Abstract
To assess the influence of lipid-lowering therapy on coronary plaque volume, and to identify the LDL and HDL targets for plaque regression to provide a comprehensive overview. The databases searched (from inception to 15 July 2020) to identify prospective studies investigating the impact of lipid-lowering therapy on coronary plaque volume and including quantitative measurement of plaque volume by intravascular ultrasound after treatment. Thirty-one studies that included 4997 patients were selected in the final analysis. Patients had significantly lower TAV (SMD: 0.123 mm3; 95% CI 0.059, 0.187; P = 0.000) and PAV (SMD: 0.123%; 95% CI 0.035, 0.212; P = 0.006) at follow-up. According to the subgroup analyses, TAV was significantly reduced in the LDL < 80 mg/dL and HDL > 45 mg/dL group (SMD: 0.163 mm3; 95% CI 0.092, 0.234; P = 0.000), and PAV was significantly reduced in the LDL < 90 mg/dL and HDL > 45 mg/dL group (SMD: 0.186%; 95% CI 0.081, 0.291; P = 0.001).Thirty-one studies that included 4997 patients were selected in the final analysis. Patients had significantly lower TAV (SMD: 0.123 mm3; 95% CI 0.059, 0.187; P = 0.000) and PAV (SMD: 0.123%; 95% CI 0.035, 0.212; P = 0.006) at follow-up. According to the subgroup analyses, TAV was significantly reduced in the LDL < 80 mg/dL and HDL > 45 mg/dL group (SMD: 0.163 mm3; 95% CI 0.092, 0.234; P = 0.000), and PAV was significantly reduced in the LDL < 90 mg/dL and HDL > 45 mg/dL group (SMD: 0.186%; 95% CI 0.081, 0.291; P = 0.001). Our meta-analysis suggests that not only should LDL be reduced to a target level of < 80 mg/dL, but HDL should be increased to a target level of > 45 mg/dL to regress coronary plaques.
Trial Registration PROSPERO identifier: CRD42019146170.
Similar content being viewed by others
Introduction
A previous study suggested that the prevalence of coronary heart disease (CHD) resulting in significant myocardial infarction (MI) morbidity and CHD mortality in American adults who are 20 years of age or older was 6.7%1. The severity of coronary atherosclerosis in patients with CHD is closely related to adverse cardiovascular events. Therefore, stabilization and regression of coronary atherosclerotic plaques by lipid-lowering therapy plays an important role in the treatment of CHD2.
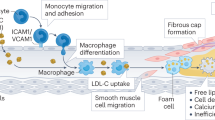
Plaque regression, which includes the removal of lipids and the necrotic core, was shown to restore endothelial function, although the cessation of intravascular smooth muscle cell proliferation is a complex process3. Coronary atherosclerotic plaque regression can be detected using various imaging techniques that can measure changes in plaque volume, and intravascular ultrasound (IVUS) is currently one of the most common of such methods4. Total atheroma volume (TAV) and percent atheroma volume (PAV) are the indices usually used to evaluate coronary plaque volume. TAV is more sensitive and PAV is more accurate5. A plaque has regressed when a reduced plaque volume is detected after treatment. Recent studies have indicated that lipid-lowering therapy can lead to the regression of a coronary atherosclerotic plaque and reduce the incidence of adverse cardiovascular events6. A recent meta-regression analysis by Bhindi et al.7 showed that a 1% reduction in mean PAV was induced by dyslipidemia therapies and was associated with a 20% reduction in the odds of major adverse cardiac events (MACE).
Statins are the cornerstone of lipid-lowering therapy, but other lipid-lowering drugs include bile acid sequestrants, ezetimibe, and PCSK9 inhibitors8. These drugs can reduce blood lipid levels through different mechanisms, including lowering total cholesterol (TC), triglycerides (TG), and low-density lipoproteins (LDL) and increasing high-density lipoproteins (HDL) to some extent. Although there have been a number of meta-analyses concerning lipid-lowering therapy and coronary plaque volume in recent years, most studies have been conservative in the drug interventions selected for inclusion in their studies. For example, they only analyzed TAV but not PAV or they only considered the effect of LDL on plaque regression but not HDL.
Therefore, we performed a meta-analysis to assess the influence of lipid-lowering therapy on coronary plaque volume (TAV and PAV) in this study, and to identify the LDL and HDL targets for plaque regression to provide a comprehensive overview.
Results
Selection of sources of evidence
Our search strategy yielded a total of 10,985 studies. There were 4989 studies after the repeated studies were excluded and 885 studies did not meet the inclusion criteria. We then excluded 57 studies because of insufficient data, 32 studies because they were animal studies, and 6 studies because they were duplicate reports of the same study population. Therefore, 31 studies (with 4997 patients in the lipid-lowering therapy group and 769 patients in the control group) that measured TAV or PAV at baseline and follow-up were included in our final analysis9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39 (Fig. S1).
Characteristics of sources of evidence
The main features of the studies are shown in Table 1. The number of patients in each study ranged from 14 to 520. TAV was measured by IVUS in 29 studies9,10,11,12,13,14,15,16,17,18,19,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,38,39, which had 4761 patients in 52 groups, and PAV was measured by IVUS in 19 studies10,14,18,20,21,22,23,24,25,29,30,31,32,33,34,36,37,38,39, which had 4226 patients in 38 groups.
Critical appraisal within sources of evidence
The quality of randomized controlled trials was assessed by the Jadad quality scale, and the quality of non-randomized controlled trials was evaluated by the Newcastle Ottawa scale (NOS). The details were shown in Table S1 and S2.
Publication bias can influence the results of a meta-analysis. Therefore, a funnel plot and Egger’s and Begg’s tests were used to evaluate the potential publication bias in the included studies. The assessment of the symmetry of the funnel plots for the TAV or PAV showed little publication bias in our results (Fig. S2). Egger’s test (TAV: P = 0.315; PAV: P = 0.272) and Begg’s test (TAV: P = 0.398; PAV: P = 0.209) both confirmed this finding.
A sensitivity analysis was conducted by performing additional meta-analyses after deleting individual studies one by one. The results of the sensitivity analysis showed that none of the studies influenced the pooled SMD, which indicated that our meta-analysis was statistically stable (Fig. S3).
Results of individual sources of evidence
The relevant data of each included study were presented in Table 1.
Synthesis of results
A total of 29 studies reported that TAV was significantly reduced in patients at follow-up (SMD: 0.123 mm3; 95% CI 0.059, 0.187; P < 0.001). There was heterogeneity among the studies (I2 = 47.0%, P < 0.001). A total of 18 studies reported a significant reduction in PAV of patients at follow-up (SMD: 0.123%; 95% CI 0.035, 0.212; P = 0.006). There was heterogeneity among the studies (I2 = 69.3%, P < 0.001).
To explore the target level of LDL for plaque regression, the included studies were divided into five groups according to the levels of LDL at follow-up: < 70, 70–80, 80–90, 90–100, > 100 mg/dL. The subgroup analysis of TAV data showed significant plaque regression in the LDL < 70 mg/dL group (SMD: 0.195 mm3; 95% CI 0.086, 0.304; P < 0.001) (I2 = 59.0%, P = 0.001, Fig. 1A) and the 70–80 mg/dL group (SMD: 0.078 mm3; 95% CI 0.003, 0.153; P = 0.042) (I2 = 0.0%, P = 0.752, Fig. 1A) at follow-up. The subgroup analysis of PAV data showed significant plaque regression in the LDL < 70 mg/dL group (SMD: 0.152%; 95% CI 0.001, 0.303; P = 0.049) (I2 = 78.9%, P < 0.001, Fig. 1B), 70–80 mg/dL group (SMD: 0.079%; 95% CI 0.003, 0.155; P = 0.042) (I2 = 0.0%, P = 0.97, Fig. 1B) and LDL 80–90 mg/dL group (SMD: 0.423%; 95% CI 0.196, 0.651; P < 0.001) (I2 = 45.1%, P = 0.141, Fig. 1B) at follow-up. The total effect was statistically significant.
In order to identify the target level of HDL for plaque regression, the included studies were divided into three groups according to the levels of HDL at follow-up: > 45, 40–45, < 40 mg/dL. The subgroup analysis of TAV data showed significant plaque regression in the HDL > 45 mg/dL group (SMD: 0.137 mm3; 95% CI 0.068, 0.205; P < 0.001) (I2 = 39.3%, P = 0.007, Fig. 2A). Meanwhile, the subgroup analysis of PAV data also showed significant plaque regression in the HDL > 45 mg/dL group (SMD: 0.166%; 95% CI 0.066, 0.266; P = 0.001) (I2 = 69.4%, P < 0.001, Fig. 2B), and the total effect was statistically significant.
To explore the combined effects of LDL reduction and HDL incrementation on plaque regression, the studies concerning TAV were divided into four groups according to the above findings: LDL < 80 mg/dL and HDL > 45 mg/dL group, LDL < 80 mg/dL and HDL < 45 mg/dL group, LDL > 80 mg/dL and HDL > 45 mg/dL group, and LDL > 80 mg/dL and HDL < 45 mg/dL group. In the meantime, we also divided the studies concerning PAV into four groups: LDL < 90 mg/dL and HDL > 45 mg/dL group, LDL < 90 mg/dL and HDL < 45 mg/dL group, LDL > 90 mg/dL and HDL > 45 mg/dL group, LDL > 90 mg/dL and HDL < 45 mg/dL group. There was a significant plaque regression in the LDL < 80 mg/dL and HDL > 45 mg/dL group (SMD: 0.163 mm3; 95% CI 0.092, 0.234; P < 0.001) (I2 = 29.5%, P = 0.088, Fig. 3A) in the subgroup analysis of TAV, and there was a significant plaque regression in the LDL < 90 mg/dL and HDL > 45 mg/dL group (SMD: 0.186%; 95% CI 0.081, 0.291; P = 0.001) (I2 = 71.3%, P < 0.001, Fig. 3B) in the subgroup analysis of PAV. The total effect was statistically significant.
A subgroup analysis of the administration of different drugs was conducted to eliminate the significant heterogeneity among the studies. The subgroup analysis concerning TAV indicated there was a significant decrease in heterogeneity in the oral administration group (SMD: 0.105 mm3; 95% CI 0.051, 0.159; P < 0.001) (I2 = 23.5%, P = 0.071, Fig. S4A) and subcutaneous injection group (SMD: 0.487 mm3; 95% CI 0.359, 0.614; P < 0.001, Fig. S4A). In addition, the heterogeneity also showed a significant decrease in the subgroup analysis concerning PAV in the oral administration group (SMD: 0.096%; 95% CI 0.033, 0.159; P = 0.003) (I2 = 33.1%, P = 0.028, Fig. S4B) and subcutaneous injection group (SMD: 0.667%; 95% CI 0.537, 0.796; P < 0.001, Fig. S4B). The total effect was statistically significant.
A regression analysis was performed to assess other potential factors that may have influenced the outcomes. Our analysis indicated that LDL levels at follow-up significantly influenced TAV and PAV (TAV: P = 0.011, tau2 = 0.0112, Adj R-squared = 43.98%, I-squared res = 30.49%; PAV: P = 0.016, tau2 = 0.0244, Adj R-squared = 24.43%, I-squared res = 51.73%, Fig. 4). At the same time, gender significantly affected TAV (P = 0.035, tau2 = 0.0195, Adj R-squared = 1.43%, I-squared res = 46.56%). The dosage of drugs (P = 0.04, tau2 = 0.0269, Adj R-squared = 19.52%, I-squared res = 53.84%) and TG levels at baseline (P = 0.04, tau2 = 0.0263, Adj R-squared = 24.95%, I-squared res = 59.11%) significantly affected PAV. Other factors, including age, region, drugs, smoking, diabetes, hypertension, and blood lipid levels (HDL, TC), did not influence the results. The details of regression analysis outcomes were shown in Table S3 and S4.
Discussion
A total of 31 studies with 4997 enrolled patients who received lipid-lowering therapy were included in our meta-analysis. The changes in coronary plaque volume were measured by IVUS, and the results showed significant coronary plaque regression in patients after receiving lipid-lowering therapy. The subgroup analysis indicated that TAV was significantly reduced when LDL at follow-up was less than 80 mg/dL and HDL was greater than or equal to 45 mg/dL, and PAV was significantly decreased when LDL at follow-up was less than 90 mg/dL and HDL was greater than or equal to 45 mg/dL. These findings were also confirmed by sensitivity analysis. Regression analysis showed that LDL levels at follow-up significantly influenced our results.
To better understand the link between lipid-lowering therapy and plaque regression, a meta-analysis was conducted to explore the changes in TAV and PAV in patients after receiving treatment, and the results showed a significant reduction in TAV and PAV at follow-up with some heterogeneity. We performed a subgroup analysis of the different types of drug administration in patients to explore the source of heterogeneity. The heterogeneity in the subgroups decreased significantly, suggesting that different drug regimens may be potential sources of heterogeneity.
In recent studies, LDL has been shown to accumulate abnormally in the vascular wall due to the dysfunction of endothelial cells. Moreover, LDL can be converted into ox-LDL, which can damage endothelial cells and smooth muscle cells, thereby causing abnormal activation of the endothelial cells, producing foam cells and eventually promoting plaque progression40. According to the latest guideline for the management of blood cholesterol and dyslipidemias, experts recommended that patients with a very high risk of arteriosclerotic cardiovascular disease (ASCVD) reduce LDL levels to below 70 mg/dL, which can delay the progress of risk factors and reduce the incidence of adverse events8. Our subgroup analysis showed that TAV was significantly reduced when the LDL levels were less than 80 mg/dL at follow-up, and PAV showed a significant decrease when the LDL levels were less than 90 mg/dL at follow-up.
In previous studies, HDL was shown to play an important role in the regression of coronary plaque by reverse cholesterol transport (RCT)3. HDL is mainly synthesized by apoAI and apoAII, which can clear or reuse cholesterol through lipid metabolism pathways, thereby reducing the progressive accumulation of cholesterol in plaque and promoting the regression of plaque41. A rise in HDL levels can reduce the incidence of cardiovascular adverse events. In the latest guideline for the management of dyslipidemias, HDL is the class I recommendation for lipid analyses in cardiovascular disease risk estimation42. In recent research, a rise in HDL level was shown to promote regression of coronary plaque and reduce the occurrence of MACE when LDL was greater than or equal to 70 mg/dL in patients receiving statin therapy43. Our subgroup analysis demonstrated a significant reduction in both TAV and PAV when HDL levels were greater than or equal to 45 mg/dL after lipid-lowering therapy.
Plaque regression is affected by various factors. In a study by Nicholls et al., a rise in HDL and reduction in TG slowed the progression of coronary atherosclerotic plaque44. A previous study also suggested that diabetes and hypertension can damage vascular endothelial function and promote the progression of coronary atherosclerotic plaque45. Changes in plaque volume at follow-up can also be affected by factors such as drug dose, method of observation, and location of the plaque. Therefore, we conducted a meta-regression analysis to assess other factors that could influence outcomes. The results indicated that LDL at follow-up affected both TAV and PAV, and gender only affected TAV, while dose of drugs and TG levels at baseline only affected PAV. Other risk factors did not influence the results.
Our analysis suggests that patients with CHD require an LDL level below 80 mg/dL and HDL above 45 mg/dL at follow-up for regression of coronary plaques to occur. TAV and PAV exhibited different target levels of LDL for plaque regression in our analysis, which may be due to differences in the number of studies included. The results have considerable significance for current CHD patient management and further research on coronary plaque regression. A recent study suggested that the regression of coronary atherosclerotic plaque in patients with stable CHD is closely related to myocardial infarction and vascular revascularization, but not significantly associated with MACE46. However, in the study by Hirohata et al., patients with atheroma progression displayed more adverse events than patients with no progression47. Therefore, combined with our research, these results suggest that for patients with CHD controlling LDL at follow-up below 80 mg/dL and HDL above 45 mg/dL can have a positive effect, improving patient prognosis. At the same time, the regression analysis also suggests the important role of LDL in plaque regression, which can provide new ideas for research on plaque regression in the future.
Limitations
This study also had several potential limitations. Most importantly, some of the studies included in the meta-analysis had a small sample size. Furthermore, some subgroup analysis included limited studies; therefore, more studies are needed to support the results. Finally, it is important to assess heterogeneity among studies, and although it may not be possible to identify all possible sources of heterogeneity, the stability of our outcomes was confirmed after adjusting for potential publication bias.
Conclusions
In general, recent meta-analyses have only considered the effect of LDL on plaque regression, whereas our meta-analysis indicates not only that LDL should be reduced to a target level of < 80 mg/dL, but also that HDL should be increased to a target level of > 45 mg/dL to regress coronary plaque.
Methods
This meta-analysis strictly abided by the PRISMA guidelines48.
Protocol and registration
The review protocol was developed according to PRISMA guidelines , and was registered in PROSPERO. The registration number was CRD42019146170.
Eligibility criteria
Studies were included if they met the following inclusion criteria: (1) the study design was a prospective clinical cohort study; (2) the impact of lipid-lowering therapy on coronary plaque volume was investigated, including quantitative measurement of plaque volume by IVUS; (3) sufficient information on blood lipids and IVUS findings at baseline and at the end of the study were presented; (4) lipid-lowering therapy was administered for at least 6 months; and (5) primary or secondary outcomes included change in total atheroma volume or percent atheroma volume.
Studies were excluded if they were: (1) non-clinical studies, observational studies, or retrospective studies; (2) duplicate reports or secondary or post hoc analyses of the same study population; or they contained (3) insufficient information on plaque volume and blood lipids (mean, SD, and sample sizes).
Information sources
The review searched studies based on PICOS (populations: CHD patients; interventions: lipid-lowering therapy; comparisons: before lipid-lowering therapy; outcomes: change in plaque volume as the first or secondary outcome; study design: prospective clinical cohort study) strategy in online databases (PubMed, EMBASE, Cochrane Library, and Web of Science) up to 15 July 2020 were systematically searched.
Search
The following search terms were searched in databases: (intravascular ultrasound OR IVUS) AND (lipid-lowering OR PCSK9 inhibitor OR PCSK9 inhibitors OR evolocumab OR alirocumab OR cholesterol absorption inhibitor OR cholesterol absorption inhibitors OR ezetimibe OR statin OR statins OR rosuvastatin OR pravastatin OR fluvastatin OR simvastatin OR atorvastatin OR pitavastatin OR lovastatin OR cerivastatin OR hydroxymethylglutaryl-CoA reductase inhibitors OR bile acid sequestrants) AND (plaque OR plaque, atherosclerotic). This analysis only included human studies and those published in English.
Selection of sources of evidence
Two reviewers (Yingrui Li and Songbai Deng) extracted data from included studies independently. When there was a disagreement on studies, the two reviewers reached a consensus through negotiation. The data extracted from each study included the sample size, LDL, HDL, TAV, and PAV at baseline and at the end of the study.
Data charting process
The Microsoft Excel was applied for a data charting form in this study. One single reviewer tested the form via 10 full-text articles. Then, both reviewers modified the form and confirmed the details of the process and data obtaining. None of reviewers found a need for additional modifications to the form.
Data items
Data about article (title, authors, year, area and study design), participant characteristics (sample size, age, BMI, gender, PAV, TAV, HDL, LDL, drug administration, smoking, diabetes and hypertension), and information on the assessment used (included population, methods for measuring participation and measurement of exposure factors) were extracted from included studies.
Critical appraisal of individual sources of evidence
The Jadad quality scale was used to assess the quality of randomized controlled trials, and the NOS was used to assess the quality of non-randomized controlled trials. The results ranged from 0 to 5 and 0 to 9, respectively, with higher scores representing better methodology quality (Table 1).
Synthesis of results
To calculate the 95% CI of the pooled standard mean difference (SMD) or weighted mean difference (WMD), we used a fixed effects model or a random effects model to perform all statistical analyses using Stata 12.0. P values < 0.05 were considered statistically significant and all P values were two-sided. The χ2 and I2 statistics were used to evaluate the heterogeneity between studies. If P was < 0.1 and I2 was > 30%, a random effects model was used; otherwise, a fixed effects model was used. Considering that one of the purposes of this study was to identify the LDL and HDL targets for plaque regression and determine the potential impact of confounding factors on the results of the study, we performed subgroup analyses of drug administration regimens and LDL and HDL levels at follow-up. Meanwhile, to examine the influence of individual studies on the total merged effects, we used a sensitivity analysis to evaluate the stability of the results. We applied Begg’s and Egger’s tests to assess publication bias in the included studies, and we assessed possible small sample effects by analyzing the symmetry of a funnel plot. P values < 0.10 were considered statistically significant49,50. Taking into account the differences between the studies, all of our analyses used a random effects model.
To explore the link between the dependent variable and the covariate, meta-regression is often used. We hypothesized that the included studies may have shown differences according to the age, gender, region, drugs and drug dosages, smoking, diabetes, hypertension, and blood lipids (LDL, HDL, TC, TG) of the patients. To evaluate the possible impact of these factors on the results of the meta-analysis, we established a regression model with the TAV or PAV value as the dependent variable (y) and the abovementioned covariate as the independent variable (x).
Patient and public involvement
Patients were not involved in the design or conduct of the study.
Data availability
No datasets were generated or analysed during the current study.
References
Benjamin, E. J. et al. Heart disease and stroke statistics—2019 update a report from the American Heart Association. Circulation 139(10), e56–e528 (2019).
Vigen, C. et al. Relation of progression of coronary artery atherosclerosis to risk of cardiovascular events (from the Monitored Atherosclerosis Regression Study). Am. J. Cardiol. 95(11), 1277–1282 (2005).
Francis, A. A. & Pierce, G. N. An integrated approach for the mechanisms responsible for atherosclerotic plaque regression. Exp. Clin. Cardiol. 16(3), 77–86 (2011).
Dohi, T. et al. Plaque regression determined by intravascular ultrasound predicts long-term outcomes of patients with acute coronary syndrome. J. Atheroscler. Thromb. 18(3), 231–239 (2011).
Nicholls, S. J. et al. Intravascular ultrasound—derived measures of coronary atherosclerotic plaque burden and clinical outcome. J. Am. Coll. Cardiol. 55, 2399–2407 (2010).
Tang, X. et al. The effect of statin therapy on plaque regression following acute coronary syndrome: A meta-analysis of prospective trials. Coron. Artery Dis. 27(8), 636–649 (2016).
Bhindi, R. et al. Coronary atheroma regression and adverse cardiac events: A systematic review and meta-regression analysis. Atherosclerosis 284, 194–201 (2019).
Grundy, S. M. et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 139(25), e1046–e1081 (2019).
Okazaki, S. et al. Early statin treatment in patients with acute coronary syndrome: Demonstration of the beneficial effect on atherosclerotic lesions by serial volumetric intravascular ultrasound analysis during half a year after coronary event: The ESTABLISH Study. Circulation 110(9), 1061–1068 (2004).
Nissen, S. E. et al. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: A randomized controlled trial. JAMA 291(9), 1071–1080 (2004).
Tani, S. et al. Effect of pravastatin on malondialdehyde-modified low-density lipoprotein levels and coronary plaque regression as determined by three-dimensional intravascular ultrasound. Am. J. Cardiol. 96(8), 1089–1094 (2005).
Yokoyama, M. et al. Plasma low-density lipoprotein reduction and structural effects on coronary atherosclerotic plaques by atorvastatin as clinically assessed with intravascular ultrasound radio-frequency signal analysis: A randomized prospective study. Am. Heart J. 150(2), 287 (2005).
Kawasaki, M. et al. Volumetric quantitative analysis of tissue characteristics of coronary plaques after statin therapy using three-dimensional integrated backscatter intravascular ultrasound. J. Am. Coll. Cardiol. 45(12), 1946–1953 (2005).
Nissen, S. E. et al. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: The ASTEROID trial. JAMA 295(13), 1556–1565 (2006).
Hong, Y. J. et al. The effects of rosuvastatin on plaque regression in patients who have a mild to moderate degree of coronary stenosis with vulnerable plaque. Korean Circ. J. 38, 366–373 (2008).
Takayama, T. et al. Effect of rosuvastatin on coronary atheroma in stable coronary artery disease: Multicenter coronary atherosclerosis study measuring effects of rosuvastatin using intravascular ultrasound in Japanese subjects (COSMOS). Circ. J. 73(11), 2110–2117 (2009).
Nasu, K. et al. Effect of fluvastatin on progression of coronary atherosclerotic plaque evaluated by virtual histology intravascular ultrasound. JACC Cardiovasc. Interv. 2(7), 689–696 (2009).
Hiro, T. et al. Effect of intensive statin therapy on regression of coronary atherosclerosis in patients with acute coronary syndrome: A multicenter randomized trial evaluated by volumetric intravascular ultrasound using pitavastatin versus atorvastatin (JAPAN-ACS [Japan assessment of pitavastatin and atorvastatin in acute coronary syndrome] study). J. Am. Coll. Cardiol. 54(4), 293–302 (2009).
Hong, M. K. et al. Effects of statin treatments on coronary plaques assessed by volumetric virtual histology intravascular ultrasound analysis. JACC Cardiovasc. Interv. 2(7), 679–688 (2009).
Hong, Y. J. et al. Comparison of effects of rosuvastatin and atorvastatin on plaque regression in Korean patients with untreated intermediate coronary stenosis. Circ. J. 75(2), 398–406 (2011).
Nicholls, S. J. et al. Effect of two intensive statin regimens on progression of coronary disease. N. Engl. J. Med. 365(22), 2078–2087 (2011).
Kovarnik, T. et al. Virtual histology evaluation of atherosclerosis regression during atorvastatin and ezetimibe administration: HEAVEN study. Circ. J. 76(1), 176–183 (2012).
Nozue, T. et al. Statin treatment for coronary artery plaque composition based on intravascular ultrasound radiofrequency data analysis. Am. Heart J. 163(2), 191–9.e1 (2012).
Lee, S. W. et al. Virtual histology findings and effects of varying doses of atorvastatin on coronary plaque volume and composition in statin-naive patients: The VENUS study. Circ. J. 76(11), 2662–2672 (2012).
Lee, C. W. et al. Comparison of effects of atorvastatin (20 mg) versus rosuvastatin (10 mg) therapy on mild coronary atherosclerotic plaques (from the ARTMAP trial). Am. J. Cardiol. 109(12), 1700–1704 (2011).
Guo, S. et al. Effects of atorvastatin on serum lipids, serum inflammation and plaque morphology in patients with stable atherosclerotic plaques. Exp. Ther. Med. 4(6), 1069–1074 (2012).
Zhang, X. et al. Intensive-dose atorvastatin regimen halts progression of atherosclerotic plaques in new-onset unstable angina with borderline vulnerable plaque lesions. J. Cardiovasc. Pharmacol. Ther. 18(2), 119–125 (2013).
Hwang, D. S. et al. Early differential changes in coronary plaque composition according to plaque stability following statin initiation in acute coronary syndrome: Classification and analysis by intravascular ultrasound-virtual histology. Yonsei Med. J. 54(2), 336–344 (2013).
Räber, L. et al. Effect of high-intensity statin therapy on atherosclerosis in non-infarct-related coronary arteries (IBIS-4): A serial intravascular ultrasonography study. Eur. Heart J. 36(8), 490–500 (2015).
Masuda, J. et al. Effect of combination therapy of ezetimibe and rosuvastatin on regression of coronary atherosclerosis in patients with coronary artery disease. Int. Heart J. 56(3), 278–285 (2015).
Tsujita, K. et al. Impact of dual lipid-lowering strategy with ezetimibe and atorvastatin on coronary plaque regression in patients with percutaneous coronary intervention: The multicenter randomized controlled PRECISE-IVUS trial. J. Am. Coll. Cardiol. 66(5), 495–507 (2015).
Matsushita, K. et al. Effects of 4 statins on regression of coronary plaque in acute coronary syndrome. Circ. J. 80(7), 1634–1643 (2016).
Oemrawsingh, R. M. et al. Integrated Biomarker and Imaging Study 3 (IBIS-3) to assess the ability of rosuvastatin to decrease necrotic core in coronary arteries. EuroIntervention 12(6), 734–739 (2016).
Nicholls, S. J. et al. Effect of evolocumab on progression of coronary disease in statin-treated patients: The GLAGOV randomized clinical trial. JAMA 316(22), 2373–2384 (2016).
Takayama, T. et al. Comparison of the effect of rosuvastatin 2.5 mg vs 20 mg on coronary plaque determined by angioscopy and intravascular ultrasound in Japanese with stable angina pectoris (from the aggressive lipid-lowering treatment approach using intensive rosuvastatin for vulnerable coronary artery plaque [ALTAIR] randomized trial). Am. J. Cardiol. 117(8), 1206–1212 (2016).
Hougaard, M. et al. Influence of ezetimibe in addition to high-dose atorvastatin therapy on plaque composition in patients with ST-segment elevation myocardial infarction assessed by serial: Intravascular ultrasound with iMap: The OCTIVUS trial. Cardiovasc. Revasc. Med. 18(2), 110–117 (2017).
Ueda, Y. et al. Effect of ezetimibe on stabilization and regression of intracoronary plaque—The ZIPANGU Study. Circ. J. 81(11), 1611–1619 (2017).
Hibi, K. et al. Effects of ezetimibe-statin combination therapy on coronary atherosclerosis in acute coronary syndrome. Circ. J. 82(3), 757–766 (2018).
Thondapu, V. et al. Comparison of rosuvastatin versus atorvastatin for coronary plaque stabilization. Am. J. Cardiol. 123(10), 1565–1571 (2019).
Barua, R. S. et al. Reactive oxygen species are involved in smoking induced dysfunction of nitric oxide biosynthesis and up regulation of endothelial nitric oxide synthase: An in vitro demonstration in human coronary artery endothelial cells. Circulation 107, 2342–2347 (2003).
Clay, M. A. et al. Cholesteryl ester transfer protein and hepatic lipase activity promote shedding of apoA-1 from HDL and subsequent formation of discoidal HDL. Biochem. Biophys. Acta. 1124, 52–58 (1992).
Ridker, P. M. et al. Reduction in C-reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: A prospective study of the JUPITER trial. Lancet 373, 1175–1182 (2009).
Puri, R. et al. The beneficial effects of raising high-density lipoprotein cholesterol depends upon achieved levels of low-density lipoprotein cholesterol during statin therapy: Implications for coronary atheroma progression and cardiovascular events. Eur. J. Prev. Cardiol. 23(5), 474–485 (2016).
Nicholls, S. J. et al. Lowering the triglyceride/high-density lipoprotein cholesterol ratio is associated with the beneficial impact of pioglitazone on progression of coronary atherosclerosis in diabetic patients: Insights from the PERISCOPE (Pioglitazone Effect on Regression of Intravascular Sonographic Coronary Obstruction Prospective Evaluation) study. J. Am. Coll. Cardiol. 57(2), 153–159 (2011).
Murcia, A. M. et al. Impact of diabetes on mortality in patients with myocardial infarction and left ventricular dysfunction. Arch. Intern. Med. 164, 2273–2279 (2004).
D’Ascenzo, F. et al. Atherosclerotic coronary plaque regression and the risk of adverse cardiovascular events: A meta-regression of randomized clinical trials. Atherosclerosis 226(1), 178–185 (2013).
Hirohata, A. et al. Four-year clinical outcomes of the OLIVUS-Ex (impact of Olmesartan on progression of coronary atherosclerosis: Evaluation by intravascular ultrasound) extension trial. Atherosclerosis 220(1), 134–138 (2012).
Moher, D. et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J. Clin. Epidemiol. 62(10), 1006–1012 (2009).
Jonathan, A. C., Sterne, M. E. & Smith, G. D. Systematic reviews in health care: Investigating and dealing with publication and other biases in meta-analysis. BMJ 323, 101–105 (2001).
Duval, S. & Tweedie, R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta- analysis. Biometrics 56, 455–463 (2000).
Acknowledgements
Not applicable.
Author information
Authors and Affiliations
Contributions
Qiang She, Yingrui Li, Yulin Yan, and Xiaodong Jing contributed to the conception or design of the work. Yingrui Li, Songbai Deng, Bin Liu, Yu Li, Jing Wang, and Jun Du contributed to the acquisition, analysis, or interpretation of data. Yingrui Li drafted the manuscript. Qiang She, Jianlin Du, and Yajie Liu critically revised the manuscript. All gave final approval and agree to be accountable for all aspects of the work ensuring integrity and accuracy.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, Y., Deng, S., Liu, B. et al. The effects of lipid-lowering therapy on coronary plaque regression: a systematic review and meta-analysis. Sci Rep 11, 7999 (2021). https://doi.org/10.1038/s41598-021-87528-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-87528-w
This article is cited by
-
Impact of coronary CT angiography in selection of treatment modalities and subsequent cardiovascular events in Thai patients with stable CAD
Clinical Research in Cardiology (2024)
-
Risk factor management and OCT characteristics of plaque vulnerability: the Holy Grail of plaque and patient vulnerability
The International Journal of Cardiovascular Imaging (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.