Abstract
Tuberculosis (TB) is still a major worldwide health threat and primarily a lung disease. The innate immune response against Mycobacterium tuberculosis (Mtb) is orchestrated by dendritic cells, macrophages, neutrophils, natural killer cells and apparently mast cells (MCs). MCs are located at mucosal sites including the lungs and contribute in host-defence against pathogens, but little is known about their role during Mtb infection. This study investigates the location and characteristics of MCs in TB lesions to assess their contribution to TB pathology. To this purpose, number, location and phenotype of MCs was studied in 11 necropsies of pulmonary TB and 3 necropsies of non-TB infected lungs that were used as controls. MCs were localised at pneumonic areas, in the granuloma periphery and particularly abundant in fibrotic tissue. Furthermore, MCs displayed intracellular Mtb and IL-17A and TGF-β immunostaining. These findings were validated by analysing, post-mortem lung tissue microarrays from 44 individuals with pulmonary TB and 25 control subjects. In affected lungs, increased numbers of MCs expressing intracellularly both tryptase and chymase were found at fibrotic sites. Altogether, our data suggest that MCs are recruited at the inflammatory site and that actively produce immune mediators such as proteases and TGF-β that may be contributing to late fibrosis in TB lesions.
Similar content being viewed by others
Introduction
Tuberculosis (TB) caused by Mycobacterium tuberculosis (Mtb), remains one of the deadliest bacterial infections worldwide1. During infection, Mtb reaches the lungs where different innate immune cells reside including mast cells (MCs)2,3,4. Mtb is phagocytosed by macrophages leading to the release of diverse cytokines, including TNFα and IL-6 that drive the inflammatory process5. To control Mtb spread, innate and adaptive immune cells surround infected phagocytic cells promoting granuloma formation5,6. The cytokines IL-17A and TNF-α are known to contribute to the process7. When granuloma containment fails, different lung injuries including pneumonia, bronchitis, caseous necrosis and eventually fibrosis prevail8,9. The fibrotic process in human lungs has been associated with the presence of TGF-β, and the proteases, tryptase and chymase10,11. The final TB pathology phase culminates in irreversible lung tissue damage manifested by necrosis and fibrosis8,9. Although many cells are involved in this process, little is known about the contribution of MCs in this pathology.
MCs are distributed in lungs and mucosal tissues and contribute to host-defence against bacterial infections12. In humans, MCs are classified as tryptase + MCs (MCT), chymase + MCs (MCC) or both tryptase + and chymase + MCs (MCTC)13. Upon bacterial exposure, they release a wide variety of cytokines and chemokines, including IL-17, TNF-α, IL-8 and TGF-β either by degranulation or canonical secretory pathways14. Additionally, during lung infections MCs are altered in numbers, phenotype and localization15,16. For instance, MC numbers are decreased in lungs of Streptococcus pneumoniae infected patients17. Furthermore, MCs are capable to phagocytose bacteria and present antigens18. Although hypothetically MCs have an important role in TB3, little has been explored. For instance, it is unknown if MCs are localized at pulmonary tuberculous human lesions and what is the predominant MC phenotype. It is also unclear whether MC cytokines, e.g. IL-17A, and TGF-β contribute to the inflammatory process, and formation and maintenance of granulomas and fibrogenesis.
In this study we examined number, distribution and phenotype of MCs in human TB-infected lungs and MC cytokine expression at granulomas and fibrotic sites in TB-infected lung tissue. Our descriptive findings demonstrated that MCs are likely to participate to the early inflammatory phase, as well as to the late fibrosis formation during TB pathology.
Materials and methods
Ethical statement and tissue procurement
All methods were performed in accordance to relevant guidelines and regulations. Lung tissue sections from 11 necropsies of deceased TB patients and 3 controls from non-TB infected necropsies were obtained from the Pathology Department files at the National Institute of Medical Sciences and Nutrition “Salvador Zubiran” in Mexico City. A specific informed consent was not required for this study, but every medical autopsy was performed with a signed authorization of the representative legal family member and tissue samples were obtained during legally authorized autopsies with signed permission by a relative who agreed tissue sample donation for the present and previous studies19. The microarray tissue study from 44 individuals with pulmonary TB and 25 control subjects were taken from the Pathology Department of the General Hospital of Mexico ‘Eduardo Liceaga’ at Mexico City, and ethical statement was approved by the local ethic committee of the Hospital Infantil de Mexico, Federico Gomez (No. HIM/2008/015). This study including all experimental protocols were approved by the in-house ethical committee at the National Institute of Medical Sciences and Nutrition “Salvador Zubiran”.
TB tissue processing
Macroscopically, lung tissue from 11 necropsies of deceased TB patients showed extensive cavitary bilateral lung disease, surrounded by numerous white nodules with irregular shapes and size that alternated with pneumonia patches. Extensive sampling was performed, obtaining several tissue fragments from different lesions and were embedded in paraffin blocks sectioned at 3 µm and mounted in glass slides. Additionally, we used post-mortem lung tissue, essentially granulomas, from 44 individuals with pulmonary TB and 25 control subjects (subjects who died as a result of any other cause without significant pulmonary disease). The lung tissues (TB and control tissue samples) were organized in a tissue microarray (MTA) as previously described20. One sample of each section (lung sections and MTA) were stained with haematoxylin and eosin (HE) to select lung lesions for study. Spare sections were left at room temperature before being used for immunoperoxidase and immunofluorescence staining. The lung tissues in this study correspond to active TB cases, not latent TB cases were included.
Immunoperoxidase staining
Lung sections were deparaffinized and treated with antigen retriever (1X; Bio SB, Santa Barbara, California) for 5 min under microwave heating. Endogenous peroxidase was blocked incubating tissue with methanol-H2O2 (9:1) for 10 min. After three washes, unspecific sites were blocked using a background sniper (BIOCARE MEDICAL; Pacheco, California) for 30 min. Slides were washed and incubated with either a rabbit anti-human chymase antibody (Ab186417, Abcam; Cambridge, United Kingdom) or a mouse anti-tryptase antibody (Ab2378, Abcam; Cambridge, United Kingdom) for 2 h. After three washes, tissue was processed using a mouse/rabbit PolyDetector DAB (3–3′-diaminobenzidine)/HRP (horseradish peroxidase) brown detection system (BSB0219, Bio SB; Santa Barbara, California) following manufacturer´s instructions. Micrographs were acquired using a LEICA DMLS microscope with a 2.5X and 40X dry objectives equipped with a LEICA DFC295 camera and analysed using an automated image analyser (QWin Leica; Wetzlar, Germany).
Immunofluorescence staining
To visualize MC phenotypes, lung tissue sections were deparaffinized, treated with DNA retriever (1X; Bio SB; Santa Barbara, California) for 5 min under heating and incubated with blocking buffer (goat serum 1:10 in PBS + tween 0.1%) for 30 min. After three washes, tissue were incubated with a rabbit anti-human chymase antibody (Ab186417, Abcam; Cambridge, United Kingdom), and a mouse anti-tryptase antibody (Ab2378, Abcam; Cambridge, United Kingdom) for 1 h followed by 1 h incubation with a goat anti-mouse antibody conjugated to Alexa 488 fluorophore (Ab150117, Abcam; Cambridge, United Kingdom) and a goat anti-rabbit antibody conjugated to Alexa 647 fluorophore (Ab150083, Abcam; Cambridge, United Kingdom). After three washes, tissue was mounted using a fluoroshield mounting media containing 4′,6-diamidino-2-phenylindole (DAPI, Abcam; Cambridge, United Kingdom). Slides were analysed using a fluorescent microscope OlympusBX41 with either 40 × and 10 × dry objectives. Images were acquired using a Zen 2.6 blue and analysed using Fiji.
To analyse cytokine expression and Mtb internalization, lung sections were deparaffinized, treated with DNA retriever (1X; Bio SB; Santa Barbara, California) for 5 min under heating and incubated with blocking buffer (goat serum 1:10 in PBS + tween 0.1%) for 30 min. After three washes, tissues were incubated with a mouse anti-tryptase antibody (Ab2378, Abcam, Cambridge, United Kingdom) and either a rabbit polyclonal anti-Mtb (CP140C, BioCare Medical), capable to recognize diverse Mtb-cell wall and secreted antigens, rabbit anti-TGF-β (Jackson Immunoresearch, Cambridge, United Kingdom) or rabbit anti-IL-17 (SC7927 Santa Cruz Lab, USA) antibodies for 1 h followed by 1 h incubation with a goat anti-mouse antibody conjugated to either Alexa 488 (Ab150117, Abcam; Cambridge, United Kingdom) or Alexa 647 fluorophores (Ab150115, Abcam; Cambridge, United Kingdom) and a goat anti-rabbit antibody conjugated to either Alexa 647 (Ab150083, Abcam; Cambridge, United Kingdom) or Alexa 488 fluorophores (Ab150081, Abcam; Cambridge, United Kingdom). After three washes, tissue was mounted using a fluoroshield mounting media containing 4′,6-diamidino-2-phenylindole (DAPI, Abcam; Cambridge, United Kingdom). Slides were analysed using a confocal microscope LSM 710 DUO, Carl Zeiss.
MC quantification
Forty-four MTAs from TB-infected lung sections from autopsy cases and 22 non-TB infected controls contained in 4 different slides were stained with HE and analysed. Five MTAs were selected as control lung tissue and 10 MTAs presenting fibrosis were selected as representative fibrotic tissue. Selected MTAs were immunostained with tryptase and chymase (as described above) and studied at 10 × magnification using a fluorescent microscope OlympusBX41 and acquired using Zen 2.6 blue software system. One high power field was taken for each MTA and all single positive (MCC or MCT) and double positive (MCTC) cells were counted per field using Fiji. MC numbers were graphed using GraphPrism.
Statistical analysis
A Shapiro–Wilk tests was performed to determine normality during phenotype quantification. Statistical analysis was achieved using Kruskal–Wallis test and a Dunn’s multiple comparison post-test (adjusted p ≤ 0.01) using GraphPad Prism 8th edition.
Compliance with Ethical standards
Authors declare that no conflict of interest happened during the present study. The present research involved the use of lung sections obtained from deceased individuals. Autopsies were selected from the Pathology Department files at the National Institute of Medical Sciences and Nutrition “Salvador Zubiran” in Mexico City. A specific informed consent was not required for this study, but every medical autopsy was performed with a signed authorization of the representative legal family member and tissue samples were obtained during legally authorized autopsies with signed permission by a relative who agreed tissue sample donation for this and previous studies19. Additional tissue was obtained from the Pathology Department of the General Hospital of Mexico ‘Eduardo Liceaga’ at Mexico City, and ethical statement was approved by the local ethic committee of the Hospital Infantil de Mexico, Federico Gomez (No. HIM/2008/015).
Experimental procedures
All methods were performed in accordance to relevant guidelines and regulations. This study including all experimental protocols were approved by the in-house ethical committee at the National Institute of Medical Sciences and Nutrition “Salvador Zubiran”.
Results
Tryptase positive mast cells are the most abundant phenotype in non-TB infected human lungs
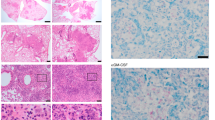
In physiological conditions MCs expressing either tryptase or chymase or both proteases reside in alveolar parenchyma. However, in pulmonary infections MC numbers and phenotype are altered21. To investigate lung MC distribution and their characteristics we studied their number, location and phenotype in autopsies from control lungs (non-TB infected). Control cases had heart attacks as death cause with lungs showing overall a normal structure with some focal patches of centrilobular emphysema (Fig. 1A). These tissues showed MCT (Fig. 1B) and lesser MCC (Fig. 1C) at alveolar walls. Both MCT and MCC were preferentially located in blood vessels adventitia. Moreover, MCT were the most abundant phenotype (median = 5 cells per field) (Fig. 1D) followed by MCTC (median = 2 cells per field), whereas MCC were not detected. In fact, all MCC observed were also tryptase + therefore MCTC (Fig. 1E). Thus, MCs expressing only chymase were rare whereas tryptase positive MCs were predominant in human lung parenchyma.
Tryptase positive mast cells are predominant in non-tuberculosis infected human lung tissue. (A) Representative micrograph HE staining of lung section from non-TB infected lung autopsy (cause of death heart attack) showing preserved alveolar structure. (B) Tryptase Immunostaining shows several positive MCs in the alveolar-capillary interstitium and in the adventitia layer of blood vessels. (C) Chymase immunostaining shows occasional positive cells. (D) Predominance of tryptase + MCs in non-tuberculous lung tissue was analysed by morphometry in five microarrays. (E) Representative immunofluorescence microarray studied with fluorescent microscopy shows MCT (green), MCC (red) and MCTC (merge) surrounding a blood vessel. Shapiro–Wilk test was done to determine normality. Statistical comparison was performed using Kruskal–Wallis test and Dunn´s multiple comparison post-test (adjusted p ≤ 0.01).
Mast cells are located at active inflammatory sites of TB-infected lungs and show intracellular mycobacterial antigens
MC numbers, location and phenotype found in non-infected lungs were used as controls to investigate MC characteristics in TB lung lesions. A table summarizing clinical data from the studied TB active necropsies is shown in Supplemental Table 1. Both MC phenotypes (MCT and MCC) were abundant at inflammatory (granulomas, pneumonia, vascular and airways walls) and fibrotic areas (Fig. 2), but absent in the proximity of necrotic sites (Supplemental Fig. 1). At pneumonic areas (Fig. 2A), numerous MCs were seen in alveolar walls and alveolar lumen (Fig. 2B,C), while in blood vessels (Fig. 2D) MCs were found in the adventitia and in bronchial airways (Fig. 2E,F). Both phenotypes were positioned below the epithelium, in the submucosa and in the muscular wall between smooth muscle cells. Furthermore, as shown in Fig. 2G, MCT stationed in inflammatory regions of TB-infected lung sections showed vacuoles containing Mtb antigens. Thus, both MCT and MCC reside in TB lung lesions and store Mtb.
Tryptase and chymase positive mast cells are located at active inflammatory lesions and show intracellular mycobacterial antigens. (A) Representative HE micrograph showing areas of tuberculous pneumonia. (B) Tryptase + MCs are present in alveolar walls and lumen. (C) Chymase + MCs show similar numbers and distribution as tryptase + MCs in the pneumonic areas. (D) Extensive inflammatory infiltrate is detected in bronchial walls. (E) Numerous tryptase + MCs are located in the bronchial submucosa. (F) Numerous chymase + MCs are in bronchial walls and neighbour alveoli. Micrographs are representative of 11 necropsies of TB patients. (G) A representative section with extensive inflammation in pneumonic areas was incubated with polyclonal anti-Mtb (Alexa 488 label) and anti-tryptase antibodies (Alexa 647 label). High power micrograph shows a MC expressing tryptase that colocalize with Mtb antigens.
Mast cells are located at the periphery of granulomas and express IL-17
Granulomas are characterized by a necrotic core containing Mtb surrounded by macrophages and lymphocytes and a fibrotic external layer20. As shown in Fig. 3, granulomas at different stages were analysed. In early or incipient granulomas (Fig. 3A), characterized by small nodular congregates of inflammatory cells, occasional MCs were seen intermixed with lymphocytes and macrophages (Fig. 3B,C). However, MCs were not observed to infiltrate typical or mature well-organized granulomas (Fig. 3D) but were located at their periphery (Fig. 3E,F). Indeed, MCs were abundant at the fibrotic outer layer of necrotic granulomas (Fig. 3F). Representative TB-infected tissue containing typical granulomas or incipient granulomas was incubated with anti-IL-17A and anti-tryptase antibodies followed by a fluorescent staining. As shown in Fig. 3G, IL-17 positive MCs were observed at neighbour inflammatory tissue near to typical granulomas or mixed with inflammatory cells in incipient granulomas. Thus, MCs are virtually absent inside mature granulomas but located at their periphery and expressing IL-17.
Mast cells are observed in granulomas and express IL-17A. Representative micrographs of different types of granulomatous lesions (incipient, mature or necrotic) were stained with HE or with anti-human anti-tryptase or anti-chymase immunoperoxidase antibodies to identify MCT or MCC. (A) Small incipient granuloma. Both MC phenotypes (B) MCT and (C) MCC are detected within the inflammatory area of incipient granulomas. (D) Mature granuloma with central necrosis. (E) MCT and MCC (F) are not infiltrating mature granulomas but both subtypes are observed in the periphery. Micrographs are representative of 11 TB necropsies. Representative section of incipient granuloma was incubated with anti-IL-17A (Alexa 488 label) and anti-tryptase antibodies (Alexa 647 label). (G) Low power micrograph shows numerous IL-17 + cells, and MCT. High power micrograph of the inset shows MCT staining positive for IL-17A.
Mast cells are in high numbers in fibrotic tissue that surrounds granulomas and cavitary lesions
MCs were constantly detected at fibrotic areas around granulomas or wall cavities supporting a number of studies that have shown MCs participate in the fibrotic process22,23. To characterize the nature of the MCs residing at fibrotic areas of TB infected tissues we determined their proteases expression. Although MCT, MCC and MCTC phenotypes were all seen in fibrotic areas (Fig. 4A), MCTC were the most abundant (median = 8.5 cells per field), followed by MCT (median = 2 cells per field) (Fig. 4B). Since non-TB infected lung controls are colonised by MCT (Fig. 1D), our data suggests a switch in proteases expression with an increase of chymase at TB-induced healing and fibrotic sites. Furthermore, by a double immunofluorescence labelling for TGF-β and tryptase, we could demonstrate that some MCs in fibrotic tissue not only express TGF-β (Fig. 4D) but exhibit a conserved but disorganized granular content suggesting their activation or partial degranulation (Fig. 4C). Thus, MCs at TB-induced lung lesions show an increased expression of chymase and pro-fibrogenic TGF-β and both may contribute to the fibrotic process.
Tryptase and chymase positive mast cells are numerous in fibrotic lesions of tuberculous lungs. (A) Representative low power micrograph of fibrotic tuberculous nodule stained with HE and immunofluorescence micrographs from the same lesion shows MCT (green), MCC (red) and MCTC (merge). (B) MCs subtypes counted in ten different high-power fields (microarrays) (40 ×) from 44 autopsy cases confirm that MCTC were the most abundant phenotype at fibrotic areas. (C) High power micrograph at fibrotic areas shows MCs that express tryptase and chymase cytoplasmic granules which are partially degranulated. Shapiro–Wilk test was done to determine normality. Representative section of a fibrotic area was incubated with anti-TGF-β (Alexa 647 label) and anti-tryptase antibodies (Alexa 488 label). (D) High power micrograph shows TGF-β colocalization with MCT. Statistical comparison was performed using Kruskal–Wallis test and Dunn´s multiple comparison post-test (adjusted *p ≤ 0.01).
Discussion
Although MCs may play a role in TB infection3, little has been explored and the available studies were performed in rodent models. Furthermore, considering the differences in MC numbers and phenotypes between mouse and human lungs it is unclear whether the described findings are relevant for human pathology21. Our study demonstrates that in TB-infected human lungs MCs are located at pneumonic areas, in proximity to granulomas and particularly abundant at fibrotic sites. Besides, we observed a MC colocalization with Mtb antigens indicating an intracellular localisation of the pathogen. In addition, MCs located in proximity to granulomatous lesions were found to express IL-17, while TGF-β positive MCs were found embedded in the fibrotic tissue.
Our observations in lung controls showing MCs surrounding blood vessels and throughout the alveolar-capillary interstitial tissue with MCT as the most abundant population are in line with current evidence that shows MCT as predominant phenotype in large airways including bronchial and alveolar, followed by MCTC which are more common at blood vessels, whereas MCC are rare or sporadically observed in human lungs24,25. Although it is not clearly understood yet, this suggests that MCT reside in the alveolar parenchyma prone to activation or differentiation upon environmental stimulus16.
Since MCs release a wide variety of cytokines, chemokines and antimicrobial molecules, they are likely to be involved in TB pathogenesis at different stages3. For instance, since our findings show that MCs locate at inflammatory areas of TB-infected human lungs, here MCs may contribute to the initial TB inflammatory stage by releasing TNF-α, IL-6, MCP-1, IL-1β, GM-CSF and IL-8. Muñoz et al. support this concept by showing the release of MC-dependent TNF and IL-6 upon Mtb infection26,27. Furthermore, the ability of MCs to contribute to immune cell recruitment was observed in Chlamydia pneumoniae lung infection characterised by a MC-dependent cellular infiltration in the lung airways12,28,29,30. Instead, the appearance of MCs at incipient granulomas and surrounding mature granulomas suggests their contribution in orchestrating granuloma formation and maintenance potentially by the secretion of IL-17, IL-6, IL-8, MCP-1, IL-10, IFN-γ and IL-1β31,32. However, MC mediators including TGF-β, IL-33, chymase and IL-1β are known to induce excessive inflammation and fibrosis and have detrimental effects31. Thus, suppressing MC-mediators release, e.g.IL-1β and TGF-β may diminish MC activation33 as proposed in SARS-COV2 infection34,35 and serve to prevent TB fibrosis.
In our study, we observe that human-lung resident MCs display intracellular Mtb fragments. This is in line with reported data26,27 showing MCs able to uptake Mtb via membrane binding through lipid rafts and CD48 receptor engagement27. Although MCs are not professional phagocytic cells, they are known to phagocytose36 and kill bacteria via acidified vacuoles37. These findings would support the role of MCs in uptaking and eliminating Mtb through phagocytosis or serve as a reservoir of intracellular Mtb during the active phase of TB infection. Although is not within the scope of this study, further research is needed to investigate whether MCs contribute to T cell activation as described in other conditions36,38,39 via Mtb-antigen presentation.
Mtb persistence promotes an ongoing cellular recruitment40 that results in the formation of early granulomas6,40. The association between MCs and TB-induced granulomas is still controversial. A positive correlation between MCs and granuloma formation was observed in tuberculous lymphadenitis tissue41 but not in tuberculous liver tissue42. Our data demonstrate that MCs infiltrate incipient granulomas and locate at the periphery or close proximity in mature or necrotic granulomas. Since different immune cells including T lymphocytes are part of the granuloma structure, a direct interaction between MCs and T cells is likely to occur at this site. MC cytokines including IL-17, and TNF-α are necessary for granuloma maturation and maintenance in mouse TB infection43. Although MC-TNF-α association was not observed (data not shown), we showed IL-17A positive MCs in the periphery of granulomas. Using an IL-17A gene-knockout mouse model, Okamoto-Yoshida et al. reported that IL-17A is necessary for granuloma maturation with γδ T cells as the major IL-17 source44. Also, granuloma formation was impaired in an IL-17A-deficient mouse model of sarcoidosis45. Therefore, we would like to propose the concept that MCs contribute to the initial step of cellular recruitment at the infection site, remain outside the inflammatory core during the adaptive immune stage, and orchestrate granuloma maturation via IL-17 expression.
In severe and chronic TB infection, fibrosis is the result of excessive inflammation11. MCs are abundant in fibrotic sites in non-infectious lung diseases46, including idiopathic pulmonary fibrosis47 and cystic fibrosis48. In addition, MCs products such as the fibroblast growth factor 2 (FGF-2)49, prostaglandin E2 (PGE2)50, IL-1β51, TGF-β10,52,53, tryptase2223,54,55 and chymase47,56,57 are known to contribute to fibrogenesis50,53,58. Our study reproduces findings described in idiopathic pulmonary fibrosis where MCs are increased in numbers and are partially degranulated at fibrotic sites46,59. Furthermore, we found a switch in MC phenotype from MCT to MCTC in fibrotic areas. In line with this, Andersson et al., correlated high MCTC numbers with lung function, tissue remodelling and TGF-β1 expression48, suggesting MCTC as important fibrosis mediators. In addition, mucosal MCs (MCT) in coculture with fibroblast lead to differentiation of MCT into connective tissue MCs (MCTC). This process is coupled with an increase in fibroblast proliferation and an enhanced collagen synthesis necessary for fibrosis generation60. Moreover, some antimicrobial drugs are known to activate MCs61 or induce differentiation62 however, none of the treatments used for the patients from the reported autopsies in this study seems to have a direct effect on MC activation or differentiation. Thus, phenotypic change from MCT to MCTC with an increase in chymase expression induced by Mtb active infection would initiate or promote fibrosis together with the release of additional fibrogenesis-specific molecules.
Conclusions
In conclusion, our results demonstrate that MCs expressing IL-17 are localizing in TB-induced human lung injuries at inflammatory sites while TGF-β positive and chymase rich MCs are stationed in the proximity of mature granulomas and embedded in fibrotic tissue. Although this is a descriptive study, our data suggest that MCs probably contribute to both, early immune cellular recruitment via IL-17A release and late fibrosis formation with differentiated MCT into MTTC.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- MCs:
-
Mast cells
- TB:
-
Tuberculosis
- MCT :
-
Mast cells expressing tryptase
- MCC :
-
Mast cells expressing chymase
- MCTC :
-
Mast cells expressing both tryptase and chymase
- Mtb:
-
Mycobacterium tuberculosis
- DC:
-
Dendritic cells
- NK cells:
-
Natural killer cells
References
Pezzella, A. T. History of pulmonary tuberculosis. Thorac. Cardiovasc. Surg. 29(1), 1–17 (2019).
Sanyaolu, A. et al. Tuberculosis: A review of current trends. EIJ 3, 000123 (2019).
Garcia-Rodriguez, K. M. et al. The role of mast cells in tuberculosis: Orchestrating innate immune crosstalk?. Front. Immunol. 8, 1290 (2017).
de Martino, M. et al. Immune response to mycobacterium tuberculosis: A narrative review. Front. Pediatr. 7, 350 (2019).
Ramakrishnan, L. Revisiting the role of the granuloma in tuberculosis. Nat. Rev. Immunol. 12(5), 352–366 (2012).
Pagán, A. J. & Ramakrishnan, L. The formation and function of granulomas. Annu. Rev. Immunol. 36, 639–665 (2018).
Shen, H. & Chen, Z. W. The crucial roles of Th17-related cytokines/signal pathways in M. tuberculosis infection. Cell. Mol. Immunol. 15(3), 216–225 (2018).
Im, J. G. et al. CT-pathology correlation of pulmonary tuberculosis. Crit. Rev. Diagn. Imaging 36(3), 227–285 (1995).
Subbian, S. et al. Lesion-specific immune response in granulomas of patients with pulmonary tuberculosis: A pilot study. PLoS ONE 10(7), e0132249 (2015).
Nakayama, S. et al. Transforming growth factor β- and interleukin 13-producing mast cells are associated with fibrosis in bone marrow. Hum. Pathol. 62, 180–186 (2017).
Wilson, M. S. & Wynn, T. A. Pulmonary fibrosis: Pathogenesis, etiology and regulation. Mucosal. Immunol. 2(2), 103–121 (2009).
Johnzon, C.-F., Rönnberg, E. & Pejler, G. The role of mast cells in bacterial infection. Am. J. Pathol. 186(1), 4–14 (2016).
Gri, G. et al. Mast cell: An emerging partner in immune interaction. Front. Immunol. 3, 120 (2012).
Krystel-Whittemore, M., Dileepan, K. N. & Wood, J. G. Mast cell: A multi-functional master cell. Front. Immunol. 6, 620 (2016).
Hsieh, F. H. et al. Human airway epithelial cell determinants of survival and functional phenotype for primary human mast cells. Proc. Natl. Acad. Sci. USA 102(40), 14380–14385 (2005).
Cruse, G. & Bradding, P. Mast cells in airway diseases and interstitial lung disease. Eur. J. Pharmacol. 778, 125–138 (2016).
van den Boogaard, F. E. et al. Mast cells impair host defense during murine streptococcus pneumoniae pneumonia. J. Infect. Dis. 210(9), 1376–1384 (2014).
Urb, M. & Sheppard, D. C. The role of mast cells in the defence against pathogens. PLoS Pathog. 8(4), e1002619 (2012).
Bini, E. I. et al. The implication of pro-inflammatory cytokines in the impaired production of gonadal androgens by patients with pulmonary tuberculosis. Tuberculosis 95(6), 701–706 (2015).
Seligson, D. et al. Expression of transcription factor Yin Yang 1 in prostate cancer. Int. J. Oncol. 27(1), 131–141 (2005).
Erjefält, J. S. Mast cells in human airways: The culprit?. Eur. Respir. Rev. 23(133), 299–307 (2014).
Abe, M. et al. Effect of mast cell–derived mediators and mast cell–related neutral proteases on human dermal fibroblast proliferation and type I collagen production. J. Allergy Clin. Immunol. 106(1), S78–S84 (2000).
Cairns, J. A. & Walls, A. F. Mast cell tryptase stimulates the synthesis of type I collagen in human lung fibroblasts. J. Clin. Investig. 99(6), 1313–1321 (1997).
Andersson, C. K. et al. Novel site-specific mast cell subpopulations in the human lung. Thorax 64(4), 297–305 (2009).
Bradding, P. Human lung mast cell heterogeneity. Thorax 64(4), 278–280 (2009).
Muñoz, S., Rivas-Santiago, B. & Enciso, J. A. Mycobacterium tuberculosis entry into mast cells through cholesterol-rich membrane microdomains. Scand. J. Immunol. 70(3), 256–263 (2009).
Muñoz, S. et al. Mast cell activation by Mycobacterium tuberculosis: Mediator release and role of CD48. J. Immunol. 170(11), 5590 (2003).
Chiba, N. et al. Mast cells play an important role in Chlamydia pneumoniae lung infection by facilitating immune cell recruitment into the airway. J. Immunol. 194(8), 3840–3851 (2015).
Abraham, S. N. & St John, A. L. Mast cell-orchestrated immunity to pathogens. Nat. Rev. Immunol. 10(6), 440–452 (2010).
Carlos, D. et al. Mast cells modulate pulmonary acute inflammation and host defense in a murine model. J. Infect. Dis. 196(9), 1361–1368 (2007).
Sasindran, S. J. & Torrelles, J. B. Mycobacterium tuberculosis infection and inflammation: What is beneficial for the host and for the bacterium?. Front. Microbiol. 2, 2–2 (2011).
Silva Miranda, M. et al. The Tuberculous Granuloma: An Unsuccessful host defence mechanism providing a safety shelter for the bacteria?. Clin. Dev. Immunol. 2012, 139127 (2012).
Gallenga, C. E. et al. Interleukin-1 family cytokines and mast cells: Activation and inhibition. J. Biol. Regul. Homeost. Agents 33(1), 1–6 (2019).
Conti, P. et al. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): Anti-inflammatory strategies. J. Biol. Regul. Homeost. Agents 34(2), 327–331 (2020).
Conti, P. et al. How to reduce the likelihood of coronavirus-19 (CoV-19 or SARS-CoV-2) infection and lung inflammation mediated by IL-1. J. Biol. Regul. Homeost. Agents 34(2), 333–338 (2020).
Galli, S. J. & Gaudenzio, N. Human mast cells as antigen-presenting cells: When is this role important in vivo?. J. Allergy Clin. Immunol. 141(1), 92–93 (2018).
Malaviya, R. et al. Mast cell phagocytosis of FimH-expressing enterobacteria. J. Immunol. 152(4), 1907–1914 (1994).
Katsoulis-Dimitriou, K. et al. Mast cell functions linking innate sensing to adaptive immunity. Cells 9, 12 (2020).
Stelekati, E. et al. Mast cell-mediated antigen presentation regulates CD8+ T cell effector functions. Immunity 31(4), 665–676 (2009).
Ehlers, S. & Schaible, U. E. The granuloma in tuberculosis: Dynamics of a host-pathogen collusion. Front. Immunol. 3, 411–411 (2013).
Taweevisit, M. & Poumsuk, U. High mast cell density associated with granulomatous formation in tuberculous lymphadenitis. Southeast Asian J. Trop. Med. Public Health 38(1), 115 (2007).
Celasun, B., Crow, J. & Scheuer, P. J. Mast cells in granulomatous liver disease. Pathol. Res. Pract. 188(1–2), 97–100 (1992).
Carlos, D. et al. TLR2-dependent mast cell activation contributes to the control of Mycobacterium tuberculosis infection. Microbes Infect. 11(8), 770–778 (2009).
Okamoto Yoshida, Y. et al. Essential role of IL-17A in the formation of a mycobacterial infection-induced granuloma in the lung. J. Immunol. 184(8), 4414–4422 (2010).
Jiang, D. et al. Inhibited IL-17A ameliorates sarcoid-like granulomatosis in mice. Eur. Respir. J. 46(suppl 59), 3310 (2015).
Pesci, A. et al. Mast cells in fibrotic lung disorders. Chest 103(4), 989–996 (1993).
Hirata, K. et al. Enhanced mast cell chymase expression in human idiopathic interstitial pneumonia. Int. J. Mol. Med. 19(4), 565–570 (2007).
Andersson, C. K. et al. Activated MCTC mast cells infiltrate diseased lung areas in cystic fibrosis and idiopathic pulmonary fibrosis. Respir. Res. 12(1), 139 (2011).
Inoue, Y. et al. Human mast cell basic fibroblast growth factor in pulmonary fibrotic disorders. Am. J. Pathol. 149(6), 2037–2054 (1996).
Bradding, P. & Pejler, G. The controversial role of mast cells in fibrosis. Immunol. Rev. 282(1), 198–231 (2018).
Conti, P. et al. IL-1 induces throboxane-A2 (TxA2) in COVID-19 causing inflammation and micro-thrombi: Inhibitory effect of the IL-1 receptor antagonist (IL-1Ra). J. Biol. Regul. Homeost. Agents 34(5), 1623–1627 (2020).
Yue, X., Shan, B. & Lasky, J. A. TGF-β: Titan of lung fibrogenesis. Curr. Enzyme Inhibit. 6(2), 67 (2010).
Overed-Sayer, C. et al. Are mast cells instrumental for fibrotic diseases?. Front. Pharmacol. 4, 174 (2014).
Bagher, M. et al. Mast cells and mast cell tryptase enhance migration of human lung fibroblasts through protease-activated receptor 2. Cell Commun. Signal 16(1), 59–59 (2018).
Wygrecka, M. et al. Mast cells and fibroblasts work in concert to aggravate pulmonary fibrosis: Role of transmembrane SCF and the PAR-2/PKC-α/Raf-1/p44/42 signaling pathway. Am. J. Pathol. 182(6), 2094–2108 (2013).
Tomimori, Y. et al. Involvement of mast cell chymase in bleomycin-induced pulmonary fibrosis in mice. Eur. J. Pharmacol. 478(2), 179–185 (2003).
Matsumoto, T. et al. Chymase inhibition prevents cardiac fibrosis and improves diastolic dysfunction in the progression of heart failure. Circulation 107(20), 2555–2558 (2003).
Hügle, T. Beyond allergy: the role of mast cells in fibrosis. Swiss Med. Wkly. 144, 13999 (2014).
Groot Kormelink, T. et al. Immunoglobulin free light chains are increased in hypersensitivity pneumonitis and idiopathic pulmonary fibrosis. PLoS ONE 6(9), e25392–e25392 (2011).
Rubinchik, E. & Levi-Schaffer, F. Mast cells and fibroblasts: Two interacting cells. Int. J. Clin. Lab. Res. 24(3), 139–142 (1994).
Zhang, T. et al. Typical antimicrobials induce mast cell degranulation and anaphylactoid reactions via MRGPRX2 and its murine homologue MRGPRB2. Eur. J. Immunol. 47(11), 1949–1958 (2017).
Bacci, S. & Romagnoli, P. Drugs acting on mast cells functions: A cell biological perspective. Inflamm. Allergy Drug. Targets 9(4), 214–228 (2010).
Acknowledgements
The authors want to thank Orsolya kiss and Mayra Silva Miranda for her support with fluorescent microscopy and Alejandro Lopez Saavedra together with the Advanced Microscopy Unit, INCan, RAI, UNAM for their support using the confocal microscope.
Funding
KMGR was supported by the National Council of Science and Technology (CONACYT) funding.
Author information
Authors and Affiliations
Contributions
K.M.G.R., R.H.P. and S.B.P. participated in the research design. Material preparation, data collection and analysis were performed by K.M.G.R. K.M.G.R. and E.I.B. conducted experiments. S.H.Y. produced micro-array tissues. K.M.G.R. and R.H.P. performed data analysis. The first draft of the manuscript was written by K.M.G.R. and R.H.P. and S.B.P. commented on previous versions of the manuscript. All authors revised and approved final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Garcia-Rodriguez, K.M., Bini, E.I., Gamboa-Domínguez, A. et al. Differential mast cell numbers and characteristics in human tuberculosis pulmonary lesions. Sci Rep 11, 10687 (2021). https://doi.org/10.1038/s41598-021-89659-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-89659-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.