Abstract
Adequate micronutrient status during adolescence can break the inter-generational cycle of malnutrition. This study evaluated the effect of community-based weekly iron-folic acid supplementation (WIFAS) on serum ferritin (SF), serum folate (SFol) and hemoglobin concentration (Hb) among adolescent girls. A community-based, individually randomized-controlled trial (RCT) was conducted in four villages of Wolaita and Hadiya zones. Adolescent girls (n = 226) aged 10–19 years were recruited and randomly assigned (n = 113/group) into: (i) WIFAS and (ii) control (no intervention) groups. Anthropometry, Hb concentration, and serum ferritin (SF), SFol, and C-reactive protein (CRP) was analyzed at baseline and endline. Baseline Hb, SF, SFol and CRP concentrations were similar in both groups (P > 0.05). About 47–49% of adolescents had marginal iron store (< 50 µg/l). Hb, SF, and SFol concentrations increased in the intervention group, but not in the control group (P < 0.05). Marginal iron store decreased from 49 to 12% after 3-months of WIFAS; whereas, the proportion of adolescents with elevated SF (> 15 µg/l) was slightly higher in the WIFAS than in the control group (P = 0.06). After adjusting for confounding factors in the multiple linear regression model, a three-months WIFAS intervention was associated with an improvement of 4.10 ng/ml in serum folate, 39.1 μg/l in serum ferritin, and 1.2 g/dl in hemoglobin concentration relative to the control group (P < 0.001). WIFAS intervention for three-months was effective in reducing iron and folate deficiency in adolescent girls. Future studies should evaluate the long-term impact of intermittent WIFAS.
Similar content being viewed by others
Introduction
Anemia is widely prevalent in low and middle-income countries (LMIC) and disproportionately affects women and children1. Anemia has been linked to adverse outcomes including poor cognitive and physical performance, increased susceptibility to disease, and perinatal complications like low-birth weight, still-birth, and preterm birth2,3. The etiology of anemia is complex and can be of nutritional origin or related to infection or chronic diseases4. Among nutritional causes, iron and folic acid deficiencies are the most common in women and relate to the higher physiological needs to compensate for menstrual losses and support a healthy pregnancy and fetal development5,6. Consequently, iron-folic acid (IFA) interventions have been the mainstay of anemia prevention interventions7.
IFA supplementation has for a long time been considered primarily for pregnant women. This is considering the increased physiological iron and folic acid requirement of the mother and the added demand to support the development of the fetus, but also to prevent neural tube defects8,9. However, in LMICs like Ethiopia, women attend their first antenatal care (ANC) visit quite late in their first trimester or often in their second trimester. Besides, adherence to recommendations is low and is often complicated by inadequate supply, social norms, and side effects10,11,12,13,14. Altogether, the late and suboptimal adherence of IFA supplementation suggests that it may be strategic to increase the iron stores of adolescent girls and non-pregnant women of reproductive age through weekly IFA supplementation15,16. This will allow women to start pregnancy with adequate iron stores and folate status; hence, lowering the risk of iron-deficiency anemia and folate deficiencies. Weekly IFA supplementation can also have the added advantage of supporting cognitive-17 and the physical-performance of adolescents18.
The focus on adolescence as a window of opportunity for preventive nutrition interventions is quite recent19. Consequently, very few studies have evaluated preventive nutrition interventions including weekly IFA supplementation, and the quality of the evidence remains low as recently evaluated by a systematic review20. Recent studies from India, Nepal, and Kenya have all shown positive effects of weekly IFA supplementation on adolescents’ hemoglobin concentration16,21,22. These studies were all school-based and hence did not capture out of school girls that may be more at risk. They also did not measure markers of iron, folate, and inflammation status. Consequently, studies on the impact of community-based weekly IFA supplementation on anemia and iron and folate status of adolescents are urgently needed. Therefore, the present study aimed to investigate the effect of community-based weekly supplementation of IFA for three-months on serum ferritin, folate and hemoglobin level among adolescent girls in Wolaita and Hadiya zones, southern Ethiopia. We hypothesized that weekly Iron-folic acid supplementation improves serum ferritin, folate, and hemoglobin concentrations.
Results
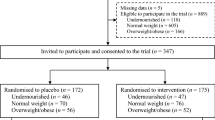
From the enrolled participants, 81% from the intervention and 99% from the control group completed the study. Nineteen adolescents discontinued the supplementation due to potential side-effects as they have reported vomit, nausea and constipation. Another two, discontinued because they left the villages where the supplementation was taking place. Study subjects were on average 14 years of age and came from an average household size of seven. Close to 80% were in high-school (grades 9–12), and about 4% had no formal education (Table 1).
There was no statistically significant difference in the mean hemoglobin, serum ferritin, serum folate, and CRP concentrations (P > 0.05), suggesting effective randomization (Table 2). Similarly, the proportion of subjects with depleted iron stores (serum ferritin < 15 µg/l), marginal and excess iron store were not statistically different between the two groups. The proportion of subjects with depleted and the excess iron store was low (≤ 5%), but close to half of the subjects had marginal iron stores. About 10% in the treatment and 13% in the control group had folate deficiency (P > 0.05).
Significant differences in hemoglobin, serum ferritin, and serum folate concentrations were observed between the intervention and the control group after 3 months of supplementation (Table 3), while no significant changes were observed in the control group. At endline, no adolescent in the intervention group had a depleted iron store or were folate deficient. The proportion of adolescents with marginal iron stores decreased from 45% at baseline to 11% at endline. During the same period, excess iron stores, reflected by a serum ferritin > 150 µg/l, increased from 3 to 10%.
The multivariate linear regression showed significant effects of the WIFAS program on endline serum folate, serum ferritin, and hemoglobin concentrations (Table 4). The lower the baseline ferritin, folate and hemoglobin concentrations, the higher the impact of the WIFAS program (P ≤ 0.001). Three months WIFAS intervention was associated with an improvement of 4.10 ng/ml in serum folate, 39.1 μg/l, and 1.2 g/dl in hemoglobin concentration relative to the control group (P < 0.001).
Discussion
We investigated the effect of community-based WIFAS intervention among adolescent girls in southern Ethiopia. To our knowledge, this is the first study to examine the effect of community-based WIFAS on hemoglobin concentration, serum ferritin and serum folate concentrations of adolescent girls in southern Ethiopia. The prevalence of low serum ferritin was surprisingly low (< 5%), but marginal iron store was highly prevalent in about half of the studied adolescents. WIFAS significantly improved hemoglobin, folate and ferritin concentrations. Baseline hemoglobin, ferritin, and folate concentration was inversely associated with endline values.
The present study showed that iron deficiency was low, despite a predominantly cereal-based diet and the low availability of iron-fortified foods, as reported elsewhere23. This finding is in line with a recent study from Hirna, Ethiopia24. Both the present and the earlier study from Hirna identified high levels of marginal iron stores. Such low iron stores can increase the risk of iron deficiency in the event of increased demands due to pregnancy or excessive blood loss. This along with folate deficiency in about 10% of the adolescents and the positive response in serum folate and ferritin in the WIFAS intervention group suggests that the intervention is adapted to the context22,25,26,27. This is further confirmed by the higher response to the intervention in those with lower serum ferritin, folate and hemoglobin at baseline.
The elimination of folate deficiency after 3 months of WIFAS further highlights the importance of WIFAS intervention. Folate deficiency in the first 2 to 3 weeks has been linked to increased risks to congenital malformations28,29,30. Although folic acid supplementation is promoted during pregnancy, the delayed first antenatal care means that most pregnant women do not get folic acid supplements at the time when they need it the most (i.e. first 2 weeks of conception). In contrast, the WIFAS program allows adolescents to have an adequate folate status; hence, reducing the risk of deficiency, if and when they become pregnant29,31. The weekly regimen is also likely to decrease any of the possible side-effects observed during daily supplementation21.
The WIFAS program has been promoted in various LMIC through school-systems25,32. Many impact evaluations have shown the benefits of such platforms for constant monitoring and reducing school dropouts. Despite the advantages that the school system provides, in LMICs like Ethiopia, a high number of adolescents are out of school and thus cannot benefit from such programs33. Unless systems’ are in place to reach out of school adolescent girls, the WIFAS intervention for those going to school only is likely to widen inequalities between adolescent girls. Our study provides evidence that a community-based WIFAS program can be successful in improving serum micronutrient status to improvement levels observed in more controlled school settings. However, the rise in high serum ferritin suggests that there is a potential risk of iron overload for a segment of the adolescent population. Whenever possible, assessing iron status prior to WIFAS interventions can minimize possible adverse effects.
The present study has several strengths and limitations. Key strengths include the use of a randomized controlled trial and the implementation of a community-based WIFAS program. The measurement of inflammation marker using CRP measures is also an additional strength that increases the reliability of serum-ferritin and -folate measures. Measurement of α-glycoprotein (AGP), in addition to CRP, could have allowed us to adjust the serum ferritin values, instead of excluding subjects with inflammation. Nevertheless, samples with CRP > 5 mg/l were excluded from the analyses. The excluded subjects represented 2.5% of the total sample, were balanced by intervention group (P > 0.05), and had similar socio-demographic characteristics than subjects retained in the analyses. The study was an effectiveness study; hence, although we know that adherence to the WIFAS was generally high; the compliance to the regimen was not strictly monitored. The long-term impact of the WIFAS benefits remains unknown and warrants to be studied.
Notwithstanding the above limitations, the present study highlighted the beneficial impact of a community-based WIFAS program. The community-based WIFAS program significantly improved serum ferritin, serum folate, and blood hemoglobin concentration among adolescent girls proving this concept in the context of Ethiopia. The present finding suggests the potential benefit of scaling-up community-based WIFAS programs in Ethiopia and beyond. Future studies should evaluate the long-term benefits of such programs.
Methods and subjects
Study area and study design
A community-based, individually randomized trial (RCT) was conducted at four Villages of Wolaita and Hadiya zones using a two-arm parallel design (one intervention group and one control group). Wolaita and Hadiya zones are two zonal administrations in Southern Nations, Nationalities, and Peoples’ Region (SNNPR). These zones are predominantly dependent on agriculture, practice mixed crop-livestock production and live in permanent settlements. Within their landholdings, community members cultivate fruits, vegetables, roots, tuber crops, and cereals. Participants were randomly assigned into intervention or control group. The intervention group took weekly IFA supplementation, and the control group received nothing. The IFA supplement contained 60 mg of elemental iron and 0.4 mg of folic acid. The IFA supplementation was monitored by four clinical nurses and two supervisors recruited for the study. IFA tablets were provided every weekend through household visits. These household visits also provided a platform to answer any questions by the study subjects. The study period was from April to September 2019. The baseline survey was conducted in April–May, followed by a three-month supplementation period (June–August), and an endline survey in September.
Ethical considerations
The study was approved by the institutional review board (IRB) of Wolaita Sodo University. All methods were performed in accordance to the Helsinki Declaration ethical principles for medical research involving human subjects. Official letter of cooperation was written to Wolaita and Hadiya zones, and the district health office. The nature of the study was fully explained to the study participants and their parents. Informed verbal and written consent/assent were obtained from parents/respondents before the interview and the intervention. The collected data were kept confidential. The trial is registered at the PanAfrican clinical trial registry (PACTR202003545370309).
Sample size and sampling
The sample sizes were calculated by using Gpower 3.0. Sample size calculations were conducted for serum ferritin, serum folate, and blood hemoglobin concentration and the estimate that gave the largest sample size estimate (i.e. hemoglobin concentration) was retained. The power calculations assumed a power of 80%, an effect size of 0.3 and 20% non-response rate, which yielded a total sample size of 226 (n = 113/group).
In the four selected villages, a list of adolescent girls who were eligible for inclusion in the study was established with the help of the health extension worker. From this list, 226 adolescent girls were selected randomly through randomly generated numbers. Then, these adolescent girls (226 in total) were randomly assigned to the intervention group (G1 = 113) and the control group (G2 = 113).
Apparently healthy adolescent girls aged 10–19 years and willing to participate in the study were recruited from four villages from Wolaita and Hadiya zones. Adolescents that smoke or drink and with a known infectious disease like HIV/AIDS, Tuberculosis, and malaria (based on blood smear) were excluded. The initial screening was supported by physicians and laboratory tests were conducted at the Wolaita Sodo University referral hospital. Adolescents with hemoglobin < 12 g/dl (anemic) and > 17 g/dl (with increased risk of excessive intake) were excluded from the study.
Anthropometric measurements
Anthropometric measures such as height, weight, and mid-upper arm circumference were measured at baseline and end line. Weight was measured to the nearest 100 g using a pre-calibrated digital scale (SECA), wearing light closing and without shoes. Height was measured in a standing position to the nearest 0.1 cm using a vertical board with a detachable sliding headpiece. Mid-upper arm circumference (MUAC) was measured using a standard MUAC tape on the upper left arm, after locating the midpoint for measurement between the end of the shoulder (acromion) and the tip of the elbow (olecranon).
Biological/laboratory sample collection and analysis
An experienced phlebotomist drew fasting venous blood (~ 5 ml), from which drops were used to determine hemoglobin concentration in-field using a portable Hemocue photometer (Hb 301 + system). The remaining blood was allowed to settle at the data collection site (nearby health post) for 45 min before centrifugation to separate the serum. The serum was stored at − 20 °C overnight and was transferred to Wolaita Sodo University referral hospital, where it was stored at − 70 °C until further analyses. Samples were then transported on dry ice to the Ethiopian Public Health Institute (EPHI), where serum ferritin was analyzed using a fully automated clinical analyzer electrochemo-iluminescence immunoassay (ECLIA, Elecsys 2010 analyzer Cobas e 411; Roche Diagnostics GmbH, Mannheim, Germany); C-reactive protein (CRP) and Folate were determined by Immune turbid metric methods with a clinical chemistry analyzer (Cobas 6000 system; Roche Diagnostic GmbH). Hemoglobin concentrations were adjusted for altitude by using the following formula:
The Hb adjustment values obtained from the above calculation was subtracted from the measured hemoglobin concentration of each individual adolescent. Subjects with Hb values < 12 g/dl were considered anemic. CRP values > 5 µg/l were considered as elevated. Serum ferritin < 15.0 μg/l was considered as depleted iron stores. Serum folate between 3 and 5.9 ng/ml indicated marginal deficiency, whereas < 3 ng/ml is considered as deficient.
Data quality assurance
The questionnaires were translated into the local languages: Wolaitegna and Hadiya and were back-translated to English to ensure the accuracy of the translation. Experienced data collectors, lab-technicians, and supervisors were recruited and trained for 4 days. The questionnaires were pre-tested and necessary changes were made prior to the actual surveys. The lead author and supervisors spot-checked the data collection and reviewed the questionnaires for completeness. Standardization of anthropometric measurements was taken. Anthropometric measurement was standardized by intensive training till the between and within measurement errors were reduced to an acceptable range. Acceptable technical error of measurement for weight was less than 0.1 kg, height was less than 0.5 cm and MUAC was less than 2 mm during standardization.
Statistical analysis
Primary outcomes were analyzed based on an intention-to-treat principle. Socio-demographic characteristics were summarized as mean ± SD (or median and range) or frequency (percentage).
A comparison of means between the two arms was analyzed using a t-test. A multiple linear regression model was used to examine the impact of weekly IFA supplementation on hemoglobin concentration, serum ferritin, and serum folate, by running separate models adjusting for confounding factors like age, family size, weight, and baseline hemoglobin, serum ferritin, and serum folate concentrations. Multicollinearity was checked by using variance inflation factor (VIF), with a VIF values < 10 reflecting that mulitcollinearity is not a serious issue. The goodness of fit of the model was also checked.
All hypothesis tests were two-sided with a P-value < 0.05 considered as statistically significant. EP-data version 4.4.2 was used for data entry and SPSS version 21.0 was for analysis.
Data availability
All relevant data are within the paper and its supporting information file.
References
Balarajan, Y. et al. Anaemia in low-income and middle-income countries. The Lancet 378(9809), 2123–2135 (2011).
Abu-Ouf, N. M. & Jan, M. M. The impact of maternal iron deficiency and iron deficiency anemia on child’s health. Saudi Med. J. 36, 146 (2015).
Scholl, T. O. & Reilly, T. Anemia, iron and pregnancy outcome. J. Nutr. 130, 443S-447S (2000).
Al-Farsi, M. A. & Lee, C. Y. Nutritional and functional properties of dates: A review. Crit. Rev. Food Sci. Nutr. 48, 877–887 (2008).
Camaschella, C. Iron-deficiency anemia. N. Engl. J. Med. 372, 1832–1843 (2015).
Sharourou, A. S. et al. Anemia: Its prevalence, causes, and management. Egypt. J. Hosp. Med. 70, 1877–1879 (2018).
Stoltzfus, R. J. Iron interventions for women and children in low-income countries. J. Nutr. 141, 756S-762S (2011).
Cesar, J. A. et al. Iron supplementation among pregnant women: Results from a population-based survey study. Rev. Bras. Epidemiol. 16, 729–736 (2013).
Young, M. et al. The effectiveness of weekly iron supplementation in pregnant women of rural northern Malawi. Trop. Doct. 30, 84–88 (2000).
Assefa, H., Abebe, S. M. & Sisay, M. Magnitude and factors associated with adherence to Iron and folic acid supplementation among pregnant women in Aykel town, northwest Ethiopia. BMC Pregn. Childbirth 19, 296 (2019).
Getachew, M. et al. Magnitude and factors associated with adherence to Iron-folic acid supplementation among pregnant women in Eritrean refugee camps, northern Ethiopia. BMC Pregn. Childbirth 18, 1–8 (2018).
Sadore, A. A., Gebretsadik, L. A. & Hussen, M. A. Compliance with iron-folate supplement and associated factors among antenatal care attendant mothers in Misha District, South Ethiopia: Community based cross-sectional study. J. Environ. Public Health 2015, 7 (2015).
Sendeku, F. W., Azeze, G. G. & Fenta, S. L. Adherence to iron-folic acid supplementation among pregnant women in Ethiopia: A systematic review and meta-analysis. BMC Pregn. Childbirth 20, 1–9 (2020).
Birhanu, T. M., Birarra, M. K. & Mekonnen, F. A. Compliance to iron and folic acid supplementation in pregnancy, Northwest Ethiopia. BMC Res. Notes 11, 1–5 (2018).
Zavaleta, N., Respicio, G. & Garcia, T. Efficacy and acceptability of two iron supplementation schedules in adolescent school girls in Lima, Peru. J. Nutr. 130, 462S-464S (2000).
Vir, S. C. et al. Weekly iron and folic acid supplementation with counseling reduces anemia in adolescent girls: A large-scale effectiveness study in Uttar Pradesh, India. Food Nutr. Bull. 29, 186–194 (2008).
Sen, A. & Kanani, S. J. Impact of iron-folic acid supplementation on cognitive abilities of school girls in Vadodara. Indian Pediatr. 46, 137–143 (2009).
Bhoite, R. M. & Iyer, U. M. Effect of deworming vs iron-folic acid supplementation plus deworming on growth, hemoglobin level, and physical work capacity of schoolchildren. Indian Pediatr. 49, 659–661 (2012).
Dorn, L. D. et al. Conceptualizing puberty as a window of opportunity for impacting health and well-being across the life span. J. Res. Adolesc. 29, 155–176 (2019).
Salam, R. A. et al. Effects of preventive nutrition interventions among adolescents on health and nutritional status in low-and middle-income countries: A systematic review. Campbell Syst. Rev. 16, 1085 (2020).
Joshi, M. & Gumashta, R. Weekly iron folate supplementation in adolescent girls—An effective nutritional measure for the management of iron deficiency anaemia. Glob. J. Health Sci. 5, 188 (2013).
Bansal, P. et al. Impact of weekly iron folic acid supplementation with and without vitamin B12 on anaemic adolescent girls: A randomised clinical trial. Eur. J. Clin. Nutr. 70, 730–737 (2016).
Halala, H. Y. et al. Low dietary diversity and its determinants among adolescent girls in Southern Ethiopia. Cogent Food Agric. 6(1), 1832824 (2020).
Seyoum, Y. et al. Iron deficiency and anemia in adolescent girls consuming predominantly plant-based diets in rural Ethiopia. Sci. Rep. 9, 1–6 (2019).
Parwati, N.M. Januraga, P., Suarjaya. The effect of school-based program of iron supplementation in preventing and controlling iron deficiency anemia among adolescent girls: Literature reviews. In 4th International Symposium on Health Research (ISHR 2019), Vol. 22, 126–132 (Atlantis Press, 2020).
Angadi, N. & Balu, P. Effectiveness of weekly iron and folic acid supplementation programme to control anemia among rural adolescent school girls of Davangere, Karnataka. Natl. J. Community Med. 10, 479–482 (2019).
Bairwa, M. et al. Directly observed iron supplementation for control of iron deficiency anemia. Indian J. Public Health 61, 37 (2017).
Oyama, K. et al. Folic acid prevents congenital malformations in the offspring of diabetic mice. Endocr. J. 56, 29–37 (2009).
Czeizel, A. E. et al. Folate deficiency and folic acid supplementation: The prevention of neural-tube defects and congenital heart defects. Nutrients 5, 4760–4775 (2013).
Huhta, J. C. & Hernandez-Robles, J. A. Homocysteine, folate, and congenital heart defects. Fetal Pediatr. Pathol. 24, 71–79 (2009).
Lassi, Z. S. et al. Folic acid supplementation during pregnancy for maternal health and pregnancy outcomes. Cochrane Database Syst. https://doi.org/10.1002/14651858.CD006896.pub2 (2013).
Kavle, J. A. & Landry, M. Community-based distribution of iron–folic acid supplementation in low-and middle-income countries: A review of evidence and programme implications. Public Health Nutr. 21, 346–354 (2018).
Erulkar, A., Medhin, G. & Negeri, L. The Journey of Out-of-School Girls in Ethiopia: Examining Migration, Livelihoods, and HIV (Addis Ababa, 2017).
Acknowledgements
The support of the Wolaita and Hadiya zone health office leaders and experts are acknowledged for their valuable cooperation during data collection and we thank all data collectors of this research and adolescent girls who were willing to participate in this study. We are grateful of the partial sponsorship obtained from the small research grant by Tufts University and the funding from the Graduate Program of the Addis Ababa University.
Author information
Authors and Affiliations
Contributions
Y.H., T.B., C.A., A.W. and K.B. conceptualized the study, looked for funding, contributed to data analyses, and thoroughly edited the manuscript. Y.H. administered the project and wrote the first draft of the manuscript. All authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Handiso, Y.H., Belachew, T., Abuye, C. et al. A community-based randomized controlled trial providing weekly iron-folic acid supplementation increased serum- ferritin, -folate and hemoglobin concentration of adolescent girls in southern Ethiopia. Sci Rep 11, 9646 (2021). https://doi.org/10.1038/s41598-021-89115-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-89115-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.