Abstract
The overexpression of hoxd13a during zebrafish fin development causes distal endochondral expansion and simultaneous reduction of the finfold, mimicking the major events thought to have happened during the fin-to-limb transition in Vertebrates. We investigated the effect of hoxd13a overexpression on putative downstream targets and found it to cause downregulation of proximal fin identity markers (meis1 and emx2) and upregulation of genes involved in skeletogenesis/patterning (fbn1, dacha) and AER/Finfold maintenance (bmps). We then show that bmp2b overexpression leads to finfold reduction, recapitulating the phenotype observed in hoxd13a-overexpressing fins. In addition, we show that during the development of the long finfold in leot1/lofdt1 mutants, hoxd13a and bmp2b are downregulated. Our results suggest that modulation of the transcription factor Hoxd13 during evolution may have been involved in finfold reduction through regulation of the Bmp signalling that then activated apoptotic mechanisms impairing finfold elongation.
Similar content being viewed by others
Introduction
The evolutionary transition from fish fins to tetrapod limbs involved sequential expansion and elaboration of the endoskeleton and simultaneous reduction of the distal ectodermal finfold (FF), formed by elongation of the embryonic apical ectodermal ridge (AER)1,2,3. This embryonic structure has signaling activity involved in maintaining cell proliferation in the underlying mesenchyme and contributing to the outgrowth and patterning of fins and limbs4,5,6. However, striking differences were detected in the developmental mechanisms of the AER in fish fins and tetrapod limbs7,8,9. During fin development, the AER is rapidly converted into a FF10,11, which is then colonized by mesenchymal cells that differentiate into actinotrichia and maturate into lepidotrichia (bony fin-rays)10,12,13. However, during limb development, the AER fails to elongate and its signaling activity persists up to the differentiation of the autopod4,9,14. The distinct behavior of the AER in fish fins and tetrapods limbs led Thorogood15 to propose the “clock model” to explain the fin-to-limb transition, according to which early FF formation hinders mesenchyme expansion, which is required to further promote distal endoskeletal formation15. Thus, a heterochronic shift in the AER-to-FF transition was proposed as essential for the origin of limbs, with the transition occurring earlier in actinopterygians, later in sarcopterygians, and being absent in tetrapods7,15,16. However, the genetic drivers of this heterochronic shift remain largely unknown.
Genes in the 5′ end of the HoxD cluster, such as Hoxd13, were shown to be essential for autopod formation in tetrapods17,18,19, in which they are expressed in two distinct phases activated either by cis-regulatory regions located in the telomeric or centromeric flanking regions of the HoxD cluster18,20,21. In the first phase, 5′Hoxd genes are transcribed exclusively in the posterior mesenchyme of the limb buds, while in the second phase, their expression domain expands distally and anteriorly occupying the entire prospective autopod region22,23.
Chondrichthyans24, basal actinopterygians25, teleosts26, and lungfishes27 were shown to have also two phases of 5′HoxD gene expression during fin development, however, their expression patterns never fully recapitulate the ones observed during tetrapod limb development28,29. These observations lead to the hypothesis that transcriptional modulation of 5′HoxD genes (and Hoxd13 in particular), through the addition of enhancer modules, was crucial for the formation of novel distal endoskeletal elements during limb evolution18,30,31,32. Freitas and colleagues address this idea using zebrafish as a pre-tetrapod representative, which lacks particular enhancer units in the telomeric landscape of the HoxD cluster highly conserved in tetrapods32. Mimicking the function of these enhancer units, they induced hoxd13a over-expression during fin development and observed expansion of the chondrogenic tissue distally and concomitant reduction of the finfold, a phenotype that resembles the morphological transformations thought to have happened during the fin-to-limb transition28,32. Likewise, Actinodin1/2 knockdown during zebrafish development fin (and1, and2) interferes with finfold formation and leads to the expression of genes involved in tetrapod digit development13. However, hoxd13a homozygous null embryos exhibit normal fins, with no visible shortening of the fin rays in adulthood, while double hoxa13a/hoxa13b or triple hoxa13a/hoxa13b/hoxd13a knockouts lack finfold development, possible due to a deficit in migration of mesenchymal cells to the finfold impairing dermal skeleton development33. Taken together, these data suggest that early silencing of hoxd13a does not affect finfold development, however time-specific overexpression, 30–32 h post-fertilization (hpf), is able to induce finfold shortening. Nevertheless, tantalizing questions remain unresolved, such as which Hoxd13-associated mechanisms are responsible for this phenotype.
Here we used a zebrafish line, allowing time-specific overexpression of hoxd13a, to evaluate the impact on putative downstream targets32,34. One of these putative targets, bmp2b, shown to have expression levels affected by hoxd13a overexpression, was then further investigated. To this end, we generated a transgenic zebrafish line that allows bmp2b overexpression specifically at 32hpf, as performed for hoxd13a, which caused a significant reduction of the finfold, accompanied by an increase in cell death. To further explore the idea that bmp2b may influence finfold size, we undertook gene expression analyses for bmp2b and hoxd13a in zebrafish mutants leot1/lofdt1, characterized by long finfolds35. These animals carry the leot1 recessive mutation located in connexin41.8 (cx41.8)36 and the lofdt2 dominant mutation located in Kcc4a, encoding K + -Cl − co-transporter and causing long finfolds37. We found that during the development of their long finfolds, both bmp2b and hoxd13a are less expressed than in Wild-Type fins (WT).
We propose an evolutionary model in which increased levels of Hoxd13 expression during fin development may have caused higher expression levels of Bmps at the distal margin of fins, promoting apoptosis and contradicting finfold elongation. In addition, higher levels of Hoxd13 may have also promoted a skeletogenic fate in the most distal cells by causing ectopic expression of fbn1 and dach genes.
Results
hoxd13a overexpression during fin development affects the expression of putative targets
Freitas and colleagues performed three independent assays aiming to overexpress hoxd13a in a spatially or temporally controlled manner32. Overexpression of hoxd13a at 32hpf, when its expression is known to expand anteriorly in zebrafish fins26, was achieved by generating transgenics in which hoxd13a was placed under the control of a hormone-inducible promoter, a col2a1 promoter, or a heat-shock protein 70 promoter (hsp70). Transgenic fish carrying any of those 3 constructs display the same morphological and gene expression phenotype, characterized by distal expression expansion of chondrogenic markers and a simultaneous decrease in the expression of finfold markers, resulting in a distal expansion of the endochondral plate and reduction of the finfold in transgenic fish.
We started by determining if the decedents of the original transgenic line, which was outcrossed with AB WT animals, still display the same fin phenotypes reported by Freitas and colleagues after heat-shock induction32. Adjusting the exposure time of the heat-shock treatments, we obtained embryos that displayed finfold reduction or truncation associated with the downregulation of the finfold marker and1, detected by in situ hybridization (ISH, n = 10) (Supplementary Information: Fig. 1A). In addition, we also dissected fins (n = 100) from wild type (Wt) and transgenic embryos (hsp70:hoxd13a) to undertake a quantitative gene expression evaluation throughout development, regarding hoxd13a, and1, and fgf8, an additional finfold marker (Supplementary Information: Fig. 1B,C). We detected much higher levels of hoxd13a transcripts in the transgenic fins than in the Wt controls at three distinct time points: 56hpf, 85hpf, and 115hpf. At the same stages, the expression of and1 and fgf8 was significantly lower in the transgenic fins than in the controls, which was in agreement with the identified phenotypes.
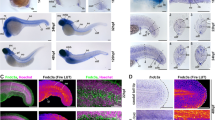
Expression levels of hoxd13a putative targets in hoxd13a-overexpressing fins (hoxd13+++) and controls (Wt) analyzed by RT-qPCR (B,C,E) and ISH (D,F,G). (A) Central role of the transcription factor Hoxd13 in the distal patterning of tetrapod limbs, based in Salsi and colleagues6. (B) meis1b appears downregulation in hoxd13+++ fins in comparison with Wt at 85 and 115 hpf. (C,D) dachA presents higher expression in hoxd13+++ fins than in Wt controls at 56 and 85 hpf (C) and is ectopically expressed in the distal margin of the transgenic fins at 85hpf (arrows), under the remaining finfold (FF) (D). (E,F) Expression analyses of bmp2a, bmp2b, bmp4, bmp7a, and bmp7b reveal upregulation of bmp2b and bmp7b in hoxd13+++ fins (E), which are ectopically expressed in the remaining finfold. Statistical significance evaluated by unpaired t-test: *p < 0.05; **p < 0.01.
Using dissected hoxd13a-overexpressing fins and controls, we then evaluated the expression of a set of putative downstream targets, identified in a Chip-to-chip assay performed in mice embryonic limbs (Meis1, Dach1, Bmp2, Bmp4, Barx1, Fbn1, and Emx2)34 (Fig. 1A). We found that meis1b is significantly downregulated in hsp70:hoxd13a fins at 85hpf and 115hpf (Fig. 1B), which suggests that hoxd13a overexpression may have caused meis1b inhibition. The tetrapod counterpart, Meis1, is required to establish proximal identity during limb patterning leading to the differentiation of the stylopod38,39 and found to be downregulated by Hoxd13 in chick limb buds38. In addition, the analyses of the three Dach1 zebrafish orthologous revealed upregulation of dacha as early as 56hpf in the transgenic fins (Fig. 1C), and its expression is maintained in the most distal mesenchyme in hoxd13a-overexpressing fins at 85hpf, contrarily to what is observed in WT controls (Fig. 1D). Thus, hoxd13a overexpression appears to positively impact dacha expression, which according to the identified role of its orthologous in tetrapods may lead to meis1b repression34. In addition, dacha may also work as a Bmp antagonist regulating finfold formation, as suggested to happen during chick limb development in which Dach1 is able to repress the BMP-mediated transcriptional control regulating the formation of the AER40.
In order to evaluate the impact of hoxd13a overexpression in the BMP-signaling, which was implicated in the abnormal expansion of the AER in chick embryos41, we analyzed the expression of Bmp2, Bmp4, and Bmp7 orthologous during fin development (Fig. 1E,F). We found significant upregulation of bmp2b and bmp7b at 85hpf, which appears ectopically expressed in the distal border of the transgenic fins (Fig. 1F). Further evidence for upregulation of the Bmp signaling in hoxd13a-overexpressing fins emerged from the immunostaining with anti-Psmad1/5 antibody, which revealed Psamd1/5 positive cells in the modified finfold from the transgenic fins not observed in the wild-type condition (Fig. 2).
We also evaluated the expression of fbn1, a gene involved in skeletogenesis in tetrapods that encodes a major component of the extracellular microfibrils42. Our analyses revealed that fbn1 is expressed in higher levels in hoxd13a-overexpressing fins than in controls at 56hpf (Supplementary Information: Fig. 2A). This suggests that hoxd13a overexpression may interfere with the formation of extracellular microfibrils at the margin of zebrafish fins. In addition, we observed lower expression levels of emx2 and barx1 in hoxd13a-overexpressing fins at three distinct developmental stages (Supplementary Information: Fig. 2B), genes involved in proximal fin patterning43. These data suggest that hoxd13 overexpression may inhibit proximal identity signals during fin development, as proposed by Freitas and colleagues32.
Overexpression of bmp2b during fin development causes finfold reduction
Taking into account the upregulation of several bmps in hoxd13a-overexpressing fins, we hypothesized that increased levels of hoxd13a may trigger bmps expression influencing finfold development. To gain insight into this hypothesis, we selected bmp2b for further functional testing, given that the tetrapod orthologous is involved in the formation of the AER44. We generated a transgenic zebrafish line, in which bmp2b was placed under the control of an hsp70 promoter and induced overexpression through heat-shock treatments at 32hpf, following the proceedings used for the transgenics allowing hoxd13a overexpression. We were able to identify finfold reduction after bmp2b overexpression not only in the pectoral fins but also in the caudal fin in 59,3% of the embryos (Supplementary Information: Fig. 3). We next performed ISH using the finfold marker and1 and measured both the finfold (stained region) and the fin endochondral plate (Fig. 3A). We found a significant reduction of the finfold in bmp2b-overexpressing fins (n = 50), while no significant differences were found in the size of the endoskeletal plate (Fig. 3B).
Phenotype and gene expression during fin development in bmp2b-overexpressing fins (bmp2b+++) and controls (Wt). (A) ISH for and1 highlights the shorter finfolds (FF) of bmp2b+++ fins in comparison with controls, similar to hoxd13+++ fins. (B) Finfold and endoskeleton measurements, after and1 ISH, revel significant reduction of the finfold in bmp2b+++ fins (n = 50; *p < 0.05). (C,D) Imunnostaining with anti-casp3 antibody (C) and subsequent counting of the casp3-positive cells in the finfold (n = 9; *p < 0.05) (D) suggests higher apoptotic activity in bmp2b+++ finfolds than in Wt controls at stage 110 hpf. (E) RT-qPCR analyses to evaluate gene expression in bmp2b+++ fins and Wt controls at 90 hpf indicate upregulation of casp3 (***p < 0.001), downregulation of finfold markers and1 and fgf8a (***p < 0.01) and non-significant alteration of ccnb1, involved in cell proliferation. Statistical significance evaluated by unpaired t-test.
We next, explored potential mechanisms involved in the finfold reduction observed in bmp2b-overexpressing fins. To evaluate if finfold reduction was due to increased cell death, we immunostained zebrafish embryos with anti-casp3 antibody and detected that bmp2b-overexpressing fins present a higher number of casp3-positive cells than controls (n = 9; Fig. 3C,D), suggesting a higher rate of apoptosis. In addition, we also evaluated the expression of casp3 by RT-qPCR in the developing fins, together with fgf8, and1, and ccnb1 (Fig. 3E). We found that, while the expression of finfold markers diminished significantly in bmp2b-overexpressing fins (fgf8a and and1), the expression of casp3 significantly increased and the expression of ccnb1 did not change significantly at 90hpf. These data suggest that bmp2b overexpression reduces the signaling activity of the finfold, which is required for the maintenance of an undifferentiated state in the underlying mesenchyme in tetrapod models45. It also reduces the expression of a gene (and1), which encodes the actinotrichia proteins required for finfold development13. Moreover, the higher expression of casp3 in bmp2b-overexpressing fins suggests increased cell death in the finfold, which may justify its reduced size in the transgenic condition. Interestingly, reduced expression of bmp2 was reported to be associated with dorsal and ventral expansions of the AER in the mutant mice (Megf7-deficient), which also presents reduced apoptotic activity46. Thus, while the overexpression of a bmp2 orthologous in zebrafish cause reduction of the finfold, its reduced expression in mice causes AER expansion and both processes relate with an impact on apoptosis.
Long-fin leot1/lofdt1 mutants express less hoxd13a and bmp2b during fin development
The zebrafish mutant leot1/lofdt1 exhibits a phenotype characterized by long finfolds (Fig. 4A). To further investigate the relationship between hoxd13a/bmp2b and finfold size, we analyzed finfold development in this mutant in comparison with WT controls (Fig. 4B–D). We performed ISH for the finfold marker and1 and measured the stained regions (Fig. 4B). We found that leot1/lofdt1 mutants show longer finfolds detectable from 48hpf onwards (n = 20, Fig. 4B,C). In addition, the expression of hox13a and bmp2b was lower in these appendages than in the wild-type condition at 86hpf, which was accompanied by lower expression of casp3 and ccnb1 (Fig. 4D). Thus, while the shortening of the finfold in our transgenic conditions seems to be associated with increased levels of hoxd13a and bmp2, the long finfold of leot1/lofdt1 mutants seem to be associated with lower levels of hoxd13a and bmp2b. This is consistent with our hypothesis suggesting that increased levels of Bmp2, mediated by the Hoxd13 transcription factor, may have been an important mechanism involved in the shortening of the finfold during evolution.
Finfold growth and gene expression in leot1/lofdt2 mutants and controls (Wt). (A) Fin phenotype comparisons in adults. (B,C) Finfold size comparison during development evaluated after and1 ISH (B), followed by measurements (C), revealing a longer finfold detectable since 48hpf in leot1/lofdt2 mutants, in comparison with controls (n = 20, *p < 0.05). (D) Gene expression analyses by RT-qPCR at 86hpf, suggest lower expression levels for hoxd13a and bmp2b in leot1/lofdt2 fins in comparison with Wt controls (***p < 0.001) detected by RT-qPCR (on the right) and ISH (on the left), which was accompanied by decreased expression of casp3, involved in apoptosis (**p < 0.01) and ccnb1, involved in proliferation (***p < 0.001). Statistical significance evaluated by unpaired t-test.
Discussion
Modulation of Hoxd13, through the elaboration of its enhancer network, has been suggested as one of the mechanisms involved in the transition from fish fins to tetrapod limbs32. Taking advantage of the current knowledge on Hoxd13 targets in tetrapods34, we tested how the overexpression of the zebrafish orthologous (hoxd13a) throughout zebrafish fin development impacts the expression of a set of 10 putative downstream targets. We found that most of these genes presented altered expression levels when in hoxd13a-overexpressing fins. The overexpression of hoxd13a leads to the downregulation of genes involved in the establishment of the proximal identity, such as meis1 and emx2, Thus, in addition, to instigating the distal cells for the fins to enter a distal-identity program, as suggested by Freitas and colleagues32, the overexpression of hoxd13a may also cause active repression of the proximal identity. Furthermore, previously chicken functional studies indicate that Dach1 participates in the repression of Meis genes, contributing to establish the identity of the distal limb domains40. Our ISH results for dacha (zebrafish Dach1 orthologous), shows that transgenic zebrafish maintain dacha expression in the most distal area of the fins, which suggests that downregulation of meis1 in hoxd13a-overexpresing fins may result from direct or indirect regulation of meis1 by hoxd13a transcription factor. In addition, in chicken, Dach1 is known as an antagonist of Bmp-mediated transcriptional control, and the cross-talk between those 2 genes is required for the PD patterning and maintenance of the AER40. Therefore, upregulation of dacha in hoxd13a-overexpressing fins may contribute to the maintenance of an AER-like structure rather than allowing the expansion of the finfold, via interference with the bmp-signaling.
We also found that fbn1 is up-regulated hoxd13a-overexpressing fins being ectopically expressed in the remaining finfold region. A similar distal increase of fbn1 expression, in response to Hoxd13 overexpression was also reported in chicken limb buds34. Given that Fbn1 encodes a major component of extracellular microfibrils42, our data suggest that the distal endochondral plate extension observed in the hoxd13a-overexpressing fins32 may stem from a hoxd13a dependent control of fbn1 expression.
Barx1 is another transcription factor shown to be regulated by Hoxd13 transcription factor during chicken limb development and its missexpression causes Barx1 downregulation in the autopod34. Our data show the inverse trend in hoxd13a-overexpressing fins: increase levels of hoxd13a lead to increased levels of barx1. Regarding bmp genes (bmp2a, bmp2b, bmp4, bmp7a, and bmp7b), we found significant upregulation of bmp2b and bmp7b at 85hpf. In addition, these genes appeared ectopically expressed in the remaining finfold of hoxd13a-overexpressing fins. Interestingly, Bmp2 seems to limit the elongation of the AER in tetrapod models41,47 and its inhibition increases its size48. This led us to hypothesize that the finfold phenotypes observed in hoxd13a-overexpressing fins result from alterations in BMPs activity and, in fact, when we overexpressed bmp2b we were able to obtain equally finfold truncation. In addition, complementary assays suggested that bmp2b overexpression generated this phenotype by incrementing apoptosis and downregulating fgf8 signaling, crucial for the survival of AER cells in tetrapod models49. Additional support for our hypothesis, suggesting involvement of hoxd13a/bmp2b interactions in finfold size definition, was obtained analyzing the formation of the long finfolds of leot1/lofdt1 mutants, in which the expression of hoxd13a and bmp2b seems to be lower compared to controls, as well as the apoptosis in these structures.
Our data hint that Hoxd13 contribution to the fin-to-limb transition may have resulted from suppression of proximal identity determinants (meis1, emx2, barx1) and upregulation of genes required for PD limb patterning (dacha) and for distal skeletogenesis (fbn1). We also present evidence that supports the hypothesis that finfold loss may have been achieved during vertebrates’ evolution through modulation of Bmps distally mediated by Hoxd13, which may have increased the apoptotic levels inhibiting finfold elongation.
Methods
Zebrafish maintenance and manipulation
Zebrafish experiments followed European Union Animal Research Guidelines and the experimental design was approved by the Ethics Committee of IBMC/I3S and DGAV (Portugal). Embryo staging followed Kimmel et al.50 and transgenesis followed previously described methods32,51. Heat-shock treatments were performed in hsp70:hoxd13a and hsp70:bmp2b lines and in AB WT at 30 hpf. To this end, three sequential heat-shock treatments were conducted at 36, 48, and 60 hpf, by placing embryos at 38.5 °C for 60 min (min.). They were then fixed in 4% PFA for ISH and Immunohistochemistry or used to collect fins for RNA extraction, subsequent cDNA synthesis, and RT-qPCR analyses.
Generation of the hsp70:bmp2b zebrafish transgenic lines
A construct harboring bmp2b, placed under the control of the heat-shock inducible hps70 promoter, was used to generate zebrafish transgenic line hsp70:bmp2b, allowing bmp2b overexpression at 30hpf. The coding sequence of bmp2b was isolated by PCR using the primers bmp2b_Fwd 5′-cggaactgactgatcatggtc-3′; bmp2b_Rev 5′-ggagattgttctcatcggcac-3′ and cloned into the pCR8/GW/TOPO vector. Correctly orientated clones were used as middle entry vectors to generate Tol2kit constructs52 along with the promoter of hsp70 (5′ entry vector), PolyA (3′ vector), and pDESTtol2CG2 as the destination vector. This vector contains a cmlc2:egfp transgenesis marker that promotes GFP expression in the heart, facilitating the identification of transgenic embryos52.
Whole-mount ISH and immunohistochemistry
Digoxigenin-labeled riboprobes were generated using a dig-UTP labeling mix and T3, T7, or SP6 RNA polymerases according to the manufacturer’s instructions (Roche). ISH was carried as previously described32,53. Immunofluorescence protocol was adapted from Mateus and colleagues54 with the following adjustments: embryos were fixed in 4% paraformaldehyde at 4 °C overnight (o/n) and then dehydrated in increasing levels of MeOH (methanol) in PBT (PBS and 0.1% Tween) and stored in 100% MeOH at – 20 °C. Embryos were then rehydrated in reverse series of MeOH /PBT solutions and washed twice in PBT for 5 min. at room temperature (RT), followed by permeabilization with 100% Acetone for 7 min. at – 20 °C. Next embryos were washed in PBT, 0.1% DMSO and 1% Bovine Serum Albumin (BSA) and subsequentially blocked in PBT, 1% DMSO, 1% BSA, and 1% goat serum for 2 h at RT. Embryos were incubated in primary antibody (rabbit anti-PSmad1/5/9, 1:100, Cell Signaling) diluted in blocking solution at 4 °C o/n. The following day embryos were washed 2 times in PBT, 0.1% DMSO, and 1% BSA for 5 min and incubated in secondary antibody (Alexa Fluor 568 anti-rabbit, 1:1000) conjugated with DAPI (1:1000) (Sigma) diluted in blocking solution o/n at 4 °C. On the next day embryos were washed 2 times in PBT, 0.1% DMSO, and 1% BSA for 5 min. Embryos were mounted in 80% Glycerol, 2% DABCO (Sigma) diluted in PBS, and then imaged in Leica SP5II confocal microscope.
RT-qPCR
RNA was extracted from pools of dissected fins (n = 100 per experimental condition) and converted into cDNA using the High-Capacity RNA-to-cDNA Kit, Thermo Fisher Scientific). RT-qPCR was performed in triplicates for each analyzed gene using Rpl13a and β-actin as reference genes and primers indicated in Supplementary Information (Table 1). Data were analyzed using Bio-Rad iQ5 Optical System Software Version 2.0 software, and relative gene expression quantification was calculated using the 2-ΔΔCt method. Statistical significance was calculated by Unpaired T-test.
References
Stewart, T. A. et al. Fin ray patterns at the fin-to-limb transition. Proc. Natl. Acad. Sci. USA 117(3), 1612–1620 (2020).
Coates, M. I. The origin of vertebrate limbs. Development 169–180 (1994).
Shubin, N. H., Daeschler, E. B. & Jenkins, F. A. Jr. The pectoral fin of Tiktaalik roseae and the origin of the tetrapod limb. Nature 440(7085), 764–771 (2006).
Mariani, F. V., Ahn, C. P. & Martin, G. R. Genetic evidence that FGFs have an instructive role in limb proximal-distal patterning. Nature 453(7193), 401–456 (2008).
Grandel, H., Draper, B. W. & Schulte-Merker, S. Dackel acts in the ectoderm of the zebrafish pectoral fin bud to maintain AER signaling. Development 127(19), 4169–4178 (2000).
Kawakami, Y. et al. MKP3 mediates the cellular response to FGF8 signalling in the vertebrate limb. Nat. Cell Biol. 5(6), 513–519 (2003).
Yano, T., Abe, G., Yokoyama, H., Kawakami, K. & Tamura, K. Mechanism of pectoral fin outgrowth in zebrafish development. Development 139(16), 2916–2925 (2012).
Casanova, J. C. et al. Apical ectodermal ridge morphogenesis in limb development is controlled by Arid3b-mediated regulation of cell movements. Development 138(6), 1195–1205 (2011).
Fernandez-Teran, M. & Ros, M. A. The apical ectodermal ridge: Morphological aspects and signaling pathways. Int. J. Dev. Biol. 52(7), 857–871 (2008).
Dane, P. J. & Tucker, J. B. Modulation of epidermal cell shaping and extracellular matrix during caudal fin morphogenesis in the zebra fish Brachydanio rerio. J. Embryol. Exp. Morphol. 87, 145–161 (1985).
Wood, A. Early pectoral fin development and morphogenesis of the apical ectodermal ridge in the killifish, Aphyosemion scheeli. Anat. Rec. 204(4), 349–356 (1982).
Wood, A. & Thorogood, P. An analysis of in vivo cell migration during teleost fin morphogenesis. J. Cell Sci. 66, 205–222 (1984).
Zhang, J. et al. Loss of fish actinotrichia proteins and the fin-to-limb transition. Nature 466(7303), 234–237 (2010).
Mariani, F. V. & Martin, G. R. Deciphering skeletal patterning: Clues from the limb. Nature 423(6937), 319–325 (2003).
Thorogood, P. Developmental Patterning of the Vertebrate Limb (Springer, 1991).
Masselink, W. et al. A somitic contribution to the apical ectodermal ridge is essential for fin formation. Nature 535(7613), 542–546 (2016).
Beccari, L. et al. A role for HOX13 proteins in the regulatory switch between TADs at the HoxD locus. Genes Dev. 30(10), 1172–1186 (2016).
Montavon, T. et al. A regulatory archipelago controls Hox genes transcription in digits. Cell 147(5), 1132–1145 (2011).
Kmita, M. et al. Early developmental arrest of mammalian limbs lacking HoxA/HoxD gene function. Nature 435(7045), 1113–1116 (2005).
Woltering, J. M., Noordermeer, D., Leleu, M. & Duboule, D. Conservation and divergence of regulatory strategies at Hox loci and the origin of tetrapod digits. PLoS Biol. 12(1), 1001773 (2014).
Montavon, T., Le Garrec, J. F., Kerszberg, M. & Duboule, D. Modeling Hox gene regulation in digits: Reverse collinearity and the molecular origin of thumbness. Genes Dev. 22(3), 346–359 (2008).
Nelson, C. E. et al. Analysis of Hox gene expression in the chick limb bud. Development 122(5), 1449–1466 (1996).
Dolle, P., Izpisua-Belmonte, J. C., Boncinelli, E. & Duboule, D. The Hox-4.8 gene is localized at the 5’ extremity of the Hox-4 complex and is expressed in the most posterior parts of the body during development. Mech. Dev. 36(1–2), 3–13 (1991).
Freitas, R., Zhang, G. & Cohn, M. J. Biphasic Hoxd gene expression in shark paired fins reveals an ancient origin of the distal limb domain. PLoS ONE 2(8), e754 (2007).
Davis, M. C., Dahn, R. D. & Shubin, N. H. An autopodial-like pattern of Hox expression in the fins of a basal actinopterygian fish. Nature 447(7143), 473–476 (2007).
Ahn, D. & Ho, R. K. Tri-phasic expression of posterior Hox genes during development of pectoral fins in zebrafish: Implications for the evolution of vertebrate paired appendages. Dev. Biol. 322(1), 220–233 (2008).
Johanson, Z. et al. Fish fingers: Digit homologues in sarcopterygian fish fins. J. Exp. Zool. B Mol. Dev. Evol. 308(6), 757–768 (2007).
Sordino, P., van der Hoeven, F. & Duboule, D. Hox gene expression in teleost fins and the origin of vertebrate digits. Nature 375(6533), 678–681 (1995).
Shubin, N., Tabin, C. & Carroll, S. Deep homology and the origins of evolutionary novelty. Nature 457(7231), 818–823 (2009).
Woltering, J. M. & Duboule, D. The origin of digits: Expression patterns versus regulatory mechanisms. Dev. Cell 18(4), 526–532 (2010).
Freitas, R., Zhang, G. J. & Cohn, M. J. Biphasic Hoxd gene expression in shark paired fins reveals an ancient origin of the distal limb domain. PLoS ONE 2(8), 1 (2007).
Freitas, R., Gomez-Marin, C., Wilson, J. M., Casares, F. & Gomez-Skarmeta, J. L. Hoxd13 contribution to the evolution of vertebrate appendages. Dev. Cell 23(6), 1219–1229 (2012).
Nakamura, T., Gehrke, A. R., Lemberg, J., Szymaszek, J. & Shubin, N. H. Digits and fin rays share common developmental histories. Nature 537(7619), 225–228 (2016).
Salsi, V., Vigano, M. A., Cocchiarella, F., Mantovani, R. & Zappavigna, V. Hoxd13 binds in vivo and regulates the expression of genes acting in key pathways for early limb and skeletal patterning. Dev. Biol. 317(2), 497–507 (2008).
Staff, Z. Mutation Details Curation of Older Features. (ed Data. ZH) (2016).
Watanabe, M. et al. Spot pattern of leopard Danio is caused by mutation in the zebrafish connexin41.8 gene. EMBO Rep. 7(9), 893–897 (2006).
Lanni, J. S. et al. Integrated K+ channel and K+Cl- cotransporter functions are required for the coordination of size and proportion during development. Dev. Biol. (2019).
Capdevila, J., Tsukui, T., Rodriquez Esteban, C., Zappavigna, V. & Izpisua, J. C. Belmonte control of vertebrate limb outgrowth by the proximal factor Meis2 and distal antagonism of BMPs by Gremlin. Mol. Cell 4(5), 839–849 (1999).
Mercader, N. et al. Conserved regulation of proximodistal limb axis development by Meis1/Hth. Nature 402(6760), 425–429 (1999).
Kida, Y., Maeda, Y., Shiraishi, T., Suzuki, T. & Ogura, T. Chick Dach1 interacts with the Smad complex and Sin3a to control AER formation and limb development along the proximodistal axis. Development 131(17), 4179–4187 (2004).
Pizette, S. & Niswander, L. BMPs negatively regulate structure and function of the limb apical ectodermal ridge. Development 126(5), 883–894 (1999).
Sakai, L. Y. & Keene, D. R. Fibrillin protein pleiotropy: Acromelic dysplasias. Matrix Biol. J. Int. Soc. Matrix Biol. 80, 6–13 (2019)
Pellegrini, M., Pantano, S., Fumi, M. P., Lucchini, F. & Forabosco, A. Agenesis of the scapula in Emx2 homozygous mutants. Dev. Biol. 232(1), 149–156 (2001).
Soshnikova, N. et al. Genetic interaction between Wnt/beta-catenin and BMP receptor signaling during formation of the AER and the dorsal-ventral axis in the limb. Genes Dev. 17(16), 1963–1968 (2003).
Moon, A. M. & Capecchi, M. R. Fgf8 is required for outgrowth and patterning of the limbs. Nat. Genet. 26(4), 455–459 (2000).
Johnson, E. B., Hammer, R. E. & Herz, J. Abnormal development of the apical ectodermal ridge and polysyndactyly in Megf7-deficient mice. Hum. Mol. Genet. 14(22), 3523–3538 (2005).
Choi, K. S., Lee, C., Maatouk, D. M. & Harfe, B. D. Bmp2, Bmp4 and Bmp7 are co-required in the mouse AER for normal digit patterning but not limb outgrowth. PLoS ONE 7(5), e37826 (2012).
Maatouk, D. M., Choi, K. S., Bouldin, C. M. & Harfe, B. D. In the limb AER Bmp2 and Bmp4 are required for dorsal-ventral patterning and interdigital cell death but not limb outgrowth. Dev. Biol. 327(2), 516–523 (2009).
Kaltcheva, M. M., Anderson, M. J., Harfe, B. D. & Lewandoski, M. BMPs are direct triggers of interdigital programmed cell death. Dev. Biol. 411(2), 266–276 (2016).
Kimmel, C. B., Ballard, W. W., Kimmel, S. R., Ullmann, B. & Schilling, T. F. Stages of embryonic development of the zebrafish. Dev. Dyn. 203(3), 253–310 (1995).
Suster, M. L., Kikuta, H., Urasaki, A., Asakawa, K. & Kawakami, K. Transgenesis in zebrafish with the tol2 transposon system. Methods Mol. Biol. (Clifton, N.J.) 561, 41–63 (2009).
Kwan, K. M. et al. The Tol2kit: A multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev. Dyn. 236(11), 3088–3099 (2007).
Thisse, B. & Thisse, C. In situ hybridization on whole-mount zebrafish embryos and young larvae. Methods Mol. Biol. 1211, 53–67 (2014).
Mateus, R. et al. BMP signaling gradient scaling in the zebrafish pectoral fin. Cell Rep. 30(12), 4292–43024297 (2020).
Acknowledgements
This work was financed by FEDER—Fundo Europeu de Desenvolvimento Regional funds through the COMPETE 2020—Operacional Programme for Competitiveness and Internationalisation (POCI), Portugal 2020, and by Portuguese funds through FCT—Fundação para a Ciência e a Tecnologia/Ministério da Ciência, Tecnologia e Ensino Superior in the framework of the project POCI-01-0145-FEDER-030562 (PTDC/BTM-TEC/30562/2017). We are grateful to Andreia Pimpão, Madalena Marques, Carla Lopes and Raquel Ramos for their assistance in some of the experiments.
Author information
Authors and Affiliations
Contributions
R.F. conceived and wrote the manuscript, supervised the work and provided funding. J.C., V.B., A.P, J.L.C, F.C. and M.F. performed the experiments. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Castro, J., Beviano, V., Paço, A. et al. Hoxd13/Bmp2-mediated mechanism involved in zebrafish finfold design. Sci Rep 11, 7165 (2021). https://doi.org/10.1038/s41598-021-86621-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-86621-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.