Abstract
Culex mosquitoes particularly Culex quinquefasciatus are important arboviral and filariasis vectors, however despite this important epidemiological role, there is still a paucity of data on their bionomics. The present study was undertaken to assess the insecticide resistance status of Cx. quinquefasciatus populations from four districts of Yaoundé (Cameroon). All Culex quinquefasciatus populations except one displayed high resistance to bendiocarb and malathion with mortalities ranging from 0 to 89% while high resistance intensity against both permethrin and deltamethrin was recorded. Molecular analyses revealed high frequencies of the ACE-1 G119S mutation (ranging from 0 to 33%) and kdr L1014F allele (ranging from 55 to 74%) in all Cx. quinquefasciatus populations. Significant overexpression was detected for cytochrome P450s genes CYP6AA7 and CYP6Z10, as well as for Esterase A and Esterase B genes. The total cuticular hydrocarbon content, a proxy of cuticular resistance, was significantly increased (compared to the S-lab strain) in one population. The study confirms strong insecticide resistance mediated by different mechanisms in Cx. quinquefasciatus populations from the city of Yaoundé. The expansion of insecticide resistance in Culex populations could affect the effectiveness of current vector control measures and stress the need for the implementation of integrated vector control strategies in urban settings.
Similar content being viewed by others
Introduction
Culex species and particularly mosquitoes of the Culex pipiens complex, are considered vectors of several diseases such as Lymphatic filariasis, West Nile Virus, Japanese Encephalitis, Saint Louis Encephalitis, Dengue and Rift Valley Fever with some being fatal in the absence of treatment and others causing lifelong disabilities and impairment1. In addition to their role as vectors, Culex species are also responsible for a high nuisance problem2,3. Indicatively Culex mosquitoes exhibit high biting rates exceeding 100 bites/person/night2,4. The Cx. pipiens complex member Culex quinquefasciatus is a prominent vector species which feeds on both humans and animals5,6, increasing its implication in pathogen transmission to both host groups. The vector displays a variety of breeding habitats including swamps, drains, pit latrin and permanent or semipermanent stagnant water bodies full of organic matters7,8, commonly found within and around African cities. Notably, the rapid unplanned urbanization of major cities in Africa, has favoured the installation of Culex quinquefasciatus within the urban environment2,9, including the cities of Yaoundé and Douala in Cameroon2,8,10.
Despite a growing interest in the promotion of integrated vector control strategies co-targeting different vector species, in Cameroon control efforts and relative entomological, epidemiological and insecticide resistance studies primarily focus on anophelines resulting in important knowledge gaps regarding Culex species and their control. In Cameroon, vector control mainly relies on the use of long-lasting insecticidal nets (LLINs) with over 35 million LLINs having been freely distributed to the population during mass distribution campaigns over the last decade11. This massive deployment of insecticide treated nets across the country and the increased use of pesticides in urban agriculture are considered to have driven insecticide resistance in Anopheles species which are now resistant to all insecticide compounds used in public health12,13,14,15. Indicatively, recent studies conducted in the city of Yaoundé reported a rapid expansion of insecticide resistance in Anopheles gambiae and An. coluzzii associated with different underlying mechanisms12,13,16,17. As Culex mosquitoes are usually found in sympatry with anophelines in sub-Saharan Africa7,18, it is possible that local Culex populations have also received high insecticide selection pressures7,18, driving the development of resistance in important vector species. A recent study on Culex mosquitoes from Cameroon points to this direction as phenotypic resistance to permethrin (mortality rate (mr) ranging from 14.25 to 66.05%) deltamethrin (mr: 2.91% to 20.78%), DDT (mr: 8.87% to 27.91%) and bendiocarb was recorded in the analysed populations8.
Insecticide resistance is primarily mediated by two molecular mechanisms: target site mutations—altering the insecticide’s target molecule and metabolic resistance—involving the increased activity of detoxification enzymes19,20. The voltage gated sodium channel (VGSC) gene point mutations L1014F, L1014S and L1014C (kdr mutations) associated with pyrethroid and secondarily organochlorine resistance in different insects have been recorded in a number of Culex populations across the world. Mutation L1014F, which upon homozygosity has been associated with operationally significant resistant phenotypes and striking ones, when in combination with P450 metabolic resistance21 has been recorded in Cx. quinquefasciatus populations from Benin, Cameroon and Zambia8,22,23 at allele frequencies ranging from 20 to 60%24.
The G119S mutation in the synaptic acetylcholinesterase gene, associated with organophosphate and carbamate resistance25,26 has also been recorded in African Culex pipiens complex populations, specifically from Morocco, Uganda and Benin albeit at low frequencies and mainly in heterozygosis23,27,28,29. G119S mutation has a high fitness cost and duplicated alleles have evolved on several occasions in Culex field populations creating a permanent state of ‘heterozygosity’, alleviating this cost26,30,31.
Detoxification enzymes belonging to the esterase and cytochrome P450 monooxygenases families have been associated with insecticide resistance in Culex mosquitoes. Overexpression of the P450 enzymes CYP9M10, CYP6AA7, CYP6Z10, CYP4H34 and Esterase A, Esterase B have been associated with pyrethroid and organophosphate/carbamate resistance respectively in Cx. quinquefasciatus mosquitoes from Saudi Arabia, USA and the Western Indian Ocean Islands20,30. To date, there are very few studies investigating metabolic resistance in Culex mosquitoes from Africa, albeit not at the molecular level. For example, synergist bioassays on Cx. pipiens populations from Tanzania and Zanzibar28,31 implicate the involvement of monooxygenases, esterases, and glutathione S-transferase in pyrethroid and DDT resistance.
A third resistance mechanism, described in Anopheles species, involves cuticular alterations resulting in reduced insecticide uptake32. Cuticle thickening has also been reported in Culex pipiens pallens lab strains33 yet no field Culex populations have been analyzed for this resistance trait.
In light of the rapid expansion of insecticide resistance in mosquito vector populations in Cameroon and the limited knowledge on the local Culex quinquefasciatus resistance status the present study was undertaken (i) to assess the pattern of insecticide resistance in Cx. quinquefasciatus populations from Yaoundé against different insecticide classes and (ii) identify possible incipient resistance (i.e. resistant alleles at low frequencies) and investigate the underlying molecular mechanisms driving this resistance (i.e. target site, metabolic, cuticular).
Results
Species identification
To confirm morphological identification, a subsample of 40 mosquitoes were genotyped. All mosquitoes processed from the four study sites (Mendong, Nkolbisson, Tongolo and Etam-Bafia) were identified as Cx. quinquefasciatus.
Susceptibility status of Culex quinquefasciatus populations
A total of 1,797 field-collected, 500 S-lab laboratory strain Culex quinquefasciatus specimens and 500 An. gambiae Kisumu strain were tested. WHO tube bioassays conducted with adult females, revealed high phenotypic insecticide resistance to pyrethroids, DDT and bendiocarb in Cx. quinquefasciatus from the four study sites (Table 1). No mortality was recorded when mosquitoes were exposed to 0.75% permethrin. Mortality rates ranging from 0 to 1.16% was recorded for 0.05% deltamethrin, 0–3% for 4% DDT and 0–14.63% for 0.1% bendiocarb. Resistance to malathion was observed in mosquitoes from Nkolbisson displaying a mortality rate of 57.5%. Mortality to malathion in the remaining populations ranged from 88 to 99%. The An. gambiae Kisumu strain was susceptible to all insecticides whereas the Cx. quinquefasciatus S-lab reference strains showed increase tolerance to permethrin and deltamethrin and high resistance to DDT.
Intensity of pyrethroid resistance in Culex quinquefasciatus populations
High pyrethroid resistance intensity was recorded in Culex quinquefasciatus populations from all four study sites (Fig. 1). The mortality rate of Cx. quinquefasciatus was found to increase with the concentration of both permethrin and deltamethrin. Depending on the site, the mortality rate varied from 2.46 to 29.34% for permethrin 5 × and from 3.75 to 38% for permethrin 10 ×. For deltamethrin, the mortality rate varied from 0 to 2.17% for deltamethrin 5 × and from 1.17 to 16.25% for deltamethrin 10 ×.
Effects of PBO synergist on the susceptibility of Culex quinquefasciatus to pyrethroids
Pre-exposure to PBO synergist significantly increased the susceptibility level of the Mendong Cx. quinquefasciatus population against deltamethrin, reporting a mortality rate of 7% (Fig. 2). Pre-exposure of the other populations to PBO did not alter the susceptibility levels against deltamethrin or permethrin.
Screening of target site mutations (kdr L1014F, L1014S, L1014C, and ace-1 G119S)
Mosquitoes resistant (bioassay survivors) to permethrin, deltamethrin or DDT were screened for kdr mutations at the 1014 VGSC locus. In Mendong, L1014F mutant allele frequency (MAF) reached 73.91%, in Nkolbisson 56.25%, in Tongolo 77.27% and in Etam-Bafia 55.00% (Table 2). The L1014S and L1014C kdr alleles were not detected in any of the samples.
The ace-1 G119S mutation was also detected in mosquito specimens resistant to malathion or bendiocarb. Apart from Mendong, where G119S mutation was not detected, subsamples of the three remaining locations display the mutation with a MAF varying from 18.48 to 33.33% (Table 2).
Detoxification gene expression analysis
The expression levels of six major detoxification genes associated with Culex metabolic resistance were compared between field Cx. quinquefasciatus populations and the S-lab susceptible laboratory strain. Detoxification genes analysed included CYP9M10, CYP6AA7, CYP6Z10, CYP4H34, Esterase A and Esterase B. The cytochrome P450 genes CYP6AA7 and CYP6Z10 were found to be upregulated (> 4.0 folds, P < 0.05) in all study sites. The CYP9M10 was not strongly overexpressed in any of the study populations. The expression of CYP4H34 gene was not detectable in any population, despite several attempts with alternative sets of primers. Esterase B was found to be overexpressed (> 10-folds) in all study sites and Esterase A in 3 of the 4 study sites (upregulation > 10-folds) (Fig. 3 and Suppl Table S2).
More precisely, the Mendong population showed a 7.54-fold and a 23.1-fold overexpression of CYP6AA7 and CYP6Z10, respectively. The Esterase B gene was also found to be significantly overexpressed in Mendong (18.7-folds) (Fig. 3 and Suppl Table S2). In Nkolbisson, the CYP6AA7 and CYP6Z10 genes were overexpressed 7.46- and 4.32- times, respectively, along with both Esterase genes (21.3-folds for Esterase A and 15.4-folds for Esterase B) (Fig. 3 and Suppl Table S2). In Tongolo, CYP6AA7 was overexpressed 10.24 times and CYP6Z10 5.01 times. Esterase A gene showed a 50.4-fold upregulation and Esterase B a 14.8-fold upregulation (Fig. 3 and Suppl Table S2). In the Etam-Bafia population a particularly robust overexpression of CYP6AA7 was detected (14.5 folds), followed by CYP6Z10 (5.36 folds). Esterase A and Esterase B genes were also overexpressed in Etam-Bafia (6.54- and 19.0- times, respectively) (Fig. 3 and Suppl Table S2).
Analysis of cuticular hydrocarbon as a marker of reduced penetration based insecticide resistance
Analysis of CHCs showed a statistically significant increase (P = 0.049) of normalised CHC content in the Tongolo population (1849 ± 70.0 ng CHCs/mg dry weight) compared to the susceptible laboratory strain (1552 ± 80.1 ng CHCs/mg dry weight). The remaining populations did not show a significant quantitative increase in their CHC profiles (Fig. 4).
Mean CHC amounts from the five Culex mosquito populations. N and M mosquitoes have higher amounts of CHCs compared to S mosquitoes normalized for their size differences, but not E specimens. T mosquitoes have significantly higher CHCs compared to the S laboratory strain. Error bars: Standard Error of mean. S: Susceptible laboratory strain, E: Etam-Bafia, N: Nkolbisson, T: Tongolo, M: Mendong. NS: Not Statistically Significant.
Discussion
Although Culex quinquefasciatus mosquitoes are predominant in most cities across Sub-Saharan Africa and they are of major epidemiological significance as vectors of important diseases like West Nile Virus and filariasis, little is known about their susceptibility to insecticides and particularly the presence and frequency of previously characterised resistance alleles in the local populations. The study objective was to assess the resistance status of Cx. quinquefasciatus populations from the city of Yaoundé against different insecticides.
High phenotypic resistance against DDT, deltamethrin, permethrin, bendiocarb, and malathion was detected in the analyzed Cx. quinquefasciatus populations.
The generated results are in line with previous bioassay records in Culex populations from the city of Yaoundé including Culex quinquefasciatus, Cx. duttoni, Cx. antennatus, Cx. perfuscus and Cx. tigripes specimens8. However here we report a significant decrease in the mortality rates of the tested Yaoundé populations, compared to the mortality rates recorded in previous studies8, indicating an ongoing process of insecticide resistance evolvement during the years 2017–2020.
Importantly, the tested populations from the four study sites of Yaoundé exhibited very high resistance intensity against permethrin and deltamethrin with mortality rates below 40% even when mosquitoes were exposed to the 10 × diagnostic concentration. This particularly low level of susceptibility to pyrethroids is of major concern with potentially operationally relevant implications in the control and elimination of diseases transmitted by these vectors.
The current expansion of pyrethroid and DDT resistance in Cx. quinquefasciatus may have derived from the increased selection pressure induced by the massive deployment of pyrethroid treated nets across the country in conjunction with the use of pyrethroid-based insecticides in agricultural pest control11.
Notably, the observed Cx. quinquefasciatus resistance profile was actually similar to that of An. gambiae populations from Yaoundé12,13,16 most likely attributed to common insecticidal pressures imposed on both vector species.
The Culex quinquefasciatus populations also displayed high resistance to both bendiocarb and malathion apart from the Mendong population which was susceptible to malathion. This is the first report of malathion resistance reported in Culex populations from Yaoundé.
Presence/frequency analyses of known target-site resistance mutations recorded the kdr allele L1014F at high frequencies in all populations. The recorded frequencies (ranging from 55–77%) were relatively increased to those reported in a previous study from Yaoundé city (frequency = 51%) conducted in 20178 indicating an ongoing pyrethroid induced selection process.
The G119S ace-1 mutation associated with both carbamate and organophosphate resistance in Cx. quinquefasciatus and An. gambiae sl.26,42,43 was also recorded in the analysed mosquitoes apart from the Mendong population. G119S was always recorded in heterozygosity, possibly due to the amplification events and fitness cost of this gene in the homozygous state26,44,45, while the non-detection of G119S in Mendong is in line with the respective population’s organophosphate susceptibility.
Use of the PBO synergist increased the deltamethrin susceptibility level of Cx. quinquefasciatus mosquitoes in some cases, indicating metabolic resistance and P450 monooxygenase enzyme might be involved in pyrethroid resistance.
In order to molecularly profile metabolic resistance, as a more specific and sensitive marker of metabolic resistance (including incipient resistance), the expression pattern of six detoxification genes (CYP9M10, CYP6AA7, CYP6Z10, CYP4H34, Esterase A and Esterase B) previously reported to be associated with insecticide resistance in Cx. quinquefasciatus41,46,47,48 were assessed in the four study populations.
The expression levels of two of the four cytochrome P450s analysed, CYP6AA7 and CYP6Z10, were found to be upregulated (> 5.0 folds) in all study sites. The CYP6AA7 gene has been shown to be overexpressed in pyrethroid resistant Cx. quinquefasciatus41 and is known to metabolize permethrin and its metabolites in vitro49 while CYP6Z10 is also considered to be involved in pyrethroid resistance in Cx. quinquefasciatus41. Although CYP4H34 and CYP9M10 genes are known pyrethroid metabolisers41 their non-expression or non- biologically relevant overexpression in the examined populations could derive from the fact that they are predominantly expressed at the larval stage and show negligible expression in adults mosquitoes41,50,51. Under the prism of P450 target site synergism21 the co-presence of L1014F and CYP6AA7, CYP6Z10 overexpression in the study populations may explain the strong resistance phenotypes against the pyrethroid insecticides.
Esterases A and B were found to be significantly overexpressed (> 10-folds, P < 0.05) in almost all populations. Esterases are considered to be implicated in organophosphate and carbamate resistance in Culex mosquitoes52,53,54 while they have also been reported to confer pyrethroid resistance in mosquitoes31,52 and in Helicorverpa armigera55.
Finally, a significant increase of CHC was detected in one study population possibly indicating that cuticle resistance may also contribute to the resistance phenotype of Cx. quinquefasciatus mosquitoes, in line with previous studies in Anopheles species32,56.
The multiple insecticide resistance mechanisms observed in Cx. quinquefasciatus populations from Yaoundé suggest high selective pressure taking place in this urban environment. In addition to insecticides, xenobiotics could also induce high resistance in this mosquito species due to its preference for organically polluted habitats at the larval stage57. The influence of organic pollution on Cx. quinquefasciatus susceptibility to insecticide has so far not been explored and requires further investigations.
Conclusion
This study revealed the high resistance profile of Cx. quinquefasciatus populations from Yaoundé to DDT, permethrin, deltamethrin, bendiocarb and malathion. Several underlying mechanisms including the target site kdr mutations L1014F and ace-1 G119S, overexpression of cytochrome P450s and esterases A and B and possibly cuticle resistance were found to be associated to this resistance, in certain populations. The multi-resistance observed in the Cx. quinquefasciatus populations could affect the efficacy of insecticide interventions, thus the development of appropriate evidence-based insecticide resistance management programs under an integrated vector control approach is recommended.
Methods
Study sites
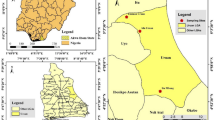
The present study took place in Yaoundé (3° 51′ N; 11° 31′ E), the capital city of Cameroon. Yaoundé is located within the Congo-Guinean phytogeographic zone characterized by a typical equatorial climate with four seasons: two rainy seasons (March to June and September to November) and two dry seasons (December to February and July to August). The city has a population estimated at about 3 million inhabitants and is situated 800 m above sea level34. The landscape of Yaoundé is characterized by an alternation of high and lowland areas frequently used for agricultural practices. Mosquitoes were collected from September to November 2019 in four districts of Yaoundé, two situated in the periphery (Nkolbisson & Mendong) and two in the city centre (Tongolo & Etam-Bafia) (Fig. 5). Nkolbisson and Mendong are located along the Mefou river. They are densely populated areas with constructions extending to the swampy area and with the edges of the Mefou river exploited for the practice of agriculture during the dry season35. Tongolo and Etam-Bafia are two districts situated in the city centre. They are highly populated areas and characterized by poor drainage, high pollution and the presence of numerous standing water collections full of organic matter.
Study sites in the city of Yaoundé, Cameroon. The administrative division of Cameroon is available in open access on the OpenStreetMap platform (https://www.openstreetmap.org/search?query=cameroon#map=6/7.406/12.283). We used the ArcGIS version 10.2.2 software (ESRI, Redland, CA, USA; https://www.esri.com/en-us/arcgis/about-arcgis/overview) to generate the map showing study sites in Yaoundé.
Mosquito collection, rearing and processing
Blood fed Cx. quinquefasciatus females collected indoors with mouth aspirators were identified morphologically to species36,37 and maintained for rearing at the insectary. All blood fed females were given access to 10% sugar solution and were maintained at 25–30 °C and 70–80% relative humidity until they became fully gravid. The produced eggs were pooled and reared in trays containing dechlorinated water. After hatching larvae were fed using Tetramin baby fish food.
After emergence, unexposed, non-blood fed females aged 3–5 days from each site were divided into three batches. The first batch consisting of 50–60 specimens was preserved in RNA later for characterization of detoxification resistance mechanisms. The second batch comprising of 100–120 specimens was killed (after few minutes in the freezer) and air-dried at room temperature for 48 h for characterization of cuticular resistance. The remaining set of mosquitoes consisting of 500–700 specimens was used in bioassay experiments. Bioassay survivors against all insecticides were preserved in 70% ethanol and used for molecular species identification and detection of target-site mutations.
Insecticide susceptibility tests
Bioassays were performed following the WHO guidelines38 with insecticides from four classes. For each mosquito population, four replicates of 20–25 F1 females each, were exposed to 0.75% permethrin, 0.05% deltamethrin, 4% DDT, 0.1% bendiocarb and 5% malathion. For each bioassay, two replicates of 20–25 female mosquitoes unexposed to any insecticide were used as an internal control. The reference Culex strain S-lab and An. gambaie Kisumu strain were tested alongside the field populations of Cx. quinquefasciatus to validate the efficacy of the impregnated papers. After 60 min exposure, mosquitoes were transferred to holding tubes and supplied with 10% glucose. The mortality rate was recorded 24 h post-exposure.
Estimating insecticide resistance intensity
To estimate the intensity of pyrethroid resistance, mosquitoes were exposed to discriminating concentrations of permethrin and deltamethrin. For each test, 4 replicates of 20–25 females aged 3–5 days were exposed to the following concentrations: 0.05% (1 ×), 0.25% (5 ×) and 0.5% (10 ×) deltamethrin, and 0.75% (1 ×), 3.75% (5 ×) and 7.5% (10 ×) permethrin for 60 min. Mortality was recorded after 24 h. These bioassays were conducted following the WHO protocol described above38.
Synergist bioassay
To assess the implication of P450 detoxification enzymes in pyrethroid resistance, bioassays with 4% PBO39, were conducted. For each mosquito population, four replicates of 20–25 F1 females mosquitoes were pre-exposed to PBO for 1 h and then transferred to tubes containing either 0.75% permethrin or 0.05% deltamethrin for 1 h. Bioassays were performed following the WHO protocol38. For each experiment, two replicates of 20–25 females mosquitoes exposed to PBO only were used as a control.
Genomic DNA and total RNA extraction
Genomic DNA was extracted from individual mosquitoes using the DNazol protocol, according to the manufacturer’s instructions (Molecular Research Center Inc). Total RNA was extracted from pooled mosquito specimens (N = 10 per pool) using the TRI reagent (TR 118, Molecular Research Center Inc), following the manufacturer’s instructions. The quantity and purity of DNA and total RNA were assessed spectrophotometrically (Nanodrop). The quality of RNA was assessed by 1.0% w/v agarose gel electrophoresis.
Identification of Culex species
Molecular identification of species was performed by applying the PCR-based assays for the identification of members of the Cx. pipiens complex described by Smith & Fonseca40. A total of 40 mosquitoes (10 from each collection site) was analysed.
Detection of L1014 kdr mutations
The presence/frequency of L1014 kdr mutations was analyzed via Sanger Sequencing. The PCRs were carried out in 25 µl reactions containing 1 unit of Kapa Taq DNA polymerase (Kapa Biosystems), 0.4 mM dNTPs,1 × KapaTaq Buffer A (containing 1.5 mM MgCl2) and 0.3 µM each of the forward and reverse primers. The amplification consisted of an initial heat activation step at 95 °C for 5 min, followed by 40 cycles of 95 °C for 30 s, 54 °C for 30 s and 72 °C for 30 s with a final extension step at 72 °C for 5 min. The sequences of the primers used were F: 5′-TGATTGTGTTCCGGGTGCTG-3′ and R: 5′-GCAATTGCACCTTTAGGTGTGG-3′. The PCR fragments 350 bp were purified using NUCLEOSPIN Gel and PCR Clean-up (Macherey–Nagel). Nucleotide sequences were determined in purified PCR products at the GeneWiz sequencing facility (Leipzig, Germany).
Detection of ACE-1 G119S mutation
Mosquitoes were tested for insensitive acetylcholinesterase (ace-1) mutations using the PCR–RFLP method as described in Weill et al.26. PCR products were digested with AluI (Minotech Biotechnology, Heraklion, Greece) restriction enzyme for 3 h and migrated on a 2.0% agarose gel.
Gene expression analysis of major detoxification genes (CYP9M10, CYP4H34, CYP6AA7, CYP6Z10, Esterase A and Esterase B)
The expression analysis of major detoxification genes was done via reverse transcription and qPCR based on SYBR Green chemistry. cDNA was synthesized using 1 μg οf total RNA, previously treated with TURBO DNase (Invitrogen, Carlsbad, CA, USA), with oligo (dT)12–18 primers and the Minotech RT system kit (Minotech Biotechnology, Heraklion, Greece), following the manufacturer’s instructions. The SYBR Green-based qPCR assays were run in duplicates in 10 μl reactions, consisting of 2 × Kapa SYBR Fast Universal qPCR Master Mix (Kapa Biosystems, Wilmington, MA, USA), forward and reverse primers specific for each gene (Suppl Table S1) at a final concentration of 200 nM as well as 10 ng of cDNA template. Ribosomal Protein L8 (RPL8) and Ribosomal protein subunit 3 (RPS3) were used for normalization purposes. Bio-Rad CFX CONNECT Real-Time PCR detection was used with a thermal protocol consisting of a 3 min polymerase activation/initial denaturation step at 95 °C, 40 cycles of denaturation and annealing/extension steps at 95 °C for 3 s, 60 °C for 30 s, followed by a melting curve analysis step. A no-template control was included in each qPCR run. All assays were previously checked for specificity (melting curve and agarose gel electrophoresis of PCR products) and reaction efficiency, dynamic range and linearity (standard curve experiments) and reproducibility (estimation of the coefficient of variation-CV by analysing a series of samples in different runs). Quality control data are provided in Suppl Table S1.
Extraction and Quantification of Cuticle Hydrocarbon (CHC) Lipids
Mosquitoes were air-dried at room temperature for 48 h and then pooled (20–25 female mosquitoes/replicate, 3 replicates for each population/strain), the dry weight of each replicate was measured, and CHC analysis was performed by GC–MS and GC-FID as previously described13,32.
Ethical clearance
All experimental protocols were approved by the Cameroon National Ethics Committee on Human health (ethical clearance N° 2016/11/832/CE/CNERSH/SP). The committee reviews.
aspects related to both human and animal and all methods were carried out in accordance with relevant guidelines and regulations.
Data analysis
The insecticide susceptibility data was interpreted as follows: 98%-100% mortality indicates susceptibility, 90%-97% mortality suggests possible resistance that needs to be confirmed, < 90% mortality suggests resistance38. Calculation of fold-changes, 95% CIs and statistical significance was performed according to the Pfaffl method41. Graphs of metabolic gene expression were constructed with the SigmaPlot software (v12.0). Differences were considered statistically significant at P < 0.05.
References
Kauffman, E. B. & Kramer, L. D. Zika virus mosquito vectors: competence, biology, and vector control. J. Infect. Dis. 216, S976–S990 (2017).
Antonio-Nkondjio, C. et al. High mosquito burden and malaria transmission in a district of the city of Douala Cameroon. BMC Infect. Dis. 12, 275 (2012).
Turell, M. J. et al. Vector competence of selected African mosquito (Diptera: Culicidae) Species for Rift Valley fever virus. J. Med. Entomol. 45, 102–108 (2008).
Mbida, A. M. et al. Preliminary investigation on aggressive culicidae fauna and malaria transmission in two wetlands of the Wouri river estuary Littoral-Cameroon. J. Entomol. Zool. Stud. 4, 105–110 (2016).
Farajollahi, A., Fonseca, D. M., Kramer, L. D. & Kilpatrick, A. M. “Bird biting” mosquitoes and human disease: a review of the role of Culex pipiens complex mosquitoes in epidemiology. Infect. Genet. Evol. 11, 1577–1585 (2011).
Weissenböck, H., Hubálek, Z., Bakonyi, T. & Nowotny, N. Zoonotic mosquito-borne flaviviruses: worldwide presence of agents with proven pathogenicity and potential candidates of future emerging diseases. Vet. Microbiol. 140, 271–280 (2010).
Antonio-Nkondjio, C., Sandjo, N. N., Awono-Ambene, P. & Wondji, C. S. Implementing a larviciding efficacy or effectiveness control intervention against malaria vectors: key parameters for success. Parasit. Vectors 11, 57 (2018).
Nchoutpouen, E. et al. Culex species diversity, susceptibility to insecticides and role as potential vector of Lymphatic filariasis in the city of Yaoundé Cameroon. PLoS Negl. Trop. Dis. 13, e0007229 (2019).
Mourou, J.-R. et al. Malaria transmission in Libreville: results of a one year survey. Malar. J. 11, 40 (2012).
Talipouo, A. et al. Comparative study of Culicidae biodiversity of Manoka island and Youpwe mainland area, Littoral Cameroon. Int. J. Biosci. 10, 9–18 (2017).
PNLP. Plan Stratégique National 2019–2023. (2019).
Antonio-Nkondjio, C. et al. Review of the evolution of insecticide resistance in main malaria vectors in Cameroon from 1990 to 2017. Parasit. Vectors 10, 472 (2017).
Bamou, R. et al. Status of insecticide resistance and its mechanisms in Anopheles gambiae and Anopheles coluzzii populations from forest settings in south Cameroon. Genes 10, 741 (2019).
Chouaïbou, M. et al. Dynamics of insecticide resistance in the malaria vector Anopheles gambiae sl from an area of extensive cotton cultivation in Northern Cameroon. Trop. Med. Int. Health 13, 476–486 (2008).
Nwane, P. et al. Trends in DDT and pyrethroid resistance in Anopheles gambiaes. s. populations from urban and agro-industrial settings in southern Cameroon. BMC Infect. Dis. 9, 163 (2009).
Antonio-Nkondjio, C. et al. Rapid evolution of pyrethroid resistance prevalence in Anopheles gambiae populations from the cities of Douala and Yaoundé (Cameroon). Malar. J. 14, 155 (2015).
Fossog, B. T. et al. Physiological correlates of ecological divergence along an urbanization gradient: differential tolerance to ammonia among molecular forms of the malaria mosquito Anopheles gambiae. BMC Ecol. 13, 1–12 (2013).
Antonio-Nkondjio, C. et al. Review of malaria situation in Cameroon: technical viewpoint on challenges and prospects for disease elimination. Parasit. Vectors 12, 501 (2019).
Hemingway, J., Hawkes, N. J., McCarroll, L. & Ranson, H. The molecular basis of insecticide resistance in mosquitoes. Insect Biochem. Mol. Biol. 34, 653–665 (2004).
Pocquet, N. et al. Multiple insecticide resistances in the disease vector Culex p. quinquefasciatus from Western Indian Ocean. PLoS ONE 8, 77855 (2013).
Samantsidis, G.-R. et al. ‘What I cannot create, I do not understand’: functionally validated synergism of metabolic and target site insecticide resistance. Proc. R. Soc. B Biol. Sci. 287, 20200838 (2020).
Corbel, V. et al. Multiple insecticide resistance mechanisms in Anopheles gambiae and Culex quinquefasciatus from Benin West Africa. Acta Trop. 101, 207–216 (2007).
Yadouléton, A. et al. Insecticide resistance status in Culex quinquefasciatus in Benin. Parasit. Vectors 8, 17 (2015).
Xu, Q., Wang, H., Zhang, L. & Liu, N. Sodium channel gene expression associated with pyrethroid resistant house flies and German cockroaches. Gene 379, 62–67 (2006).
Martinez-Torres, D. et al. Voltage-dependent Na+ channels in pyrethroid-resistant Culex pipiens L. mosquitoes. Pestic. Sci. 55, 1012–1020 (1999).
Weill, M. et al. The unique mutation in ace-1 giving high insecticide resistance is easily detectable in mosquito vectors. Insect Mol. Biol. 13, 1–7 (2004).
Djogbénou, L., Akogbéto, M. & Chandre, F. Presence of insensitive acetylcholinesterase in wild populations of Culex pipiens quinquefasciatus from Benin. Acta Trop. 107, 272–274 (2008).
Jones, C. M. et al. Insecticide resistance in Culex quinquefasciatus from Zanzibar: implications for vector control programmes. Parasit. Vectors 5, 78 (2012).
Tmimi, F.-Z. et al. Insecticide resistance and target site mutations (G119S ace-1 and L1014F kdr) of Culex pipiens in Morocco. Parasit. Vectors 11, 51 (2018).
Kothera, L. et al. Using targeted next-generation sequencing to characterize genetic differences associated with insecticide resistance in Culex quinquefasciatus populations from the southern U.S. PLoS ONE 14, (2019).
Matowo, N. S. et al. Fine-scale spatial and temporal variations in insecticide resistance in Culex pipiens complex mosquitoes in rural south-eastern Tanzania. Parasit. Vectors 12, 413 (2019).
Balabanidou, V. et al. Cytochrome P450 associated with insecticide resistance catalyzes cuticular hydrocarbon production in Anopheles gambiae. Proc. Natl. Acad. Sci. 113, 9268–9273 (2016).
Huang, Y. et al. Culex pipiens pallens cuticular protein CPLCG5 participates in pyrethroid resistance by forming a rigid matrix. Parasit. Vectors 11, 6 (2018).
Cameroun fiche pays populationData.net 2020. https://www.populationdata.net/pays/cameroun/.
Djamouko-Djonkam, L. et al. Implication of Anopheles funestus in malaria transmission in the city of Yaoundé Cameroon. Parasite 27, 91 (2011).
Edwards, F. W. Mosquitoes of the Ethiopian Region. III.-Culicine adults and pupae. Mosquitoes Ethiop. Reg. III-Culicine Adults Pupae (1941).
Jupp, P. G. Mosquitoes of Southern Africa: culicinae and toxorhynchitinae. (Ekogilde Publishers, 1996).
Organization, W. H. Test procedures for insecticide resistance monitoring in malaria vector mosquitoes. (2016).
Feyereisen, R. Insect P450 inhibitors and insecticides: challenges and opportunities. Pest Manag. Sci. 71, 793–800 (2015).
Smith, J. L. & Fonseca, D. M. Rapid assays for identification of members of the Culex (Culex) pipiens complex, their hybrids, and other sibling species (Diptera: Culicidae). Am. J. Trop. Med. Hyg. 70, 339–345 (2004).
Scott, J. G., Yoshimizu, M. H. & Kasai, S. Pyrethroid resistance in Culex pipiens mosquitoes. Pestic. Biochem. Physiol. 120, 68–76 (2015).
Bisset, J., Rodríguez, M. M. & Fernández, D. Selection of insensitive acetylcholinesterase as a resistance mechanism in Aedes aegypti (Diptera: Culicidae) from Santiago de Cuba. J. Med. Entomol. 43, 1185–1189 (2006).
Low, V. L. et al. Current susceptibility status of Malaysian Culex quinquefasciatus (Diptera: Culicidae) against DDT, propoxur, malathion, and permethrin. J. Med. Entomol. 50, 103–111 (2013).
Djogbénou, L., Noel, V. & Agnew, P. Costs of insensitive acetylcholinesterase insecticide resistance for the malaria vector Anopheles gambiae homozygous for the G119S mutation. Malar. J. 9, 12 (2010).
Labbé, P. et al. Independent duplications of the acetylcholinesterase gene conferring insecticide resistance in the mosquito Culex pipiens. Mol. Biol. Evol. 24, 1056–1067 (2007).
Delannay, C. et al. Multiple insecticide resistance in Culex quinquefasciatus populations from Guadeloupe (French West Indies) and associated mechanisms. PLoS ONE 13, e0199615 (2018).
Georghiou, G. P. & Pasteur, N. Organophosphate Resistance and Esterase Pattern in a Natural Population of the Southern House Mosquito from California. J. Econ. Entomol. 73, 489–492 (1980).
Xu, W. et al. Cypermethrin resistance conferred by increased target insensitivity and metabolic detoxification in Culex pipiens pallens Coq. Pestic. Biochem. Physiol. 142, 77–82 (2017).
Gong, Y., Li, T., Feng, Y. & Liu, N. The function of two P450s, CYP9M10 and CYP6AA7, in the permethrin resistance of Culex quinquefasciatus. Sci. Rep. 7, 1–12 (2017).
Komagata, O., Kasai, S. & Tomita, T. Overexpression of cytochrome P450 genes in pyrethroid-resistant Culex quinquefasciatus. Insect Biochem. Mol. Biol. 40, 146–152 (2010).
Liu, N., Li, T., Reid, W. R., Yang, T. & Zhang, L. Multiple Cytochrome P450 Genes: their constitutive overexpression and permethrin induction in insecticide resistant mosquitoes culex quinquefasciatus. PLoS ONE 6, e23403 (2011).
Gordon, J. R. & Ottea, J. Association of esterases with insecticide resistance in Culex quinquefasciatus (Diptera: Culicidae). J. Econ. Entomol. 105, 971–978 (2012).
Mouches, C. et al. Characterization of amplification core and esterase B1 gene responsible for insecticide resistance in Culex. Proc. Natl. Acad. Sci. 87, 2574–2578 (1990).
Pasteur, N., Nancé, E. & Bons, N. Tissue localization of overproduced esterases in the mosquito Culex pipiens (Diptera: Culicidae). J. Med. Entomol. 38, 791–801 (2001).
Achaleke, J., Martin, T., Ghogomu, R. T., Vaissayre, M. & Brévault, T. Esterase-mediated resistance to pyrethroids in field populations of Helicoverpa armigera (Lepidoptera: Noctuidae) from Central Africa. Pest Manag. Sci. Former. Pestic. Sci. 65, 1147–1154 (2009).
Simma, E. A. et al. Genome-wide gene expression profiling reveals that cuticle alterations and P450 detoxification are associated with deltamethrin and DDT resistance in Anopheles arabiensis populations from Ethiopia. PEST Manag. Sci. 75, 1808–1818 (2019).
Subra, R. Biology and control of Culex pipiens quinquefasciatus Say, 1823 (Diptera, Culicidae) with special reference to Africa. Int. J. Trop. Insect Sci. 1, 319–338 (1981).
Acknowledgements
This study was partly sponsored by a Wellcome Trust Senior Fellowship in Public Health and Tropical Medicine (202687/Z/16/Z) to CAN. Part of this research has received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement N° 731060 (INFRAVEC2) to AT. The funding bodies did not have any role in the design, collection of data, analysis and interpretation of data and in writing of the manuscript.
Author information
Authors and Affiliations
Contributions
A.T. and C.A.N. conceptualized and designed the study; A.T., E.N., B.D.T., E.K. and R.B. performed the field experiments. A.T., K.M., V.B., S.B. and E.A.F. performed laboratory experiments. A.T. and K.M. performed the statistical analysis. K.M., S.K., C.S.W., P.A.A., E.A.F. and J.V. critically reviewed and amended the manuscript. A.T. and C.A.N. interpreted, analysed data and wrote the manuscript with input from all authors. All the authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Talipouo, A., Mavridis, K., Nchoutpouen, E. et al. High insecticide resistance mediated by different mechanisms in Culex quinquefasciatus populations from the city of Yaoundé, Cameroon. Sci Rep 11, 7322 (2021). https://doi.org/10.1038/s41598-021-86850-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-86850-7
This article is cited by
-
Effects of Alphacypermethrin selection on fitness traits of Culex quinquefasciatus (Diptera: Culicidae)
Applied Entomology and Zoology (2024)
-
Efficacy of a ‘lethal house lure’ against Culex quinquefasciatus from Bouaké city, Côte d’Ivoire
Parasites & Vectors (2023)
-
Overexpression of cytochrome P450 and esterase genes involved in permethrin resistance in larvae and adults of Culex quinquefasciatus
Parasitology Research (2023)
-
Rapid evolution of insecticide resistance and patterns of pesticides usage in agriculture in the city of Yaoundé, Cameroon
Parasites & Vectors (2022)
-
Phenotypic insecticide resistance status of the Culex pipiens complex: a European perspective
Parasites & Vectors (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.