addition, we found that one NE child had unclear T1 images and one NE child and eight healthy participants had incomplete registration.. Consequently, they were excluded from the study.
All the participants were interviewed and filled out a questionnaire that required demographic information and clinical history. In this questionnaire, the frequency of NE (0 for no enuresis; 1 for 1–3 times a month; 2 for once a week; 3 for twice a week; 4 for three times a week; 5 for four times a week; 6 for five times a week; 7 for six times a week; 8 for once per day; 9 for twice per night; 10 for three times per night; 11 for four times per night; 12 for five times per night; 13 for above five times per night) and urinary intention-related wakefulness (0 for no enuresis; 1 for wake-up after urinating less than half of urine volume; 2 for wake-up after urinating more than half of urine volume; 3 for wake-up after emptying the bladder; 4 for inability to wake up after emptying the bladder) were collected. (More details could be found in supplementary materials).
The protocol for this research had been approved by the Ethics Committees of Shanghai Children's Medical Center and was consistent with the provisions of the Declaration of Helsinki and relevant policies in China. All of the participants and their caregivers provided written informed consent before the investigation. Besides, they were informed that the whole survey involvement would be confidential and voluntary and reassured about their right to withdraw from the survey at any time.
Data collection
The rs-fMRI scanning parameters were repetition time (TR) = 2000 ms; echo time (TE) = 30 ms; flip angle = 90°; FOV = 22 × 22 cm2; acquisition matrix = 64 × 64; voxel size = 3.4 × 3.4 × 3 mm3; slice number = 32, volume number = 210, scan time = 420 s. T1- weighted image acquisition for all individuals, the imagining parameters are: TR = 440 ms; TE = 2.46 ms; flip angle = 90°; acquisition matrix = 256 × 320; FOV = 22 × 22 cm2. During the scan, the subjects were instructed to keep eyes closed.
Data preprocessing
Preprocessing of resting-state functional data was performed using the DPABI toolbox60 in MATLAB R2017b (MathWorks, Natick, Mass). The first 10 time points of each functional time series were discarded for magnetization equilibrium and participant adaptation. Slice timing correction and realignment were applied to the remaining volumes. Afterward, the corrected functional images were spatially normalized to the standard Montreal Neurological Institute (MNI) space with a resampled voxel size of 3 × 3 × 3 mm3 by using unified segmentation to the T1 image. 5 subjects with the head movement larger than 3.0 mm of translation or 3.0° of rotation and 8 subjects with poor registrations were ruled out from the study. Linear trends within the time series were removed after spatial normalization. Then, regression models were used to to remove variances regarding head motion parameters from the time series of each voxel61. The mean value of the time series of each voxel was added back in this step. Preprocessed functional data were band-pass filtered (The fALFF was not filtered).
Data analysis
Voxel-wise whole-brain analytic metrics were applied to further data analysis. In this study, fALFF, ReHo and PerAF were calculated using RESTplus62 within five different frequency bands: typical band (0.01–0.08 Hz), slow-5 band (0.010–0.027 Hz), slow-4 (0.027–0.073 Hz), slow-3 (0.073–0.198 Hz), and slow-2 (0.198–0.25 Hz). Each metric except PerAF of each voxel was then divided by the global mean metric value of each individual for standardization purposes. Finally, the data were smoothed with a Gaussian kernel of 4 mm full width at half-maximum (FWHM).
Statistical analysis
To compare the fALFF, ReHo and PerAF maps between HCs and patients with NE respectively, two-sample t-tests were performed within the five different frequency bands mentioned above to identify the regions with significant differences. Gender was treated as covariates during the group comparisons to minimize their potential effect on our results. The resultant T-maps were corrected using the Gaussian random field (GRF) theory (voxel P < 0.05, cluster P < 0.05). Pearson correlation analysis was performed with clinical/physiological/biochemical characteristics of the patients (including frequency of nocturnal enuresis, urinary intention-related wakefulness). All the statistical analysis was performed using DPABI60.
References
Austin, P. F. et al. The standardization of terminology of lower urinary tract function in children and adolescents: update report from the Standardization Committee of the International Children’s Continence Society. J. Urol. 191, 1863 (2014).
Austin, P. F. et al. The standardization of terminology of lower urinary tract function in children and adolescents: Update report from the standardization committee of the International Children’s Continence Society. Neurourol. Urodyn. 35, 471 (2016).
Zhang, A. et al. Nocturnal enuresis in obese children: a nation-wide epidemiological study from China. Sci. Rep. 9, 8414 (2019).
Cortes, E., Sahai, A., Pontari, M. & Kelleher, C. The psychology of LUTS: ICI-RS 2011. Neurourol. Urodyn. 31, 340 (2012).
Schulpen, T. W. The burden of nocturnal enuresis. Acta Paediatr. 86, 981 (1997).
Lei, D. et al. Spontaneous brain activity changes in children with primary monosymptomatic nocturnal enuresis: a resting-state fMRI study. Neurourol. Urodyn. 31, 99 (2012).
Bi, X. A., Liu, Y., Xie, Y., Hu, X. & Jiang, Q. Morbigenous brain region and gene detection with a genetically evolved random neural network cluster approach in late mild cognitive impairment. Bioinformatics 36, 2561 (2020).
Bi, X. A., Hu, X., Wu, H. & Wang, Y. Multimodal data analysis of Alzheimer’s disease based on clustering evolutionary random forest. IEEE J. Biomed. Health Inform. 24, 2973 (2020).
Ogawa, S. & Lee, T. M. Magnetic resonance imaging of blood vessels at high fields: In vivo and in vitro measurements and image simulation. Magn. Reson. Med. 16, 9 (1990).
Logothetis, N. K., Pauls, J., Augath, M., Trinath, T. & Oeltermann, A. Neurophysiological investigation of the basis of the fMRI signal. Nature 412, 150 (2001).
Zhu, J., Zhao, Y. P. & Zhang, Y. Q. The rs-fMRI study of effects of fornix and hippocampus-related brain function after the transcallosal interforniceal approach. Brain Res. Bull. 150, 207 (2019).
Qing, Z. et al. The impact of spatial normalization strategies on the temporal features of the resting-state functional MRI: Spatial normalization before rs-fMRI features calculation may reduce the reliability. Front. Neurosci. 13, 1249 (2019).
Zang, Y., Jiang, T., Lu, Y., He, Y. & Tian, L. Regional homogeneity approach to fMRI data analysis. Neuroimage 22, 394 (2004).
Zang, Y. F. et al. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 29, 83 (2007).
Zou, Q. H. et al. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: Fractional ALFF. J. Neurosci. Methods 172, 137 (2008).
Jia, X. Z. et al. Percent amplitude of fluctuation: A simple measure for resting-state fMRI signal at single voxel level. PLoS ONE 15, e227021 (2020).
Zhao, N. et al. Intra- and inter-scanner reliability of voxel-wise whole-brain analytic metrics for resting state fMRI. Front. Neuroinform. 12, 54 (2018).
Mao, N. et al. Aberrant Resting-State Brain Function in Adolescent Depression. Front. Psychol. 11, 1784 (2020).
Egorova, N., Veldsman, M., Cumming, T. & Brodtmann, A. Fractional amplitude of low-frequency fluctuations (fALFF) in post-stroke depression. Neuroimage Clin. 16, 116 (2017).
Lian, N. et al. A comparative study of magnetic resonance imaging on the gray matter and resting-state function in prodromal and first-episode schizophrenia. Am. J. Med. Genet. B Neuropsychiatr. Genet. 177, 537 (2018).
Wang, S. et al. Abnormal regional homogeneity as a potential imaging biomarker for adolescent-onset schizophrenia: A resting-state fMRI study and support vector machine analysis. Schizophr Res. 192, 179 (2018).
Tu, M. C. et al. Attention and functional connectivity among patients with early-stage subcortical ischemic vascular disease and Alzheimer’s disease. Front Aging Neurosci. 12, 239 (2020).
Yang, L. et al. Frequency-dependent changes in fractional amplitude of low-frequency oscillations in Alzheimer’s disease: a resting-state fMRI study. Brain Imaging Behav. 14, 2187 (2020).
Cheng, W., Ji, X., Zhang, J. & Feng, J. Individual classification of ADHD patients by integrating multiscale neuroimaging markers and advanced pattern recognition techniques. Front Syst. Neurosci. 6, 58 (2012).
Wang, J. B. et al. Inconsistency in Abnormal Brain Activity across Cohorts of ADHD-200 in Children with Attention Deficit Hyperactivity Disorder. Front. Neurosci. 11, 320 (2017).
Zhu, W. et al. Study on neuropathological mechanisms of primary monosymptomatic nocturnal enuresis in children using cerebral resting-state functional magnetic resonance imaging. Sci. Rep. 9, 19141 (2019).
Jiang, K. et al. Amplitude of low-frequency fluctuation of resting-state fMRI in primary nocturnal enuresis and attention deficit hyperactivity disorder. Int. J. Dev. Neurosci. 80, 235 (2020).
Yu, R. et al. Frequency-specific alternations in the amplitude of low-frequency fluctuations in schizophrenia. Hum. Brain Mapp. 35, 627 (2014).
Russo, D. et al. Opposing Changes in the Functional Architecture of Large-Scale Networks in Bipolar Mania and Depression. Schizophr Bull 46, 971 (2020).
Zuo, X. N. et al. The oscillating brain: Complex and reliable. Neuroimage 49, 1432 (2010).
Chen, H. et al. Multivariate classification of autism spectrum disorder using frequency-specific resting-state functional connectivity—A multi-center study. Prog Neuropsychopharmacol Biol Psychiatry 64, 1 (2016).
Zhang, Y., Yang, R. & Cai, X. Frequency-specific alternations in the moment-to-moment BOLD signals variability in schizophrenia. Brain. Imaging Behav. 15, 68 (2021).
Yeung, C. K., Chiu, H. N. & Sit, F. K. Bladder dysfunction in children with refractory monosymptomatic primary nocturnal enuresis. J. Urol. 162, 1049–1054 (1999).
Neveus, T. Pathogenesis of enuresis: Towards a new understanding. Int. J. Urol. 24, 174 (2017).
Jiang, K. et al. Degree centrality and voxel-mirrored homotopic connectivity in children with nocturnal enuresis: A functional MRI study. Neurol. India 66, 1359 (2018).
Lei, D. et al. Altered brain activation during response inhibition in children with primary nocturnal enuresis: An fMRI study. Hum. Brain Mapp. 33, 2913 (2012).
Lei, D. et al. Connectome-Scale Assessments of Functional Connectivity in Children with Primary Monosymptomatic Nocturnal Enuresis. Biomed Res. Int. 2015, 463708 (2015).
Jakicic, J. M. et al. Effect of a stepped-care intervention approach on weight loss in adults: a randomized clinical trial. JAMA 307, 2617 (2012).
Ersche, K. D. et al. Cognitive dysfunction and anxious-impulsive personality traits are endophenotypes for drug dependence. Am. J. Psychiatry 169, 926 (2012).
Ji, G. J. et al. Classification of schizophrenia by intersubject correlation in functional connectome. Hum. Brain Mapp. 40, 2347 (2019).
Li, R. et al. Abnormal dynamics of functional connectivity density in children with benign epilepsy with centrotemporal spikes. Brain Imaging Behav. 13, 985 (2019).
Knyazev, G. G. Motivation, emotion, and their inhibitory control mirrored in brain oscillations. Neurosci. Biobehav. Rev. 31, 377 (2007).
Huang, J. et al. Identifying resting-state multifrequency biomarkers via tree-guided group sparse learning for schizophrenia classification. IEEE J. Biomed. Health Inform. 23, 342 (2019).
Zago, T. et al. Cerebellar pathology and micturitional disorders: anatomotopographic and functional correlations. Arch. Ital. Urol. Androl. 82, 177 (2010).
Yu, B. et al. Aberrant whole-brain functional connectivity and intelligence structure in children with primary nocturnal enuresis. PLoS ONE 8, e51924 (2013).
Wang, M. et al. Morphometric magnetic resonance imaging study in children with primary monosymptomatic nocturnal enuresis. Front. Pediatr. 6, 103 (2018).
Tadic, S. D., Tannenbaum, C., Resnick, N. M. & Griffiths, D. Brain responses to bladder filling in older women without urgency incontinence. Neurourol. Urodyn. 32, 435 (2013).
Zhao, L., Liao, L. & Gao, Y., Brain functional connectivity during storage based on resting state functional magnetic resonance imaging with synchronous urodynamic testing in healthy volunteers. Brain Imaging Behav. https://doi.org/10.1007/s11682-020-00362-y (2020).
Yeung, C. K., Sreedhar, B., Leung, V. T. & Metreweli, C. Ultrasound bladder measurements in patients with primary nocturnal enuresis: a urodynamic and treatment outcome correlation. J. Urol. 171, 2589 (2004).
de Groat, W. C., Griffiths, D. & Yoshimura, N. Neural control of the lower urinary tract. Compr. Physiol. 5, 327 (2015).
Yeung, C. K., Diao, M. & Sreedhar, B. Cortical arousal in children with severe enuresis. N. Engl. J. Med. 358, 2414 (2008).
Grivaz, P., Blanke, O. & Serino, A. Common and distinct brain regions processing multisensory bodily signals for peripersonal space and body ownership. Neuroimage 147, 602 (2017).
Gregoriou, G. G., Paneri, S. & Sapountzis, P. Oscillatory synchrony as a mechanism of attentional processing. Brain Res. 1626, 165 (2015).
Zhong, Y. et al. Altered regional synchronization in epileptic patients with generalized tonic-clonic seizures. Epilepsy Res. 97, 83 (2011).
Lei, D. et al. Changes in the brain microstructure of children with primary monosymptomatic nocturnal enuresis: a diffusion tensor imaging study. PLoS ONE 7, e31023 (2012).
Zhang, A. et al., Functional connectivity of thalamus in children with primary nocturnal enuresis: results from a resting-state fMRI study. Brain Imaging Behav. https://doi.org/10.1007/s11682-020-00262-1 (2020).
Yu, B. et al. Abnormal thalamic functional connectivity during light non-rapid eye movement sleep in children with primary nocturnal enuresis. J. Am. Acad. Child Adolesc. Psychiatry 59, 660 (2020).
Shapleske, J., Rossell, S. L., Woodruff, P. W. & David, A. S. The planum temporale: A systematic, quantitative review of its structural, functional and clinical significance. Brain Res. Brain Res. Rev. 29, 26 (1999).
Arning, L. et al. PCSK6 VNTR polymorphism is associated with degree of handedness but not direction of handedness. PLoS ONE 8, e67251 (2013).
Yan, C. G., Wang, X. D., Zuo, X. N. & Zang, Y. F. DPABI: Data processing & analysis for (resting-state) brain imaging. Neuroinformatics 14, 339 (2016).
Friston, K. J., Williams, S., Howard, R., Frackowiak, R. S. & Turner, R. Movement-related effects in fMRI time-series. Magn. Reson. Med. 35, 346 (1996).
Jia, X. et al. RESTplus: an improved toolkit for resting-state functional magnetic resonance imaging data processing. Sci. Bull. 64, 953 (2019).
Acknowledgements
This study was funded by grants from the Science and Technology Commission of Shanghai Municipality (16411952800, 18411960200 and 20ZR1434700), the Development of Science and Technology in Pu Dong District (PKJ2017-Y06), National Natural Science Foundation of China (82001898).
Author information
Authors and Affiliations
Contributions
X.Z. and J.S. co-designed the study, completed the analysis, and drafted the manuscript; Y.L. contributed to reviewing and revising the manuscript; M.W. and X.D. participated in the acquisition of the data; J.M. and X.J. co-designed the study, reviewed and revised the manuscript. All authors reviewed the manuscript and approved the final version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zheng, X., Sun, J., Lv, Y. et al. Frequency-specific alterations of the resting-state BOLD signals in nocturnal enuresis: an fMRI Study. Sci Rep 11, 12042 (2021). https://doi.org/10.1038/s41598-021-90546-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-90546-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.