Abstract
Now there is no clinical scale for early prediction of refractory Mycoplasma pneumoniae pneumonia (RMPP). The aim of this study is to identify indicators and develop an early predictive scale for RMPP in hospitalized children. First we conducted a retrospective cohort study of children with M. pneumoniae pneumonia admitted to Children’s Hospital of Nanjing Medical University, China in 2016. Children were divided into two groups, according to whether their pneumonia were refractory and the results were used to develop an early predictive scale. Second we conducted a prospective study to validate the predictive scale for RMPP in children in 2018. 618 children were included in the retrospective study, of which 73 with RMPP. Six prognostic indicators were identified and included in the prognostic assessment scale. The sensitivity of the prognostic assessment scale was 74.0% (54/73), and the specificity was 88.3% (481/545) in the retrospective study. 944 children were included in the prospective cohort, including 92 with RMPP, the sensitivity of the prognostic assessment scale was 78.3% (72/92) and the specificity was 86.2% (734/852). The prognostic assessment scale for RMPP has high diagnostic accuracy and is suitable for use in standard clinical practice.
Similar content being viewed by others
Introduction
Mycoplasma pneumoniae is one of the important pathogens that cause childhood community acquired pneumonia. The incidence of M. pneumoniae infection does not differ by sex, but it varies substantially by age. It is most common in preschool and school age children. The infection rate of pneumonia in children over 5 years old can be as high as 50%1,2. Pneumonia caused by M. pneumoniae infection is generally self-limiting, but sometimes is refractory. After regular treatment, lung lesions can still recur or be prolonged, resulting in residual structural and/or functional lung damage, often manifested as mosaic signs and bronchiectasis3. These sequelae often cause repeated lung infections in children, and have a significant impact on the lung function of adults, which is also closely related to the occurrence of asthma4,5,6. With the incidence of refractory M. pneumoniae pneumonia in children steadily increasing and some case fatalities, early diagnosis and treatment of refractory M. pneumoniae pneumonia is particularly important7.
For the prognosis of adult community acquired pneumonia, A variety of predictive indicators such as the Pneumonia Severity Index and CURB-65 score have been developed to determine the prognosis of community-acquired pneumonia in adults8,9. However, given the practicality of these scales and age limitations, they cannot be directly applied to children.
There has been some research on the predictors of refractory M. pneumoniae pneumonia. Large-scale pulmonary morphogenesis, extrapulmonary complications, and elevated CRP and LDH are clinically relevant risk factors for refractory M. pneumoniae pneumonia10,11,12. However, the current prediction methods often use only a single indicator to judge the prognosis, or there are few clinical data and no prospective verification. The indicators included in some studies are not readily available clinically, and in some studies, the outcome was complications caused by refractory M. pneumoniae pneumonia, rather than predictors of refractory M. pneumoniae pneumonia13,14. Therefore, the aim of this study was to use multiple simple indicators to develop a scale for early prediction of refractory M. pneumoniae pneumonia in hospitalized children.
Methods
Ethics
The study was approved by the institutional ethics committee of Children’s Hospital Affiliated to Nanjing Medical University (Approval number: 201801126-1), and was registered in the Chinese Clinical Trial Registry (Registration number: ChiCTR1800015673). All methods were performed in accordance with the Declaration of Helsinki.
Informed consent
Informed consent was obtained from all subjects or, if subjects are under 18, from a parent and/or legal guardian.
Patients and groups
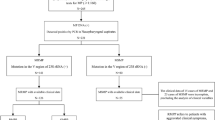
A flowchart of our research is provided in Fig. 1A. We conducted a retrospective cohort study among children admitted to the Children's Hospital of Nanjing Medical University with M. pneumoniae pneumonia from January to December 2016. This was followed by a prospective cohort from January to December 2018. All children were first seen in Children’s Hospital. M. pneumoniae infection was confirmed by polymerase chain reaction testing of nasopharyngeal swab specimens.
(A) Study flow. CAP, community-acquired pneumonia; RMPP, refractory M. pneumoniae pneumonia. (B) The first line is the score corresponding to each indicator value. The following is the index included in the scale, and finally the calculated total score and predicted probability. When using, the table should be scaled up and printed on paper, and the score should be calculated using a tool such as a ruler. (C/D) Scale for predicting refractory M. pneumoniae pneumonia by receiver operator characteristic curves. (C) In the retrospective cohort; (D) In the prospective cohort.
Patients with immune deficiencies, chronic diseases, heart diseases or who were using immunosuppressive drugs were excluded. All those enrolled had negative tuberculosis IgM or purified protein derivative tests. In addition, their nasopharyngeal secretions were negative for respiratory syncytial viruses, influenza viruses, adenovirus, parainfluenza virus, and Chlamydia trachomatis. The subjects also had negative bacterial cultures of nasopharyngeal secretions and double-negative blood cultures. Consent for participation was obtained.
Refractory M. pneumoniae pneumonia is defined as a case with prolonged fever accompanied by deterioration of radiological findings despite appropriate management and treatment with a macrolide antibiotic for ≥ 7 days15. On the basis of this definition, we reviewed patients’ medical records and divided them into 2 groups: RMPP group and non-RMPP group.
Data collection and study variables
We collected data on demographic and clinical characteristics including age, sex, fever days on admission, and chest imaging findings; and laboratory test results including complete blood count, C-reactive protein, alanine aminotransferase, aspartate aminotransferase, lactate dehydrogenase and creatine kinase. After preliminary screening of all indicators, statistically significant indicators were selected for regression analysis.
Respiratory pathogens
Nasopharyngeal aspirates were tested for respiratory pathogens using a real-time, multiplex polymerase chain reaction assay in our hospital’s clinical virology laboratory. The specific pathogens identified included influenza A and B, respiratory syncytial viruses, adenovirus, parainfluenza virus, C. trachomatis, and M. pneumoniae. A positive polymerase chain reaction result for M. pneumoniae was a copy number of > 2,500/mL(ACON Biotech Co.,Ltd, Hangzhou, China). Bacterial culture results based on nasopharyngeal aspirates and blood were obtained from the hospital’s microbiology laboratory16.
Statistical analysis
Statistical analysis was performed with SPSS Version 20.0 (IBM Corp, Armonk, NY, USA) and R Version 3.5.3 (R Foundation for Statistical Computing, Vienna, Austria), and P < 0.05 was considered statistically significant. Categorical variables were analyzed using the chi-square test. Normally distributed continuous data were analyzed using t tests, and non-normally distributed measurement data were analyzed using Mann–Whitney U tests.
Multivariate analysis was performed using a stepwise logistic regression model. R software was used to transform the final regression model into a nomogram. Receiver operating characteristic (ROC) curves were used to analyze the regression model for prediction of refractory M. pneumoniae pneumonia. Calculate the sensitivity and specificity of the predictive scale.
Results
Patient characteristics and laboratory findings
The clinical characteristics of the two cohort patients are shown in Table 1.We enrolled 618 patients in retrospective cohort. There were 73 patients in the RMPP group, and 545 patients in the non-RMPP group. The characteristics of the patients in the retrospective cohort on admission are summarized in Table 2.
There was no significant difference in sex distribution between the 2 groups. The average age and fever days were significantly greater in the RMPP group than that in the non-RMPP group. Compared with the non-RMPP group, significantly more patients in the RMPP group had atelectasis or lobar or segmental lung consolidation, and moderate to large pleural effusions than those in the non-RMPP group.
Compared with the non-RMPP group, the RMPP group showed significantly higher levels of C-reactive protein, neutrophil %, neutrophils (absolute value), aspartate aminotransferase, alanine aminotransferase, and lactate dehydrogenase. The other laboratory findings did not differ significantly between the two groups.
Chest imaging score
In order to be able to incorporate chest imaging findings into regression analysis, we created a new indicator, the chest imaging score (Table 3).
A small amount of pleural effusion: the angle of the costal diaphragm becomes dull; a medium amount of effusion: a large uniform dense shadow in the lower pleural cavity, the upper boundary is curved, the concave surface is upward, and the highest point is in the armpit; Even shadow, the mediastinum is pushed to the opposite side; Large-area lung consolidation: occupying a segment of the lung or above the range of the lung lobes (range over 2/3 of the lung lobes), can involve single or multilobe lesions17,18,19.
Logistic regression and nomogram
All variables that were statistically significant in the comparison between groups were considered for inclusion in the logistic regression analysis. The variables were screened using the maximum likelihood ratio forward stepwise regression method. Finally, age, fever days, C-reactive protein, alanine aminotransferase, lactate dehydrogenase and chest imaging score were included in the predictive model. (Table 4). The final predictive model is shown as a nomogram in Fig. 1B.
Prospective cohort
From January to December 2018, 944 children admitted to our hospital with M. pneumoniae pneumonia were enrolled in the prospective cohort study. The characteristics of the patients in the prospective cohort are shown in Table 5.
Receiver-operating characteristic curve analysis
In the retrospective cohort, the area under the curve for the predictive scale was 0.899 (95% CI 0.860–0.937) as determined by ROC curve analysis (Fig. 1C). In the prospective cohort, the area under the curve was 0.871 (95% CI 0.830–0.911, Fig. 1D).
The optimal cutoff of the scale for predicting refractory M. pneumoniae pneumonia was 0.2, with a sensitivity of 74.0%, specificity of 88.3%, and consistency rate of 86.6% in the retrospective cohort. The optimal cutoff in the prospective cohort was also 0.2, with a sensitivity of 78.3%, specificity of 86.2%, and consistency rate of 85.4% (Table 6).
Discussion
Currently, the majority viewpoint is that the main pathogenic mechanism for the lung damage that occurs in some children with M. pneumoniae pneumonia is due to inflammatory damage mediated by human autoimmune function20. The symptoms of Mycoplasma pneumoniae pneumonia have a rapid onset and are changeable. After treatment, M. pneumoniae pneumonia can also cause serious complications21,22,23,24.
In order to early predict refractory M. pneumoniae pneumonia and reduce the incidence of complications and long-term lung damage, we identified 6 prognostic indicators, including age, fever days, CRP, ATL, LDH, and chest imaging findings. The incidence of refractory M. pneumoniae pneumonia in the retrospective cohort increased with age, suggesting that the pathogenic mechanism in refractory M. pneumoniae infection is related to an excessive immune response25. A persistent fever and CRP are common clinical indicators of infection. LDH is also considered to replace inflammatory cytokines such as IL-18 as useful indicators for predicting refractory M. pneumoniae pneumonia26.These indicators were higher in those in the RMPP group than in those in the non-RMPP group, indicating that the children with refractory M. pneumoniae pneumonia have a more pronounced inflammatory responses. Hepatic dysfunction is a common extrapulmonary injury after M. pneumoniae infection. Both AST and ALT can reflect hepatocyte function, but ALT is often considered to be a specific indicator of liver injury in patients with M. pneumoniae pneumonia27.
There are many factors affecting the prognosis of children with pneumonia, but because there is no support for big data, there are no established criteria for predicting which children are at risk of a poor outcome. Some existing prediction scales often lack the universality of clinical application because of a bias of the original data, or are derived from the improved adult scale and has a narrower scope of application28,29,30. Some previous reports have also shown that increasing age, severe chest imaging findings, and elevated inflammatory markers can effectively predict the occurrence of refractory M. pneumoniae pneumonia and its complications. Clinical features combined with laboratory results can improve the diagnosis of refractory M. pneumoniae pneumonia31.
The predictive power of the scale obtained in this study on refractory M. pneumoniae pneumonia has good performance in both retrospective and prospective cohorts. The area under the ROC curve in the retrospective and prospective cohort was 0.899 and 0.875, respectively, indicating that the predictive scale can correctly distinguish between children with refractory M. pneumoniae pneumonia and those with simple disease. The scale has high sensitivity and specificity in the two cohorts. Compared with other studies, the clinical indicators included in this study are relatively simple and easy to obtain, which is more conducive to application in clinical work.
Conclusions
In summary, we finally included six readily available clinical indicators to predict refractory M. pneumoniae pneumonia. This predictive scale helps to determine whether a child will develop refractory M. pneumoniae pneumonia early in the disease. In the retrospective and prospective cohort, the scale has good discrimination, high sensitivity and specificity.
Abbreviations
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- CK:
-
Creatine kinase
- HB:
-
Hemoglobin
- LDH:
-
Lactate dehydrogenase
- MPP:
-
M. pneumoniae Pneumonia
- RMPP:
-
Refractory M. pneumoniae pneumonia
- WBC:
-
White blood cells
References
Jain, S. et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N. Engl. J. Med. 372(9), 835–845 (2015).
Korppi, M., Heiskanen-Kosma, T. & Kleemola, M. Incidence of community-acquired pneumonia in children caused by Mycoplasma pneumoniae: serological results of a prospective, population-based study in primary health care. Respirology 9(1), 109–114 (2004).
You, S. Y., Jwa, H. J., Yang, E. A., Kil, H. R. & Lee, J. H. Effects of methylprednisolone pulse therapy on refractory mycoplasma pneumoniae pneumonia in children. Allergy Asthma Immunol. Res. 6(1), 22–26 (2014).
Vervloet, L. A., Marguet, C. & Camargos, P. A. Infection by Mycoplasma pneumoniae and its importance as an etiological agent in childhood community-acquired pneumonias. Braz. J. Infect. Dis. Off. Publ. Braz. Soc. Infect. Dis. 11(5), 507–514 (2007).
Wang, X. et al. Necrotizing pneumonia caused by refractory Mycoplasma pneumonia pneumonia in children. World J. Pediat. WJP. 14(4), 344–349 (2018).
Kim, C. K. et al. Late abnormal findings on high-resolution computed tomography after Mycoplasma pneumonia. Pediatrics 105(2), 372–378 (2000).
Eibach, D. et al. Increased detection of Mycoplasma pneumoniae infection in children, Lyon, France, 2010 to 2011. Euro Surv. Bull. Eur. sur les maladies Trans. Eur. Commun. Dis. Bull. 17(8), 20094 (2012).
Aujesky, D. & Fine, M. J. The pneumonia severity index: a decade after the initial derivation and validation. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 47, S133–S139 (2008).
Lim, W. S. et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax 58(5), 377–382 (2003).
Saraya, T. et al. The correlation between chest X-ray scores and the clinical findings in children and adults with mycoplasma pneumoniae pneumonia. Int. Med. 56(21), 2845–2849 (2017).
Shimizu, T., Kida, Y. & Kuwano, K. Cytoadherence-dependent induction of inflammatory responses by Mycoplasma pneumoniae. Immunology 133(1), 51–61 (2011).
Lu, A., Wang, C., Zhang, X., Wang, L. & Qian, L. Lactate dehydrogenase as a biomarker for prediction of refractory mycoplasma pneumoniae pneumonia in children. Respir. Care 60(10), 1469–1475 (2015).
Cheng, S. et al. Development and validation of a simple-to-use nomogram for predicting refractory Mycoplasma pneumoniae pneumonia in children. Pediatr. Pulmonol. 55(4), 968–974 (2020).
Xu, X. et al. Nomogram for prediction of bronchial mucus plugs in children with mycoplasma pneumoniae pneumonia. Sci. Rep. 10(1), 4579 (2020).
Tamura, A. et al. Methylprednisolone pulse therapy for refractory Mycoplasma pneumoniae pneumonia in children. J. Infect. 57(3), 223–228 (2008).
[Guidelines for management of community acquired pneumonia in children (the revised edition of 2013) (I)]. Zhonghua er ke za zhi = Chinese journal of pediatrics. 2013;51(10):745–52.
Cho, Y. J. et al. Correlation between chest radiographic findings and clinical features in hospitalized children with Mycoplasma pneumoniae pneumonia. PLoS ONE 14(8), e0219463 (2019).
Yoon, I. A. et al. Radiologic findings as a determinant and no effect of macrolide resistance on clinical course of Mycoplasma pneumoniae pneumonia. BMC Infect. Dis. 17(1), 402 (2017).
Zhou, Y. et al. More complications occur in macrolide-resistant than in macrolide-sensitive Mycoplasma pneumoniae pneumonia. Antimicrob. Agents Chemother. 58(2), 1034–1038 (2014).
Zhang, Y. et al. Cytokines as the good predictors of refractory Mycoplasma pneumoniae pneumonia in school-aged children. Sci. Rep. 6, 37037 (2016).
San Martin, I., Zarikian, S. E., Herranz, M. & Moreno-Galarraga, L. Necrotizing pneumonia due to Mycoplasma in children: an uncommon presentation of a common disease. Adv. Respir. Med. 86, 305–309 (2018).
Han, X., He, B. & Wang, F. Mycoplasma pneumonia associated with hemolytic anemia: case report and literature review. Zhonghua jie he he hu xi za zhi Zhonghua jiehe he huxi zazhi Chin. J. Tuberculosis Respir. Dis. 34(11), 832–836 (2011).
Sarah, M. et al. Mycoplasma pneumonia and pulmonary embolism in a child due to acquired prothrombotic factors. Pediatr. Pulmonol. 43(2), 200–202 (2007).
Jin, X., Zou, Y., Zhai, J., Liu, J. & Huang, B. Refractory Mycoplasma pneumoniae pneumonia with concomitant acute cerebral infarction in a child: a case report and literature review. Medicine 97(13), e0103 (2018).
Pechous, R. D. With friends like these: the complex role of neutrophils in the progression of severe pneumonia. Front. Cell. Infect. Microbiol. 7, 160 (2017).
Barker, A. F. et al. Aztreonam for inhalation solution in patients with non-cystic fibrosis bronchiectasis (AIR-BX1 and AIR-BX2): two randomised double-blind, placebo-controlled phase 3 trials. Lancet Respir. Med. 2(9), 738–749 (2014).
Daxboeck, F., Gattringer, R., Mustafa, S., Bauer, C. & Assadian, O. Elevated serum alanine aminotransferase (ALT) levels in patients with serologically verified Mycoplasma pneumoniae pneumonia. Clin. Microbiol. Infect. 11(6), 507–510 (2010).
Reed, C. et al. Development of the Respiratory Index of Severity in Children (RISC) score among young children with respiratory infections in South Africa. PLoS ONE 7(1), e27793 (2012).
Hooli, S. et al. Predicting hospitalised paediatric pneumonia mortality risk: an external validation of risc and mrisc, and local tool development (RISC-Malawi) from Malawi. PLoS ONE 11(12), e0168126 (2016).
Rubulotta, F., Ramsay, D. & Williams, M. D. PIRO score for community-acquired pneumonia: a new prediction rule for assessment of severity in intensive care unit patients with community-acquired pneumonia. Crit. Care Med. 38(4), 1236 (2010).
Meyer Sauteur, P. M. et al. Improved diagnostics help to identify clinical features and biomarkers that predict Mycoplasma pneumoniae community-acquired pneumonia in children. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 71, 1675–1754 (2019).
Funding
This work was supported, in part, by Grants from Jiangsu Province Special Funds for Key Program (Social Development) (BE2019607), Key Projects of Nanjing Health and Planning Commission (ZKX18041), Jiangsu Province Young Medical Talents (QNRC2016087).
Author information
Authors and Affiliations
Contributions
Y.B. performed experiments, statistical analysis, made the figures and tables and drafted manuscripts; Y.Z. participated in the study design, collected and interpreted the clinical information, determined the clinical status for each children involved in the study; X.M. participated in the revision of the article and the supplement of the data. J.X., Y.G., T.H., S.Z., and X.W. participated in the collection of clinical data; D.Z. participated in study design and contributed to the interpretation of data; F.L. designed the study, analyzed the data and revised the manuscript. All authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bi, Y., Zhu, Y., Ma, X. et al. Development of a scale for early prediction of refractory Mycoplasma pneumoniae pneumonia in hospitalized children. Sci Rep 11, 6595 (2021). https://doi.org/10.1038/s41598-021-86086-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-86086-5
This article is cited by
-
Integrative study of pulmonary microbiome, transcriptome and clinical outcomes in Mycoplasma pneumoniae pneumonia
Respiratory Research (2024)
-
Recognition of refractory Mycoplasma pneumoniae pneumonia among Myocoplasma pneumoniae pneumonia in hospitalized children: development and validation of a predictive nomogram model
BMC Pulmonary Medicine (2023)
-
miRNA, lncRNA and circRNA: targeted molecules with therapeutic promises in Mycoplasma pneumoniae infection
Archives of Microbiology (2023)
-
Development and validation of a nomogram for predicting Mycoplasma pneumoniae pneumonia in adults
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.