Abstract
The study investigated the effect of single and chronic (10 sessions) whole-body cryotherapy (WBC; 3-min, − 110 °C) on amino acid (AA) profile, myostatin, fibroblast growth factor 21 (FGF21), and concentrations of brain-derived neurotrophic factor (BDNF), irisin and adiponectin in relation to glucose homeostasis. Thirty-five, healthy men were randomly split into experimental (young: 28 ± 7 years and middle-aged: 51 ± 3 years) and control groups. Blood samples were taken before and 1 h after the first and last (10th) WBC session. Baseline myostatin correlated significantly with visceral fat area, glucose, insulin, HOMA-IR and irisin (all p < 0.05). The single session of WBC induced temporary changes in AA profile, whereas chronic exposure lowered valine and asparagine concentrations (p < 0.01 and p = 0.01, respectively) compared to the baseline. The chronic WBC reduced fasting glucose (p = 0.04), FGF21 (− 35.8%, p = 0.06) and myostatin (-18.2%, p = 0.06). Still, the effects were age-dependent. The decrease of myostatin was more pronounced in middle-aged participants (p < 0.01). Concentrations of irisin and adiponectin increased in response to chronic WBC, while BDNF level remained unchanged. By improving the adipo-myokine profile, chronic WBC may reduce effectively the risk of the metabolic syndrome associated with hyperinsulinemia, increased levels of valine and asparagine, and muscle atrophy.
Similar content being viewed by others
Introduction
Insulin resistance (IR) occurs when higher circulating insulin levels are necessary to achieve the integrated glucose-lowering response1. IR results in a compensatory increased release of insulin by pancreatic β-cells and hyperinsulinemia, which is thought to precede the development of type 2 diabetes (T2DM) by 10 to 15 years2. Obesity, age and physical inactivity are the most prominent factors exacerbating the risk of developing IR3. These factors are codependent. Ageing is associated with a reduced activity, which contributes to lower total energy expenditure4 and may lead to fat tissue accumulation5, especially visceral fat area (VFA). This condition significantly affects development of the age-related IR6. Routine screening tests including fasting glucose concentration and glycated hemoglobin (HbA1C) are most commonly used to detect this condition7. The oral glucose tolerance test may also be applied for this purpose, but is performed less frequently due to being poorly tolerated by the patients as well as being time consuming8.
Serum amino acids (AA’s) are considered to be useful laboratory biomarkers in detecting early disruptions of glucose homeostasis9. Serving as an energy source, AA’s can be used for gluconeogenesis during catabolic states10, and influence insulin and glucagon secretion11. Increased levels of AA’s have been observed in all stages of diabetes, including early pre-diabetic IR12. Insulin reduces concetrations of amino acids in circulation by stimulating their transport to cells13. In particular, the elevated circulating branched-chain amino acids (BCAA’s) are considered to be reliable predictors of the T2DM development in normoglycemic subjects14. A cross-sectional study including both normoglycemic and T2DM individuals demonstrated higher concentrations of serum BCAA’s and also the aromatic AA’s (tyrosine and phenylalanine) in individuals with impaired fasting glycaemia and IR15. The authors reported a decrease in glycine in all T2DM individuals, contrary to the observed increase of AA’s after the meal15. This result was attributed to an increased hepatic clearance of postprandial glycine to replenish a conjugated bile acid pool in the gall bladder16. Likewise, increased plasma AA’s levels of alanine, proline and glutamate/glutamine were observed in a group of 263 men with different stages of diabetes, including early prediabetic IR12.
Due to changes in AA’s concentrations and inhibited insulin action, individuals with IR may also exhibit skeletal mass disfunction and obesity related sarcopenia17. Myostatin is one of the factors which contributes to the development of sarcopenia18. It is a skeletal muscle-derived member of the transforming growth factor β superfamily, which inhibits protein synthesis via an impaired mammalian target of rapamicyn (mTOR) signaling19. Circulating myostatin was previously demonstrated to be correlated with indices of IR20. A study in animal models showed that blocking the myostatin receptor induced an elevation of brown adipose tissue (BAT), an improvement of its mitochondrial function, and better cold tolerance, which altogether contributed to an enhanced energy expenditure21. Similarly, myostatin propeptide which inhibits its activity prevents the development of diet-induced obesity and insulin resistance in transgenic animals17.
Together with physical activity22, cold exposure might improve insulin sensitivity and counteract the inflammatory status associated with obesity. By increasing peripheral insulin sensitivity as well as BAT mass and activity, cold-induced adaptive thermogenesis may be a potential therapy for T2DM23. Similar to cold water immersion24, WBC reduces superficial body temperature leading to changes in tissue blood flow. It does so by means of vasoconstriction at the skin and an increased metabolic rate caused by shivering to maintain a constant core temperature (around 37 °C)25,26, ultimately affecting the expression of myokines27 and adipokines28. These physiological responses provide a theoretical base for applying cold exposure as a possible therapeutic strategy in individuals with metabolic diseases29.
Health benefits of cold exposure are releated to shifts in fibroblast growth factor 21 (FGF21) and irisin30,31. It has been proven that the secretion of FGF21 is stimulated by nonshivering thermogenesis and irisin, in turn, by shivering thermogenesis30. FGF21 regulates expression of genes involved in gluconeogenesis, lipogenesis, lipolysis and fatty acid oxidation32. It is also a metabolic regulator with anti-diabetic properties capable of stimulating enhanced glucose uptake in adipocytes33. FGF21 enhances energy expenditure by increasing the core body temperature and decreasing the respiratory quotient34. Dulian et al. (2015) noted an increase of irisin level in response to 10 sessions of WBC in obese, inactive men, which was also positively correlated withsubcutaneous fat tissue31.
Data on the influence of cold exposure on AA profile’s and myostatin are limited35. We previously reported that the effect of WBC on adipokines depended on participants’ cardiorespiratory fitness36 , expressed in relative maximal oxygen uptake (VO2max). Therefore, for this study, we recruited only men with comparable levels of aerobic capacity. As such, the main purpose was to examine whether both acute and chronic WBC affected changes in blood AA’s and myostatin concentration and the secondary purpose was to establish whether the induced changes were be associated with glucose homeostasis. We hypothesized that both a single and chronic WBC exposures would induce an imporvement in glucose metabolism, related to changes in blood myokines and adipokines concentrations, which would allow considering WBC as a preventative strategy against IR and development of T2DM.
Results
Significant differences in measured insulin sensivity indicators were noted among participants at baseline. Lower glucose (95.7 ± 8.6 vs 106.5 ± 7.0 mg∙mL-1; p = 0.002), insulin concentrations (6.3 ± 2.5 vs 9.0 ± 2.1 µU∙mL-1; p = 0.01) and HOMA-IR (0.8 vs 1.2) were evident in younger participants (YG) compared to middle-aged individuals respectively (MG; supplementary Table S1). These differences were also visible in myokine concentrations. At baseline, BDNF was significantly higher in YG than in MG subjects (p = 0.01), while the trend was opposite for myostatin (p < 0.01; Fig. 1a,b). Conversely, irisin and adiponectin concentrations did not differ at baseline between the two groups (Fig. 1c,d). In WBC-EXP group, baseline concentrations of BDNF and irisin was negatively correlated (r = -0.75, p < 0.01; Fig. 2a), which was not observed at the end of chronic WBC (r = -0.12; p = 0.58; Fig. 2b). In turn, irisin concentration correlated positively at baseline with the amount of fat tissue (percentage of body fat, PBF% as well in absolute kilograms) only in MG subjects (r = 0.58, p = 0.01; supplementary Table S2).
Group- and age-related changes post a single session of the WBC in concentrations of (a) BDNF; (b) myostatin; (c) irisin and (d) adiponectin; recorded before (I) and 1 h after the first (Ih) as well as before (II) and 1 h after the last (IIh) WBC session. WBC-EXP (n = 22) included young (YG, n = 9) and middle aged (MG, n = 13) participants. Data are presented as mean ± SD; *statistical significance in the group; #statistical difference between groups at a time point, +statistical significance in the group MG vs WBC-CON.
In the whole group of participants myostatin concentration, regardless of the age, correlated significantly with VFA (r = 0.70, p < 0.01), glucose homeostasis indicators such as glucose (r = 0.69, p = 0.00), insulin (r = 0.46, p = 0.01) and HOMA-IR (r = 0.53, p < 0.01) and irisin (r = 0.65, p < 0.01; Table 1).
Effects of a single session of WBC
In our assessment, we considered the analysis of blood samples collected before and 1hour after the first (acute) and the last (chronic) WBC exposure.
Changes in resposne to the first session of WBC
The effect of the first WBC session on myokines concentrations is presented in Fig. 1. Irisin (p = 0.02) and myostatin (p = 0.03) concentrations increased significantly in the WBC-EXP group. This was not the case for BDNF concentration. However, when considering the age groups, the first WBC session resulted in a pronounced drop of BDNF and a significant increase of irisin (p = 0.01) concentration in YG participants, but not in MG individuals (Fig. 1a,c). A single WBC exposure also decreased FGF21 level in the WBC-EXP group (from 280.4 ± 160.5 to 239.7 ± 166.6 pg∙mL-1, p = 0.07; Fig. 3a). Changes in AA profile’s in response to a single session of WBC are presented in Table 2. Elevated levels of alanine, isoleucine, tryptophan, lysine, tyrosine, phenylalanine, methionine, arginine and threonine were recorded. The effect size expressed by Cohen’s d value ranged from medium to large.
Changes in the concentration of FGF21 (data are presented as mean ± SEM) recorded: (a) at each point of blood collection: (I) before WBC, (Ih) 1 h after the first WBC, (II) before the last WBC and (IIh) 1 h after the last WBC; (b) in the WBC-EXP group with age-dependent changes before the first (I) and the last (II) session of WBC. *p < 0.05 significant differences between time point measurements.
Changes in resposne to the last session of WBC
Before the last WBC session, the circulating level of irisin remained elevated in YG (p = 0.01) but not MG subjects (Fig. 1c). Additionally, in MG individuals, the concentration of irisin correlated significantly with PBF% (r = 0.58, p < 0.01; supplementary Table S2). In YG subjects, a positive relationship between skeletal muscle mass (SMM) and irisin concentration was observed 1 h after the last exposure (r = 0.78, p < 0.01; supplementary Table S2). Blood analysis of the last WBC session revealed a continued decrease of FGF21 (p < 0.01; Fig. 3a). Adiponectin also tended towards a decrease (p = 0.04) contrary to the effect observed after the chronic WBC exposure (Fig. 1d). The Cohen’s d effect size was large for both proteins (1.08 for adiponectin and 1.12 for FGF21). The last WBC session affected circulating levels of alanine, leucine, lysine, valine, asparagine, glycine and proline, which increased significantly with the exception of glycine concentration, which declined (p = 0.01; Table 2). Moreover, at this time point, the level of alanine was significantly higher in MG subjects compared to YG individuals (163.5 ± 44.9 vs 116.3 ± 19.3 µmol∙L−1, respectively, p = 0.05). The Cohen’s d effect size for the AA’s was medium (> 0.5 but < 0.8), except for asparagine and glycine, where the observed change was small (0.27 and 0.26, respectively).
Effects of chronic WBC
Our assessment of the effect of chronic WBC is based on the analysis of blood samples collected at rest before the first and the last exposure (completing nine sessions, before 10th session). A comparison of PBF% before and after chronic WBC exposure showed a reduction in the WBC-EXP group (19.3 ± 6.1 to 18.8 ± 6.0%, p = 0.03,\({\eta }_{p}^{2}=\) 0.14). Chronic WBC exposure also resulted in a reduction of VFA (88.74 ± 40.39 to 84.41 ± 39.56 cm2, p = 0.03, \({\eta }_{p}^{2}=\) 0.13). Changes in glucose homeostasis indicators (glucose, insulin, HOMA indicators) and the lipid profile from the initial to the final WBC session are presented in Table 3. Fasting glucose level significantly decreased (p = 0.04, \({\eta }_{p}^{2}=\) 0.13), whereas the lipid profile was not affected. Additionally, a significant reduction of insulin (from 9.0 ± 2.1 to 6.9 ± 2.1 µmol∙L−1, p = 0.01, \({\eta }_{p}^{2}=\) 0.28) and HOMA-IR (from 1.21 ± 0.3 to 0.92 ± 0.3, p = 0.01, \({\eta }_{p}^{2}=\) 0.28) was recorded only in MG subjects (Supplementary Table S1). In the WBC-EXP group, HOMA-S increased by 19.6% compared to the baseline for all participants (p = 0.08). HOMA-B increased significantly only in YG individuals (from 71.6 ± 13.1 to 90.4 ± 21.4%, p = 0.01, \({\eta }_{p}^{2}=\) 0.3; Supplementary Table S1).
Table 4 presents changes in biochemical markers and AA profile’s recorded at baseline and in blood collected before the last WBC session. Ulike following acute WBC exposure, chronic WBC did not affect BDNF. The elevated level of irisin induced by the first cryosession was maintained among YG participants (p = 0.04). FGF21 concentration continued to drop throughout the intervention (baseline WBC p = 0.57 vs final WBC session p < 0.01; Fig. 3a,b). Chronic WBC exposure was also accompanied by a significant increase of adiponectin (46.8%, p = 0.05, \({\eta }_{p}^{2}=\) 0.09) in comparison to the WBC-CON group. Further, chronic WBC caused a decline in the circulating myostatin concentration but only in MG subjects (-30%, p < 0.01; effect size was equal 0.58; Fig. 1b). The opposite- upward trend was noted in the whole WBC-CON group. Interestingly, chronic WBC exposure blunted the difference in myostatin concentration recorded at baseline between YG and MG subjects.
Regarding changes in AA profile’s, the concentrations of valine (p < 0.01) and asparagine (p < 0.01) were significantly lower in the WBC-EXP than in the WBC-CON upon the last session of WBC. At this point in time, a positive correlation between valine and myostatin was recorded in the WBC-EXP group (r = 0.60; Table 1). The remaining AA’s were not affected by the intervention (Table 4).
Discussion
Our results demonstrate that chronic WBC exposure had a positive effect on glucose homeostasis in normoglycemic participants. This exposure caused a significant decrease of blood glucose concentration and ameliorated most of the measured indicators of glucose homeostasis. There was also a significant reduction of glucose, evident in the WBC-CON, but still the decrease noted among experimental WBC-EXP group was two-fold higher compared to the WBC-CON group. Significant reductions of insulin and HOMA-IR values were particularly visible among MG participants subject to WBC. The level of these factors was elevated at baseline compared to YG subjects, thus the effect of the intervention in MG participants was more pronounced. Beneficial changes in glucose homeostasis may be connected with the activation of the hypothalamic–pituitary–adrenal axis and the sympathetic nervous system27. A recently published paper by Yoneshiro et al. (2019) revealed that cold exposure significantly reduced plasma concentrations of valine, leucine and isoleucine. The authors relied on plasma metabolomics in obese mice and measured the acitivity of BAT, which displayed the highest valine oxidation in cold exposure, relative to other metabolic organs. In a follow up study, these observations were also verified on humans37. Therefore, a WBC induced reduction in glucose concentration evident in our study may have modified the activity of white as well as BAT resulting in reduced PBF% and VFA.
To the best of our knowledge, our study is the first to assess strictly the effect of WBC on blood concentrations of AA’s in men. Previously, only one study demonstrated a significant drop of tryptophan and valine after 10 sessions of WBC combined with volleyball training38. In the present study, we assessed AA concentrations based on their role in glucose homeostasis. Similar to previous research in animal models (Yoneshiro et al. 2019), we noted a drop of valine in the WBC-EXP group following chronic WBC exposure compared to the WBC-CON group. In the present study, the observed decrease in valine following WBC likely occurred either because WBC could have induced the conversion of valine to β-aminoisobutyric acid, which is a myokine involved in the browning of fat39. Or because cold treatment stimulated the activity of mitochondrial BCAA enzymes such as the branched-chain α-keto acid dehydrogenase complex in the white adipose tissue37. Hence, the observed decrease of valine could have been associated with a statistically significant decrease of glucose concentration accompanied by the downward trend of insulin and HOMA-IR recorded in the MG part of the WBC-EXP group. At the same time, we noted a significant drop of VFA in the whole WBC-EXP group. This reduction in the amount of VFA might have had also a diminishing effect on its endocronical action.
Circulating concentrations of almost half of the AA’s increased significantly 1 h after the first WBC session. It is possible that, at this point in time, the protein breakdown peaked and AA’s were released into the bloodstream. This hypothesis is supported by the fact that this trend of change was also observed 1 h after the last session of WBC. Among all of the AA’s only changes in alanine followed the same trend in response to first as well last session of WBC. Increased metabolism of BCAA in skeletal muscle during WBC, which is manifested by a decrease in serum valine, may lead to increased alanine formation40. Thus, alanine can be transported to the liver to act as a substrate in the gluconeogenesis process. Nevertheless, chronic WBC exposure did not affect alanine expression in the present study. Meanwhile, only concentrations of valine and asparagine were reduced following the chronic WBC. This response might be beneficial in IR individuals because a previous study revealed that high concentrations of BCAA, phenylalanine, tyrosine, alanine, ornithine and lysine were associated with an increased risk of T2DM41. Further, valine and asparagine belong to an AAs signature associated with T2DM risk and progression. Particularly, while increased valine levels together with isoleucine and leucine predict T2DM risk, increased asparagine is associated with a progression of diabetes (along with aspartic acid, glutamine and glutamate)42. It is possible to hypothesize that if chronic WBC exposure is capable of reducing AA expression in normoglycemic participants, a similar response in hyperglycemic individuals would be beneficial. Therefore, the beneficial effects of WBC on metabolism can be marked by the improved AA profile.
Together with the improvement of AA profile’s, we noted a drop of myostatin among MG participans of WBC-EXP group. In addition to regulating muscle cell growth, myostatin has been shown to inhibit glucose uptake43, which suggests that it may contribute to systemic IR. Elevated myostatin levels were registered in pathological conditions characteristic of the metabolic deregulations such as obesity, T2DM and aging44. Our results are consistent with those findings. We observed a significant correlation between myostatin and most of the glucose homeostasis indicators at baseline. Also, at baseline, MG exhibited higher concentrations of myostatin than YG ones. These data are consistent with the findings of Yarasheski et al. (2002), who reported elevated serum myostatin in advance-aged men compared with younger subjects45. Interestingly, following chronic WBC exposure, serum myostatin dropped in MG subjects to the level recorded in their YG counterparts. This response could be beneficial, particularly in MG individuals at risk of hyperglycaemia.
In addition to skeletal muscles, BAT is a significant source of myostatin46,47. Cold exposure has been shown to up-regulate the transcription of interferon regulatory factor 4 (IRF4) in BAT, leading to inhibition of the myostatin expression46. In comparison, heat exposure (30 °C) or loss of IRF4 function have been reported to result in an elevation of serum myostatin46. Report of Kong et al. (2018) revealed that BAT can secrete significant amounts of myostatin into the blood; therefore, cold treatment can be expected to inhibit the secretion of myostatin from BAT46. In contrast, Zak et al. (2018) previously observed that the synthesis of myostatin in skeletal muscles was not sensitive to temperature48. Based on these reports, our data suggest that the effects of WBC were related to its impact on BAT rather than skeletal muscles. Importantly, the changes observed in serum myostatin were age-dependent. Sliwicka et al. (2020) observed that shifts in myostatin induced by cold treatment and/or physical exercise were only temporary in young men and returned to the baseline level within 24 h following cryotherapy/exercise27,49. In the present study, changes in myostatin concentration were more pronounced in MG participants, subject to chronic WBC, after which they exhibited serum myostatin at the level observed in YG subjects at baseline.
Myostatin acts through the inhibition of Akt kinase, which can lead to the activation of FOXO3a, a transcription factor that induces the expression of atrogin-1 gene encoding for a protein strongly linked to muscle atrophy. Thus, reducing the expression of myostatin through chronic WBC exposure can possibly improve the uptake of AA’s in MG individuals, and indirectly, ameliorate insulin sensitivity50.
The improvement of glucose homeostasis was accompanied by changes in FGF21 concentration in the present study. These results are partly comparable to those reported by Shabkhiz et al. (2020)51. They observed a decrease of FGF21 and myostatin, which suppressed IR in elderly men after 12-weeks of resistance training51. On the other hand, elevated circulating levels of FGF21 have been reported in the elderly52 and in T2DM patients53. In the present study MG participants demonstrated an elevated concentration of FGF21 compared to YG counterparts at baseline. WBC induced a drop of FGF21 among all participants, however, these changes were age- dependent. Previously, Hollstein aet al. (2020) also observed a decrease in plasma FGF21 after a long-term cooling session (24 h inside a calorimeter at 19.0 ± 0.3 °C) in overweight and obese participants54. Others have reported conflicting effects on FGF21; with one study reporting an increased secretion of FGF21 (12 h exposure to 24 °C or 19 °C in a whole-room indirect calorimeter)55, while a second study reported a decrease in FGF21 (cooling vest ~ 14.5 °C for 1-2 h)28. The disparity in the aforementioned results could be a result of the cooling protocols which differed significantly from the extremely low temperature applied in our WBC intervention. Furthermore, the variations in FGF21 concentrations can also be attributed to the different time points at which FGF21 was measured particulary because the circadian rhythm modulates a nightly increase and daily decrease in FGF2156. In order to standardize our data collection, and in an attempt to mitigate the impact of circadian rhythm on FGF21 concentration as well as other tested markers57,58, we collected blood samples at the same time of day on each day of our data collection.
Together with FGF21, irisin represents a link between myostatin and glucose metabolism59. Lee et al. (2014) demonstrated that both irisin and FGF21 are cold-modulated factors that participate in the regulation of glucose metabolism30. In the present study, we observed a significant increase in serum irisin 1 h after the first WBC session, particularly among YG participants. This effect was sustained throughout the study protocol. We also observed two-fold higher values of irisin at baseline in MG participants compared to YG. This observation is consistent with that of Huth et al. (2015), who found a positive correlation between irisin, age and obesity markers, which all correlated inversely with insulin sensitivity60. Changes in irisin concentration in response to WBC can be linked with the two sources of this protein: skeletal muscles30 and fat tissue31,61. The correlations recorded in WBC-EXP group in our study would suggest that the origin of irisin during cold exposure depended on body composition. In our previous study, we concluded that the effect of WBC on irisin concentration depended on participants’ physical fitness level31, thus men with a similar level of relative VO2max were recruited for this experiment. Chronic WBC caused significant increase in irisin concentration, but we did not observe any correlations of this change with body composition or fitness level.
It is worth noting that the elevated concentration of irisin at baseline was accompanied by a lower level of BDNF in MG participants, who were also characterized by higher adiposity compared to the YG individuals. This relationship was also confirmed by a statistically significant, inverse correlation between irisin and BDNF in the whole experimental group. BDNF is hypothesized to be a growth factor with a strong influence on peripheral metabolism, including fat oxidation and the subsequent effect on adipose tissue62. Krabble et al. (2007) noted low levels of circulating BDNF in individuals with both obesity and T2DM63. Moreover, Pedersen et al. (2009) observed an inverse correlation between plasma BDNF and glucose, which raises a possibility that high plasma glucose levels would negatively influence BDNF concentration62. Significantly higher glucose concentration among MG subjects recorded at baseline in our study, partly confirm these findings. BDNF did not change in response to the single and chronic exposure to WBC.
Chronic cold exposure caused an increase of the level of adiponectin in the WBC-EXP group, yet no significant changes were observed in the WBC-CON group. Similarly, Imbeault et al. (2009) observed an increase in adiponectin levels in young healthy men during a 2 h period of cold exposure (both 4 °C and 10 °C) which was inhibited by glucose ingestion64. Adiponectin is considered a marker of systemic insulin sensitivity65. In the present study, the elevation in adiponectin concentration was accompanied by a decrease of insulin and glucose in the WBC-EXP group at the conclusion of the tenth WBC exposure. Despite the differences in PBF% among YG and MG participants, the mean change of adipokine during WBC exposure did not differ significantly between these two cohorts. Nevertheless, a trend towards an increase in adiponectin among MG individuals compared with YG individuals (mean increase of 54.6% vs 35.5%, respectively) was noticed. Adiponectin is one of the most abundant adipokines secreted by adipocytes65. Our findings suggest that the amount of body adipose tissue may have affected the relative increase of circulating adiponectin during WBC.
To conclude, the both the acute and chronic WBC protocol led to an improvement in glucose homeostasis indicators together with a reduction of valine and asparagine (Fig. 4). These changes were accompanied by a decline of serum myostatin concentration. This effect was more pronounced amongst the MG participants. Our intervention is not without limitations. We did not perform fat or muscle tissue biopsy, which means that we cannot clearly determine the source of the indicators observed in the blood. Further research could address these limitations, in particular determine the longevity of the WBC-induced effects on reduce myostatin level, changes in blood AA profile, improvement of glucose homeostasis and explore other factors modulating these effects. Overall, our results support the use of WBC to induce at least a short-term improvement in the metabolic profile that may feed into more complex preventive strategies, including physical activity and eventually, pharmacologic interventions, against the risk of development of IR and T2DM.
Methods
Subjects
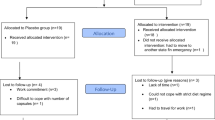
Thirty-five healthy, untrained, male participants, who had not experienced WBC in the previous 12 months, took part in the experiment. Prior to participation in the study, volunteers completed a medical screening in order to eliminate those with contraindications to cold exposure (e.g. cardiovascular disease, blood pressure > 160/100 mmHg, stroke or cold intolerance)66. Using an online software https://www.graphpad.com/quickcalcs/randMenu/), the participants were randomly assigned to either the experimental WBC group (WBC-EXP, n = 22; age = 40 ± 13.5 years; BMI = 26.1 ± 3.9 kg∙m2; PBF% = 19.3 ± 6.1%) or the control group (WBC-CON, n = 13; age = 30.1 ± 7.4 years; BMI = 23.5 ± 2.5 kg∙m2; PBF% = 17.2 ± 5.9%). The WBC-EXP group was further divided intoeither young (YG; n = 9; age = 28 ± 7 years) or middle-aged (MG; n = 13; age = 51 ± 3 years). Considering previous reports, which revealed that the effect of WBC on circulating myokines and adipokines was depended on participants’ cardiorespiratory fitness36, that the participants in the present study all had a similar relative VO2max (WBC-CON 47.4 ± 4.6 mL kg-1 min-1; WBC-EXP 46.5 ± 5.1 mL kg-1 min-1). The study protocol was approved by the Bioethical Committee of the Regional Medical Society in Gdansk KB-28/17 and was conducted in accordance with the Declaration of Helsinki. This experiment was conducted as an arm of the clinical trial registered in the ClinicalTrials.gov: NCT04375969 on 6 May 2020. A written, informed consent was obtained from all subjects. A schematic representation of the experimental protocol is presented in Fig. 5.
The experiment schedule. Blood collection: (I) before the first WBC session, (Ih) 1 h after the first WBC, (II) before the last WBC session and (IIh) 1 h after the last WBC session. Baseline assessment: body composition assessment and cardiorespiratory fitness measurement. Final assesment: body composition asesssement.
Body composition assessment
Body mass and body composition, skeletal muscle mass, PBF% and VFA67,68 were estimated using a multi-frequency impedance analyser (In Body 720, Biospace, Korea). Measurements were taken on the first day of data collection and after the final session of WBC with the participants in a fasted state. During the measurement, subjects wore only shorts and remained barefoot. The impedance of segments of different body parts (trunk, arms and legs) was measured at six different frequencies (1, 5, 50, 250,500, and 1000 kHz) using an eight-polar tactile-electrode. This method can be used as a surrogate of dual-energy X-ray absorptiometry69 because of greater availability and smaller individual error produced by InBody analyzer, which makes it equally precise.
Cardiorespiratory fitness measurement
In order to standarize the study group in terms of cardiorespiratory fitness, participants performed a graded cycle test on a cycle ergometer (884E Sprint Bike Monark, Sweden) to determine their VO2max. The test was conducted three days prior to the start of the first WBC exposure. The VO2max test began with a 5-min warm up at a workload of 1.5 W·kg−1 and a pedalling cadence of 60 rpm. The load increased progressively by 25 W·min−1 until an individual reached the point of volitional exhaustion. Pulmonary gas exchange was measured during the test (MetaMax 3B, Cortex, Germany)36. The highest value of relative oxygen uptake was taken into consideration when assigning experiment and control groups.
Blood analysis and collection
Blood samples were taken on the first day of the WBC treatment (both prior to and 1 h after the first session) and on the last day of the final WBC session (also both prior to and 1 h after the last, 10th session). Samples (approx. 20 ml per person during each collection) were collected from the antecubital vein uding a needle into vacutainer tubes with K3EDTA (Becton, Dickinson & Co., Franklin Lakes, NJ, USA) for plasma analysis, and into vacutainer’s with sodium fluoride to estimate glucose concentration and SSTTM II Advance for serum analysis. Samples were centrifuged at 2000 g at 4 °C for 10 min and then stored at −80 °C.
Serum FGF21, myostatin and BDNF were determined by enzyme immunoassay methods using commercial kits (R&D Systems, USA; catalog no. DF2100, DGDF80 and DBD00, respectively) in accordance with manufacturer’s instructions. The detection limits were 8.69 pg∙mL−1 for FGF21, 2.25 pg∙mL−1 for myostatin and < 20 pg∙mL−1 for BDNF. The average intra-assay coefficient of variation (CV) was 3.5–3.9% for FGF21 and 5% for myostatin and BDNF. For myostatin measurements, samples were diluted in a 1:4 ratio (in 1 N HCL, 1.2 N NaOH/0.5 M HEPES and Calibrator Diluent RD5-26) prior to the analysis according to the manufacturer’s instruction.
Quantification of serum irisin and plasma adiponectin was determined via the enzyme immunoassay method using commercially available kits from Phoenix Pharmaceuticals Inc, USA (catalog no. EK 067–29 and EK- ADI-01, respectively) according to the manufacturer´s protocol. For irisin, intra-assay CV was 4–6% and inter-assay CV was 8–10%. For adiponectin intra-assay and inter-assay CV’s were < 10% and < 15% respectively, and detection sensitivity was 5.32 pg mL−1. AA profile was conducted based on the ion-pair reversed phase high performance liquid chromatography combined with the tandem mass spectrometry IP-RP HPLC–MS/MS (TSQ Vantage Thermo Scientific, USA). The procedure was executed following the protocol already described by Gmiat et al.70.
Glucose level was assessed using the Cobas 6000 analyser. To determine insulin concentration the immunoassay kit from DiaMetra (catalogue no DKO076, Perugia, Italy) was used. The intra-assay CV was ≤ 5% and the inter-assay CV was ≤ 10%. Homeostasis model assessments for insulin sensitivity (HOMA-S), β-cell function (HOMA-B) and insulin resistance (HOMA-IR) were obtained from paired fasting glucose and insulin levels using the updated software HOMA calculator, version 2.2.3, copyright by The University of Oxford (www.dtu.ox.ac.uk/homacalculator). Normal values are 100% for HOMA-S and HOMA-B and 1.0 for HOMA-IR71.
Whole-body cryotherapy procedure
WBC sessions took place in a cryogenic chamber (Zimmer MedizinSysteme, Elecpol) at the Pomeranian Rheumatologic Centre in Sopot, Poland. The treatments were performed five days in a row, with a two-day rest period, followed by five more consecutive days, for a total of 10 sessions completed over two weeks (Fig. 5). Sessions took place at the same time of day (in the morning between 7:30 am and 8:00 am after a light breakfast). Each session was preceded by a 30-s adaptation in the chamber at − 60 °C. The cryotherapy exposure in the main chamber lasted 3 min at − 110 °C. Participants wore shorts, socks, gloves and a hat to protect their hands, feet and ears againts frostbite. According to the instructions, they moved slowly on a circle, changing direction of the motion every 1 minute72. Participants did not engage in any other recovery treatment, throughout the duration of the study.
Statistical analysis
Statistical analyses were performed using a dedicated software package (Statistica 13.1 software, TIBCO Software, Palo Alto, California, USA). The sample size of the study group was predetermined using a power calculation in the software G ∗ power version 3.1.9.473 (a priori repeated-measures within-between interaction, α = 0.05, 1-β = 0.95, r = 0.6, f = 0.25, ε = 1, with a further 20% surcharge due to the possibility of the participant not completing the experiment). Shapiro–Wilk tests were used to assess the homogeneity of dispersion from normal distribution. Brown–Forsythe test was used to evaluate the homogeneity of variance. To analyse the effect of a single cryotherapy session, we used paired tests for a homogenous sample. For a heterogeneous sample, Wilcoxon signed-rank test was used. In the second phase of analysis, for lipid profile, amino acid profile and glucose homeostasis indicators two (group: WBC-EXP, WBC-CON) x two (time: before and after 2 weeks) analyses of repeated measurements of variances (ANOVA) were calculated. In case of a significant group x time interaction, for homogenous results Tukey’s post hoc tests for unequal sample sizes were performed to identify significantly different results. For heterogeneous results, ANOVA Friedman’s test and Dunn-Bonferroni post-hoc test were used. The effect size (partial eta squared, \({\eta }_{p}^{2}\)) was also calculated, with \({\eta }_{p}^{2}\)≥0.01 indicating a small effect; ≥ 0.059 indicating a medium effect; and ≥ 0.138 indicating a large effect74. A similar analysis was done for age groups (YG and MG) in the WBC-EXP treatment group. Relationships between variables were evaluated using the Spearman correlation coefficient. Additionally, the effect size (Cohen’s d) was calculated, with d ≥ 0.2 indicating small effect; ≥ 0.5 indicating medium effect; and ≥ 0.8 indicating large effect. The level of significance was set at p < 0.05. In the descriptive analysis, data are reported as a mean ± standard deviation (SD).
References
Petersen, M. C. & Shulman, G. I. Mechanisms of insulin action and insulin resistance. Physiol. Rev. 98, 2133–2223. https://doi.org/10.1152/physrev.00063.2017 (2018).
Freeman, A. M. & Pennings, N. in StatPearls (2020).
Fletcher, B., Gulanick, M. & Lamendola, C. Risk factors for type 2 diabetes mellitus. J. Cardiovasc. Nurs. 16, 17–23. https://doi.org/10.1097/00005082-200201000-00003 (2002).
Slawik, M. & Vidal-Puig, A. J. Lipotoxicity, overnutrition and energy metabolism in aging. Ageing Res. Rev. 5, 144–164. https://doi.org/10.1016/j.arr.2006.03.004 (2006).
Barzilai, N., Huffman, D. M., Muzumdar, R. H. & Bartke, A. The critical role of metabolic pathways in aging. Diabetes 61, 1315–1322. https://doi.org/10.2337/db11-1300 (2012).
Arum, O. et al. Specific suppression of insulin sensitivity in growth hormone receptor gene-disrupted (GHR-KO) mice attenuates phenotypic features of slow aging. Aging Cell 13, 981–1000. https://doi.org/10.1111/acel.12262 (2014).
Waugh, N. R., Shyangdan, D., Taylor-Phillips, S., Suri, G. & Hall, B. Screening for type 2 diabetes: a short report for the National Screening Committee. Health Technol Assess 17, 1–90. https://doi.org/10.3310/hta17350 (2013).
Pippitt, K., Li, M. & Gurgle, H. E. Diabetes mellitus: screening and diagnosis. Am. Fam. Physician 93, 103–109 (2016).
Gar, C. et al. Serum and plasma amino acids as markers of prediabetes, insulin resistance, and incident diabetes. Crit. Rev. Clin. Lab. Sci. 55, 21–32. https://doi.org/10.1080/10408363.2017.1414143 (2018).
Schutz, Y. Protein turnover, ureagenesis and gluconeogenesis. Int. J. Vitamin Nutrit. Res. 81, 101–107. https://doi.org/10.1024/0300-9831/a000064 (2011).
Gannon, M. C. & Nuttall, F. Q. Amino acid ingestion and glucose metabolism–a review. IUBMB Life 62, 660–668. https://doi.org/10.1002/iub.375 (2010).
Tai, E. S. et al. Insulin resistance is associated with a metabolic profile of altered protein metabolism in Chinese and Asian-Indian men. Diabetologia 53, 757–767. https://doi.org/10.1007/s00125-009-1637-8 (2010).
Javed, K. & Fairweather, S. J. Amino acid transporters in the regulation of insulin secretion and signalling. Biochem. Soc. Trans. 47, 571–590. https://doi.org/10.1042/BST20180250 (2019).
Wang, T. J. et al. Metabolite profiles and the risk of developing diabetes. Nat. Med. 17, 448–453. https://doi.org/10.1038/nm.2307 (2011).
Mook-Kanamori, D. O. et al. Type 2 diabetes is associated with postprandial amino acid measures. Arch. Biochem. Biophys. 589, 138–144. https://doi.org/10.1016/j.abb.2015.08.003 (2016).
Alves, A., Bassot, A., Bulteau, A. L., Pirola, L. & Morio, B. Glycine metabolism and its alterations in obesity and metabolic diseases. Nutrients 11, doi:https://doi.org/10.3390/nu11061356 (2019).
Zhao, B., Wall, R. J. & Yang, J. Transgenic expression of myostatin propeptide prevents diet-induced obesity and insulin resistance. Biochem. Biophys. Res. Commun. 337, 248–255. https://doi.org/10.1016/j.bbrc.2005.09.044 (2005).
Cleasby, M. E., Jamieson, P. M. & Atherton, P. J. Insulin resistance and sarcopenia: mechanistic links between common co-morbidities. J. Endocrinol. 229, R67-81. https://doi.org/10.1530/JOE-15-0533 (2016).
Trendelenburg, A. U. et al. Myostatin reduces Akt/TORC1/p70S6K signaling, inhibiting myoblast differentiation and myotube size. Am. J. Physiol. Cell Physiol. 296, C1258-1270. https://doi.org/10.1152/ajpcell.00105.2009 (2009).
Amor, M. et al. Serum myostatin is upregulated in obesity and correlates with insulin resistance in humans. Exp. Clin. Endocrinol. Diabetes 127, 550–556, doi:https://doi.org/10.1055/a-0641-5546 (2019).
Fournier, B. et al. Blockade of the activin receptor IIb activates functional brown adipogenesis and thermogenesis by inducing mitochondrial oxidative metabolism. Mol. Cell. Biol. 32, 2871–2879. https://doi.org/10.1128/MCB.06575-11 (2012).
Pedersen, B. K. Anti-inflammatory effects of exercise: role in diabetes and cardiovascular disease. Eur. J. Clin. Invest. 47, 600–611. https://doi.org/10.1111/eci.12781 (2017).
Hanssen, M. J. et al. Short-term cold acclimation improves insulin sensitivity in patients with type 2 diabetes mellitus. Nat. Med. 21, 863–865. https://doi.org/10.1038/nm.3891 (2015).
Costello, J. T., Culligan, K., Selfe, J. & Donnelly, A. E. Muscle, skin and core temperature after -110 degrees c cold air and 8 degrees c water treatment. PLoS ONE 7, e48190. https://doi.org/10.1371/journal.pone.0048190 (2012).
Brychta, R. J. & Chen, K. Y. Cold-induced thermogenesis in humans. Eur. J. Clin. Nutr. 71, 345–352. https://doi.org/10.1038/ejcn.2016.223 (2017).
Mawhinney, C. et al. Influence of cold-water immersion on limb and cutaneous blood flow after exercise. Med. Sci. Sports Exerc. 45, 2277–2285. https://doi.org/10.1249/MSS.0b013e31829d8e2e (2013).
Sliwicka, E., Cison, T., Straburzynska-Lupa, A. & Pilaczynska-Szczesniak, L. Effects of whole-body cryotherapy on 25-hydroxyvitamin D, irisin, myostatin, and interleukin-6 levels in healthy young men of different fitness levels. Sci. Rep. 10, 6175. https://doi.org/10.1038/s41598-020-63002-x (2020).
Sun, L. et al. Fibroblast growth factor-21, leptin, and adiponectin responses to acute cold-induced brown adipose tissue activation. J. Clin. Endocrinol. Metabol.https://doi.org/10.1210/clinem/dgaa005 (2020).
Wiecek, M., Szymura, J., Sproull, J. & Szygula, Z. Whole-body cryotherapy is an effective method of reducing abdominal obesity in menopausal women with metabolic syndrome. J. Clin. Med. 9, doi:https://doi.org/10.3390/jcm9092797 (2020).
Lee, P. et al. Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab. 19, 302–309. https://doi.org/10.1016/j.cmet.2013.12.017 (2014).
Dulian, K. et al. The whole body cryostimulation modifies irisin concentration and reduces inflammation in middle aged, obese men. Cryobiology 71, 398–404. https://doi.org/10.1016/j.cryobiol.2015.10.143 (2015).
Potthoff, M. J. et al. FGF21 induces PGC-1alpha and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proc. Natl. Acad. Sci. U.S.A. 106, 10853–10858. https://doi.org/10.1073/pnas.0904187106 (2009).
Kharitonenkov, A. et al. FGF-21 as a novel metabolic regulator. J. Clin. Investig. 115, 1627–1635. https://doi.org/10.1172/JCI23606 (2005).
Coskun, T. et al. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology 149, 6018–6027. https://doi.org/10.1210/en.2008-0816 (2008).
Jaworska, J. et al. Short-term resistance training supported by whole-body cryostimulation induced a decrease in myostatin concentration and an increase in isokinetic muscle strength. Int. J. Environ. Res. Public Health. https://doi.org/10.3390/ijerph17155496 (2020).
Ziemann, E. et al. Whole-body cryostimulation as an effective method of reducing low-grade inflammation in obese men. J. Physiol. Sci. JPS 63, 333–343. https://doi.org/10.1007/s12576-013-0269-4 (2013).
Yoneshiro, T. et al. BCAA catabolism in brown fat controls energy homeostasis through SLC25A44. Nature 572, 614–619. https://doi.org/10.1038/s41586-019-1503-x (2019).
Jaworska, J. et al. A 2-week specific volleyball training supported by the whole body cryostimulation protocol induced an increase of growth factors and counteracted deterioration of physical performance. Front. Physiol. 9, 1711. https://doi.org/10.3389/fphys.2018.01711 (2018).
Stautemas, J. et al. Acute aerobic exercise leads to increased plasma levels of R- and S-beta-aminoisobutyric acid in humans. Front. Physiol. 10, 1240. https://doi.org/10.3389/fphys.2019.01240 (2019).
Gannon, N. P., Schnuck, J. K. & Vaughan, R. A. BCAA metabolism and insulin sensitivity - dysregulated by metabolic status?. Mol. Nutr. Food Res. 62, e1700756. https://doi.org/10.1002/mnfr.201700756 (2018).
Chen, S. et al. Serum amino acid profiles and risk of type 2 diabetes among Japanese adults in the Hitachi Health Study. Sci. Rep. 9, 7010. https://doi.org/10.1038/s41598-019-43431-z (2019).
Owei, I., Umekwe, N., Stentz, F., Wan, J. & Dagogo-Jack, S. Amino acid signature predictive of incident prediabetes: a case-control study nested within the longitudinal pathobiology of prediabetes in a biracial cohort. Metab. Clin. Exp. 98, 76–83, doi:https://doi.org/10.1016/j.metabol.2019.06.011 (2019).
Camporez, J. P. et al. Anti-myostatin antibody increases muscle mass and strength and improves insulin sensitivity in old mice. Proc. Natl. Acad. Sci. U.S.A. 113, 2212–2217. https://doi.org/10.1073/pnas.1525795113 (2016).
Gomarasca, M., Banfi, G. & Lombardi, G. Myokines: the endocrine coupling of skeletal muscle and bone. Adv. Clin. Chem. 94, 155–218. https://doi.org/10.1016/bs.acc.2019.07.010 (2020).
Yarasheski, K. E., Bhasin, S., Sinha-Hikim, I., Pak-Loduca, J. & Gonzalez-Cadavid, N. F. Serum myostatin-immunoreactive protein is increased in 60–92 year old women and men with muscle wasting. J. Nutr. Health Aging 6, 343–348 (2002).
Kong, X. et al. Brown Adipose Tissue Controls Skeletal Muscle Function via the Secretion of Myostatin. Cell metabolism 28, 631–643 e633, doi:https://doi.org/10.1016/j.cmet.2018.07.004 (2018).
McPherron, A. C., Lawler, A. M. & Lee, S. J. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 387, 83–90. https://doi.org/10.1038/387083a0 (1997).
Zak, R. B. et al. Impact of local heating and cooling on skeletal muscle transcriptional response related to myogenesis and proteolysis. Eur. J. Appl. Physiol. 118, 101–109. https://doi.org/10.1007/s00421-017-3749-z (2018).
Matsakas, A. & Diel, P. The growth factor myostatin, a key regulator in skeletal muscle growth and homeostasis. Int. J. Sports Med. 26, 83–89. https://doi.org/10.1055/s-2004-830451 (2005).
Sakuma, K., Aoi, W. & Yamaguchi, A. Molecular mechanism of sarcopenia and cachexia: recent research advances. Pflugers Arch. 469, 573–591. https://doi.org/10.1007/s00424-016-1933-3 (2017).
Shabkhiz, F., Khalafi, M., Rosenkranz, S., Karimi, P. & Moghadami, K. Resistance training attenuates circulating FGF-21 and myostatin and improves insulin resistance in elderly men with and without type 2 diabetes mellitus: a randomized controlled clinical trial. Eur. J. Sport Sci., 1–14, doi:https://doi.org/10.1080/17461391.2020.1762755 (2020).
Hanks, L. J. et al. Circulating levels of fibroblast growth factor-21 increase with age independently of body composition indices among healthy individuals. J. Clin. Transl. Endocrinol. 2, 77–82. https://doi.org/10.1016/j.jcte.2015.02.001 (2015).
Wang, Y. S. et al. Increased serum/plasma fibroblast growth factor 21 in type 2 diabetes mellitus: a systematic review and meta-analysis. Postgrad. Med. J. 95, 134–139. https://doi.org/10.1136/postgradmedj-2018-136002 (2019).
Hollstein, T. et al. The metabolic responses to 24-h fasting and mild cold exposure in overweight individuals are correlated and accompanied by changes in FGF21 concentration. Diabetes https://doi.org/10.2337/db20-0153 (2020).
Lee, P. et al. Mild cold exposure modulates fibroblast growth factor 21 (FGF21) diurnal rhythm in humans: relationship between FGF21 levels, lipolysis, and cold-induced thermogenesis. J. Clin. Endocrinol. Metab. 98, E98-102. https://doi.org/10.1210/jc.2012-3107 (2013).
Keuper, M., Haring, H. U. & Staiger, H. Circulating FGF21 Levels in human health and metabolic disease. Exp. Clin. Endocrinol. Diabetes doi:https://doi.org/10.1055/a-0879-2968 (2019).
Lombardi, G., Sansoni, V. & Banfi, G. Measuring myokines with cardiovascular functions: pre-analytical variables affecting the analytical output. Ann. Transl. Med. 5, 299. https://doi.org/10.21037/atm.2017.07.11 (2017).
Lombardi, G., Barbaro, M., Locatelli, M. & Banfi, G. Novel bone metabolism-associated hormones: the importance of the pre-analytical phase for understanding their physiological roles. Endocrine 56, 460–484. https://doi.org/10.1007/s12020-017-1239-z (2017).
Dong, J., Dong, Y., Chen, F., Mitch, W. E. & Zhang, L. Inhibition of myostatin in mice improves insulin sensitivity via irisin-mediated cross talk between muscle and adipose tissues. Int. J. Obes. (Lond.) 40, 434–442. https://doi.org/10.1038/ijo.2015.200 (2016).
Huth, C. et al. Irisin is more strongly predicted by muscle oxidative potential than adiposity in non-diabetic men. J. Physiol. Biochem. 71, 559–568. https://doi.org/10.1007/s13105-015-0402-3 (2015).
Roca-Rivada, A. et al. FNDC5/irisin is not only a myokine but also an adipokine. PLoS ONE 8, e60563. https://doi.org/10.1371/journal.pone.0060563 (2013).
Pedersen, B. K. et al. Role of exercise-induced brain-derived neurotrophic factor production in the regulation of energy homeostasis in mammals. Exp. Physiol. 94, 1153–1160. https://doi.org/10.1113/expphysiol.2009.048561 (2009).
Krabbe, K. S. et al. Brain-derived neurotrophic factor (BDNF) and type 2 diabetes. Diabetologia 50, 431–438. https://doi.org/10.1007/s00125-006-0537-4 (2007).
Imbeault, P., Depault, I. & Haman, F. Cold exposure increases adiponectin levels in men. Metabol. Clin. Exp. 58, 552–559, doi:https://doi.org/10.1016/j.metabol.2008.11.017 (2009).
Fang, H. & Judd, R. L. Adiponectin regulation and function. Compr. Physiol. 8, 1031–1063. https://doi.org/10.1002/cphy.c170046 (2018).
Lubkowska, A., Dudzinska, W., Bryczkowska, I. & Dolegowska, B. Body composition, lipid profile, adipokine concentration, and antioxidant capacity changes during interventions to treat overweight with exercise programme and whole-body cryostimulation. Oxid. Med. Cell. Longev. 2015, 803197. https://doi.org/10.1155/2015/803197 (2015).
Ogawa, H. et al. InBody 720 as a new method of evaluating visceral obesity. Hepatogastroenterology 58, 42–44 (2011).
Park, K. S. et al. Comparison between two methods of bioelectrical impedance analyses for accuracy in measuring abdominal visceral fat area. J. Diabetes Complications 30, 343–349. https://doi.org/10.1016/j.jdiacomp.2015.10.014 (2016).
McLester, C. N., Nickerson, B. S., Kliszczewicz, B. M. & McLester, J. R. Reliability and agreement of various inbody body composition analyzers as compared to dual-energy x-ray absorptiometry in healthy men and women. J. Clin. Densitom 23, 443–450. https://doi.org/10.1016/j.jocd.2018.10.008 (2020).
Gmiat, A. et al. Improvement of cognitive functions in response to a regular Nordic walking training in elderly women - A change dependent on the training experience. Exp. Gerontol. 104, 105–112. https://doi.org/10.1016/j.exger.2018.02.006 (2018).
Wallace, T. M., Levy, J. C. & Matthews, D. R. Use and abuse of HOMA modeling. Diabetes Care 27, 1487–1495. https://doi.org/10.2337/diacare.27.6.1487 (2004).
Lombardi, G., Ziemann, E. & Banfi, G. Physical activity and bone health: what is the role of immune system? A narrative review of the third way. Front. Endocrinol. 10, 60. https://doi.org/10.3389/fendo.2019.00060 (2019).
Beck, T. W. The importance of a priori sample size estimation in strength and conditioning research. J. Strength Cond. Res. 27, 2323–2337. https://doi.org/10.1519/JSC.0b013e318278eea0 (2013).
Cohen, J. Statistical power analysis for the behavioral sciences. 2nd edn, (L. Erlbaum Associates, 1988).
Acknowledgements
This work has been funded by a grant from National Science Centre OPUS 13, Project No 2017/25/B/NZ7/02309. The authors would like to express thanks to the medical staff of the hospital where the cold exposure took place, especially Aleksandra Kasinska and Iwona Piekarska, for their help in conducting the research, and all of the participants for their engagement in the experiment.
Author information
Authors and Affiliations
Contributions
MK, EZ designed the study and performed the research. MK, EZ, JA, SP, GL performed the research and wrote the paper. MK, GL, MŻ, JA, EZ designed, drafted, and critically revised the manuscript. MK, EZ, SP, KŻ, JK analysed the data. MŻ, JK, KŻ, SP performed the research. All authors have read and approved the final version of the manuscript and agree with the order of presentation of the authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kozłowska, M., Kortas, J., Żychowska, M. et al. Beneficial effects of whole-body cryotherapy on glucose homeostasis and amino acid profile are associated with a reduced myostatin serum concentration. Sci Rep 11, 7097 (2021). https://doi.org/10.1038/s41598-021-86430-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-86430-9
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.