Abstract
To evaluate the effects of alcohol consumption on disease activity in rheumatoid arthritis. EMBASE, Pubmed, the Cochrane Library, and Web of Science were searched until July 29, 2020. English language studies that reported disease activity outcomes in rheumatoid arthritis were included. Studies were excluded if they were reviews, case reports, had fewer than 20 patients, or reported on prevalence but not disease activity in RA. Forest plots were used to determine pooled mean difference and were generated on RevMan5.3. Linear regression was used to determine correlations between alcohol and antibody status, gender, and smoking status. The search identified 4126 citations of which 14 were included. The pooled mean difference in DAS28 (95% CI) was 0.34 (0.24, 0.44) (p < 10−5) between drinkers and non-drinkers with lower DAS28 in non-drinkers, 0.33 (0.05, 0.62) (p = 0.02) between heavy drinkers and non-drinkers with lower DAS28 in heavy drinkers, and 0.00 (− 0.30, 0.30) (p = 0.98) between low- and high-risk drinkers. The mean difference of HAQ assessments was significantly different between those who drink alcohol compared to those who do not, with drinkers reporting lower HAQ scores (0.3 (0.18, 0.41), p < 10−5). There was no significant correlation between drinking and gender, smoking status, or antibody positivity. Alcohol consumption is associated with lower disease activity and self-reported health assessment in rheumatoid arthritis. However, drinking has no correlation with smoking, gender, or antibody status.
Similar content being viewed by others
Introduction
Rheumatoid Arthritis (RA) is a chronic inflammatory condition which if left untreated can lead to joint inflammation, damage and a reduction in life expectancy1,2. Both genetic and environmental risk factors contribute to the aetiology of RA with their impact varying depending on a patient’s rheumatoid factor (RF) and whether they have antibodies to citrullinated protein antigen (ACPA)3,4. The most recognised environmental risk factor associated with RA is smoking5. Studies have shown that smoking increases the risk of developing ACPA positivity as well as decreases response to treatment6,7,8. Other environmental risk factors which have been studied in relation to the development of RA include alcohol intake, diet, vitamin D and education levels9,10,11,12. However, the impact of these risk factors on both the development and severity of RA remains unclear.
Several studies have assessed the role of alcohol consumption and its association with the risk of developing RA, with many suggesting that alcohol is associated with the incidence of RA13,14,15,16,17. A previous meta-analysis investigating the protective effect of alcohol on developing RA showed alcohol intake was inversely associated with ACPA-positive RA, proposing a protective effect18.
The influence of environmental factors on disease activity is less understood. Alcohol has been shown to downregulate the synthesis of pro-inflammatory cytokines and in mouse models it has been associated with a reduction in joint destruction and disease activity14,19,20. In a Swedish study, alcohol consumption is shown to be associated with a reduction in disease activity in women but not men15. Another study suggests that frequency of alcohol consumption is inversely associated with radiological damage and disease activity13. However, other studies suggest that alcohol has no association with disease activity21. This study presents a systematic review and a meta-analysis to evaluate the relationship between alcohol intake and disease activity in RA.
Methods
Study selection
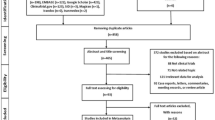
The PICO (population, intervention, control, and outcomes) methodology was used to generate the research question comparing the mean difference in disease activity in RA patients with different levels of alcohol consumption. Search terms were generated using exploded search terms on EMBASE for topics pertaining to alcohol consumption in RA and are listed in “Appendix 1”. EMBASE, Pubmed, the Cochrane Library, and Web of Science were searched from their inceptions until July 29, 2020. Studies reporting on alcohol consumption and disease activity in a cohort of RA patients were included for further investigation.
Inclusion criteria and data extraction
English language studies were included if they presented disease activity outcome measures in patients with RA including: the disease activity score based on a 28 joint count (DAS28), C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), tender joint count (TJC), swollen joint count (SJC), rheumatoid factor (RF) positivity, Anti-citrullinated protein antibody (ACPA) positivity, and/or health assessment questionnaire (HAQ). Observational studies that were either longitudinal or cross sectional were included. Studies were excluded if they were case reports or review articles, if they had fewer than 20 patients or if they reported on the prevalence of RA, not its disease activity. The strengthening the reporting of observational studies in epidemiology (STROBE) checklist was used to evaluate individual study bias22. Data were extracted from baseline assessment of alcohol consumption.
Statistical analysis
RevMan5.3 was used to generate forest plots from 95% confidence intervals of extracted data using mean differences. I-squared and tau-squared statistics were used to evaluate variance and heterogeneity across included studies. Random effects models were used to generate forest plots. Linear regression was used on SPSS26 to evaluate correlations between alcohol consumption and other factors including proportions of smoking, serology, and gender within each cohort.
Ethics approval and consent to participate
None was needed as it is a systematic review.
Results
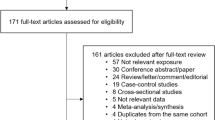
The literature search identified 4126 papers of which 14 were included for analysis (Fig. 1, Table 1)23. These papers represented data from 16,347 patients. Heterogeneity, according to the I2 statistic, was between 97 and 99% and thus a random effects model was used to calculate pooled mean differences and prevalence.
There was significantly lower disease activity in those who drink alcohol compared to those who do not (Fig. 2). The pooled mean difference in disease activity (95% CI) according to the DAS28 was 0.34 (0.24, 0.44) between non-drinkers and drinkers (p < 10−5) with non-drinkers having a higher mean DAS28. Between non-drinkers and heavy drinkers, the pooled mean difference in DAS28 was 0.33 (0.05, 0.62) (p = 0.02) with non-drinkers having a higher mean DAS28. Among those who drink, the mean difference between low-risk drinkers and high-risk drinkers was 0 (− 0.3, 0.3) (p = 0.98).
There was also a significant difference in HAQ between non-drinkers and drinkers with those who drink having lower HAQ scores (Fig. 3). The pooled mean difference in HAQ between non-drinkers and drinkers was 0.3 (0.18, 0.41) (p = < 10−5) whereby non-drinkers had higher HAQ scores. Between non-drinkers and heavy drinkers, the pooled mean difference in HAQ was 0.21 (0.05, 0.38) (p = 0.01) with non-drinkers having higher HAQ scores. Among those who drink, the mean difference between low-risk drinkers and high-risk drinkers was − 0.08 (− 0.25, 0.1) (p = 0.41).
When analyzing the consumption habits of the cohorts, the proportion of those who drank was 0.55 (0.44, 0.67). The total pooled proportion of the cohorts considered high risk drinkers was 0.25 (0.14, 0.35).
Across cohorts, gender (p = 0.057) and smoking status (p = 0.734) were not correlated to patient drinking status. High risk drinking was weakly correlated with male gender (R2 = 0.0027, p = 0.022) but was not correlated with smoking status (p = 0.59). RF positivity (p = 0.424, R2 = 0.22) and ACPA positivity (p = 0.230, R2 = 0.59) were not significantly associated with increases in disease activity.
STROBE scores for studies ranged between 15 and 20. CRP, ESR, and joint counts were not correlated with alcohol consumption.
Discussion
Our systematic analysis supported a relationship between alcohol consumption and disease activity in RA, where disease activity and HAQ scores are lower in those who consume alcohol compared to those who do not. Despite no significant difference seen between levels of alcohol consumption, both HAQ and DAS28 CRP were significantly lower in non-drinkers compared to those who consume alcohol.
Previous studies in Sweden and the UK have shown that moderate alcohol intake was associated with lower disease activity and improved quality of life measures15. A Swedish study showed that there was an association between alcohol consumption and better health related quality of life in women but not in men15. Measures of disease activity including the modified HAQ were found to be inversely proportional to alcohol consumption in both men and women in the 873 patients with RA in the United Kingdom15. This association was also present in our systematic review, with lower HAQ scores in patients who drink compared to non-drinkers.
Some studies have suggested an inverse relationship between alcohol consumption and RA development, which is predominately related to ACPA positive patients13,18. However, when looking at the ACPA status, alcohol consumption and disease activity of patients, there was no correlation between APCA positive patients’ alcohol consumption and disease activity. ACPA status was inconsistently reported in a portion of evaluated studies, therefore caution when interpreting results is required. A previous meta-analysis suggests an inverse relationship between alcohol consumption and the development of RA in ACPA positive individuals18. ACPA positive RA has been associated with environmental risk factors including smoking status and alcohol intake4. Smoking has been associated with more aggressive disease and poorer outcomes in patients with RA34,35. In this study, there was no relationship between alcohol intake and smoking amongst RA patients.
Strengths of this work include its robust search strategy. A broad search was conducted across four databases to identify all possible citations and included both abstracts and conference presentations. The double extraction protocol used for article selection minimizes selection bias and allows for a thorough evaluation. While the heterogeneity of studies was high, a random effects model to calculate pooled statistics was used. This model relaxes the assumption that the true relationship between alcohol consumption and disease activity does not vary between studies, however it does not alone control for confounding36. Therefore, regression was performed to assess the relationship between alcohol consumption and other demographic information such as gender, smoking status, and RF/ACPA positivity. Since these studies were mostly cross sectional, age could not be meaningfully assessed in regression models.
Our study is not without limitations. Individual studies categorize drinking habits differently and different regions have varying standards for what they consider to be high-risk drinking. In the UK, men who consume more than 14 standard drinks and women who consume more than 10 per week are considered to be at risk37. We did not differentiate between the different international standards for drinking risk within our analysis as the breadth of literature to do so was unavailable. The mean age also varied between cohorts, which could have an influence on alcohol consumption. However, in linear regression models, gender, smoking status, and antibody status had no relationship with alcohol consumption in RA. The study could also be susceptible to the ecological fallacy, each study may have its own unique relationship that may be diluted when making pooled comparisons. There could be confounding variables which potentially mask further differences seen in the individual studies38. In addition, studies that met the inclusion criteria were all conducted after 2010. This is coincidental as older studies did not consistently compare groups for analysis.
Overall, there is an association with alcohol consumption and better disease activity scores in patients with RA. The pooled proportion of those with RA who drink alcohol in these cohorts is 56%. When comparing demographic data to alcohol consumption there is no significant correlation between alcohol use and smoking nor gender. In addition, no correlation between alcohol consumption and antibody status was observed.
Data availability
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
References
Myasoedova, E., Davis, J. M., Crowson, C. S. & Gabriel, S. E. Epidemiology of rheumatoid arthritis: rheumatoid arthritis and mortality. Curr. Rheumatol. Rep. 12, 379–385 (2010).
Davis, J. M. Rheumatoid arthritis: a severe disease that preventive approaches would greatly benefit. Clin. Ther. 41, 1240–1245 (2019).
Liao, K. P., Alfredsson, L. & Karlson, E. W. Environmental influences on risk for rheumatoid arthritis. Curr. Opin. Rheumatol. 21, 279–283 (2009).
Pedersen, M. et al. Environmental risk factors differ between rheumatoid arthritis with and without auto-antibodies against cyclic citrullinated peptides. Arthritis Res. Ther. 8, R133 (2006).
Sugiyama, D. et al. Impact of smoking as a risk factor for developing rheumatoid arthritis: a meta-analysis of observational studies. Ann. Rheum. Dis. 69, 70–81 (2010).
Silman, A. J., Newman, J. & MacGregor, A. J. Cigarette smoking increases the risk of rheumatoid arthritis. Results from a nationwide study of disease-discordant twins. Arthritis Rheum. 39, 732–735 (1996).
Chang, K. et al. Smoking and rheumatoid arthritis. Int. J. Mol. Sci. 15, 22279–22295 (2014).
Vittecoq, O., Richard, L., Banse, C. & Lequerré, T. The impact of smoking on rheumatoid arthritis outcomes. Jt. Bone Spine 85, 135–138 (2018).
Bergström, U., Jacobsson, L. T. H., Nilsson, J. Å., Wirfält, E. & Turesson, C. Smoking, low formal level of education, alcohol consumption, and the risk of rheumatoid arthritis. Scand. J. Rheumatol. 42, 123–130 (2013).
Johansson, K., Askling, J., Alfredsson, L., Di Giuseppe, D. & EIRA Study Group. Mediterranean diet and risk of rheumatoid arthritis: a population-based case-control study. Arthritis Res. Ther. 20, 175 (2018).
Deane, K. D. et al. Genetic and environmental risk factors for rheumatoid arthritis. Best Pract. Res. Clin. Rheumatol. 31, 3–18 (2017).
Lee, Y. H. & Bae, S.-C. Vitamin D level in rheumatoid arthritis and its correlation with the disease activity: a meta-analysis. Clin. Exp. Rheumatol. 34, 827–833 (2016).
Maxwell, J. R., Gowers, I. R., Moore, D. J. & Wilson, A. G. Alcohol consumption is inversely associated with risk and severity of rheumatoid arthritis. Rheumatology (Oxford) 49, 2140–2146 (2010).
Källberg, H. et al. Alcohol consumption is associated with decreased risk of rheumatoid arthritis: results from two Scandinavian case-control studies. Ann. Rheum. Dis. 68, 222–227 (2009).
Bergman, S., Symeonidou, S., Andersson, M. L., Söderlin, M. K. & Barfot Study Group. Alcohol consumption is associated with lower self-reported disease activity and better health-related quality of life in female rheumatoid arthritis patients in Sweden: data from BARFOT, a multicenter study on early RA. BMC Musculoskelet. Disord. 14, 218 (2013).
Lu, B. et al. Associations of smoking and alcohol consumption with disease activity and functional status in rheumatoid arthritis. J. Rheumatol. 41, 24–30 (2014).
Di Giuseppe, D., Alfredsson, L., Bottai, M., Askling, J. & Wolk, A. Long term alcohol intake and risk of rheumatoid arthritis in women: a population based cohort study. BMJ 345, e4230 (2012).
Scott, I. C. et al. The protective effect of alcohol on developing rheumatoid arthritis: a systematic review and meta-analysis. Rheumatology (Oxford) 52, 856–867 (2013).
Jonsson, I.-M. et al. Ethanol prevents development of destructive arthritis. Proc. Natl. Acad. Sci. U. S. A. 104, 258–263 (2007).
Morris, N. L. et al. Alcohol and inflammatory responses: summary of the 2013 Alcohol and Immunology Research Interest Group (AIRIG) meeting. Alcohol 49, 1–6 (2015).
Baker, J. F. et al. Changes in alcohol use and associations with disease activity, health status, and mortality in rheumatoid arthritis. Arthritis Care Res. (Hoboken) 72, 301–308 (2020).
von Elm, E. et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 370, 1453–1457 (2007).
Moher, D., Liberati, A., Tetzlaff, J. & Altman, D. G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int. J. Surg. 8, 336–341 (2010).
Gunter, S. et al. Cardiovascular risk factors and disease characteristics are consistently associated with arterial function in rheumatoid arthritis. J. Rheumatol. 44, 1125–1133 (2017).
Larsson, I. & Andersson, M. L. E. Reasons to stop drinking alcohol among patients with rheumatoid arthritis in Sweden: a mixed-methods study. BMJ Open 8, e024367 (2018).
Mangnus, L., van Steenbergen, H. W., Nieuwenhuis, W. P., Reijnierse, M. & van der Helm-van Mil, A. H. M. Moderate use of alcohol is associated with lower levels of C reactive protein but not with less severe joint inflammation: a cross-sectional study in early RA and healthy volunteers. RMD Open 4, e000577 (2018).
Davis, M. L. R. et al. Associations of alcohol use with radiographic disease progression in african americans with recent-onset rheumatoid arthritis. J. Rheumatol. 40, 1498–1504 (2013).
Nissen, M. J., Gabay, C., Scherer, A., Finckh, A. & Swiss Clinical Quality Management Project in Rheumatoid Arthritis. The effect of alcohol on radiographic progression in rheumatoid arthritis. Arthritis Rheum. 62, 1265–1272 (2010).
Pala, O. et al. FRI0170 effect of alcohol on response to therapy with TNF-a inhibitors for rheumatoid arthritis: results from corrona registry. Ann. Rheum. Dis. 71, 369.1-369 (2013).
Sageloli, F. et al. Moderate alcohol consumption is associated with increased radiological progression in women, but not in men, with early rheumatoid arthritis: results from the ESPOIR cohort (Étude et Suivi des Polyarthrites Indifférenciées Récentes). Scand. J. Rheumatol. 47, 440–446 (2018).
Shimizu, Y. et al. SAT0079 association of alcohol consumption and disease activity in japanese patients with rheumatoid arthritis: analysis of the iorra cohort. In Poster Presentations 798.2–799 (BMJ Publishing Group Ltd and European League Against Rheumatism, 2017). https://doi.org/10.1136/annrheumdis-2017-eular.2340.
Taşpınar, Ö. et al. Evaluation in terms of dietary habits of rheumatic process: a clinical study. BMR 31, 23–27 (2018).
Yoshimura, H. et al. THU0695 differences of lifestyle habits of smoking, drinking alcohol and caffeinated coffee consumption between rheumatoid arthritis patients and healthy control—tomorrow study. In Poster Presentations 645.1–645 (BMJ Publishing Group Ltd and European League Against Rheumatism, 2019). https://doi.org/10.1136/annrheumdis-2019-eular.4138.
Gianfrancesco, M. A. et al. Smoking is associated with higher disease activity in rheumatoid arthritis: a longitudinal study controlling for time-varying covariates. J. Rheumatol. 46, 370–375 (2019).
Rydell, E., Forslind, K., Nilsson, J. -Å., Jacobsson, L. T. H. & Turesson, C. Smoking, body mass index, disease activity, and the risk of rapid radiographic progression in patients with early rheumatoid arthritis. Arthritis Res. Ther. 20, 82 (2018).
Riley, R. D., Higgins, J. P. T. & Deeks, J. J. Interpretation of random effects meta-analyses. BMJ 342, d549 (2011).
UK Chief Medical Officers’ Health Department. Low Risk Drinking Guidelines (2016). https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/489795/summary.pdf
Reade, M. C., Delaney, A., Bailey, M. J. & Angus, D. C. Bench-to-bedside review: avoiding pitfalls in critical care meta-analysis—funnel plots, risk estimates, types of heterogeneity, baseline risk and the ecologic fallacy. Crit. Care 12, 220 (2008).
Acknowledgements
We’d like to thank the professional and research staff at the Bone and Joint Unit and Centre for Rheumatic Disease at St. Vincent’s University Hospital, Dublin 4, Ireland.
Funding
The Centre for Arthritis and Rheumatic Disease.
Author information
Authors and Affiliations
Contributions
J.N.T. and E.R.Z. performed the literature search, data extraction, and were involved with manuscript writing. They are considered co-first authors. A.E.G., K.M., and M.A.T. were involved with statistical analysis, study design, and manuscript writing. D.J.V. was involved with study design and manuscript writing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Turk, J.N., Zahavi, E.R., Gorman, A.E. et al. Exploring the effect of alcohol on disease activity and outcomes in rheumatoid arthritis through systematic review and meta-analysis. Sci Rep 11, 10474 (2021). https://doi.org/10.1038/s41598-021-89618-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-89618-1
This article is cited by
-
Patients’ perspectives on adherence to cardiovascular screening consultation and lifestyle changes
Archives of Public Health (2024)
-
Prevalence and sociodemographic correlates of antinuclear antibody testing by indirect immunofluorescence or solid-phase assays in a Spanish population: the Camargo Cohort
Immunologic Research (2024)
-
Cardiovascular health worsening in patients with autoimmune rheumatological diseases during the COVID-19 pandemic
Clinical Rheumatology (2023)
-
Mortality and morbidity in ageing men: Biology, Lifestyle and Environment
Reviews in Endocrine and Metabolic Disorders (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.