Abstract
The objective of this study is to assess prognostic value of surgery for elderly oral tongue squamous cell carcinomas (OTSCC) patients. Patients with OTSCC were extracted from the SEER database between 2010 and 2014. The distributions of categorical demographic and clinicopathological characteristics were determined for different age groups: the 75–79, 80–84, and 85–102 years old groups. Univariate and multivariate analyses were performed to determine the effects of each variable on survival. A total of 1064 patients were analyzed. 75–79 years old patients tended to be male and rate of surgery declined with advancing age (P < 0.001). 75–79 years old patients more frequently presented with advanced stage compared to their older peers (P = 0.002). Compared to surgery groups, the hazard ratios for no surgery groups were 2.856 (95% CI 2.267–3.599; (P < 0.001)) for OS and 3.687 (95% CI 2.561–5.308; (P < 0.001)) for CSS in multivariable analysis. In subgroup analysis, the effect of no surgery was significantly associated with a higher risk of poor CSS in patients aged 75–79 years, 80–84 years and 85–102 years (P < 0.001, respectively). Our results showed that there were a series of factors contributing to poor outcomes in the elderly OTSCC patients, including clinicopathological characteristics and surgical management. Surgical resection is significantly associated with an improved OS and CSS, but further exploration in larger prospective clinical trials and better prognostic and predictive tools for select old patients for surgery are needed.
Similar content being viewed by others
Introduction
Oral tongue squamous cell carcinoma (OTSCC) is one of the most frequent head and neck cancers, accounting for 20% of these cancers1,2,3. Approximately 24% to 55% of the patients were reported to have locally advanced disease at presentation, and the 5-year overall survival is 15% to 45%4,5. The primary therapeutic strategy for patients has been surgery, and systemic therapy has been reserved based on a multitude of factors.
The malignancy is rare below the age of 40, with a mean age at diagnosis of 50–60 years old1,5,6,7. The poor prognosis associated with advanced age was thought to be due to poor immunologic defense against cancer, more aggressive histological type8, or less aggressive therapy9,10,11,12,13. By the year 2030, 20% of Americans will be older than 65 years, with those aged > 85 years representing the fastest-growing subset14. The older population experiences the greatest suffering caused by this cancer; however, elderly patients are greatly underrepresented in the scientific data and age-restrictive exclusion criteria are commonplace in clinical trials15. Therefore, the aim of this study is to assess the impact of surgery on long-term overall survival (OS) and cancer-specific survival (CSS) in a large cohort of elderly patients with OTSCC.
Results
Clinical characteristics of all patients
A total of 1064 OTSCC patients were included in this population-based study, with a median age of 81 years old (range 75–102 years old). 438 (41.2%), 342 (32.1%), and 284 (26.7%) patients were aged 75–79, 80–84, and 85–102 years old, respectively. Among the cohort of the patients, 65.9% and 34.1% patients were stage I–II and stage III–IV, respectively. Most of OTSCC occurs on the anterior 2/3 of tongue (27.7%). 75–79 years old patients tended to be male and rate of surgery declined with advancing age (P < 0.001, respectively). 75–79 years old patients more frequently presented with advanced stage compared to their older peers (P = 0.002). Only 16.8% (179/1064) didn’t receive surgery to primary tumor. The clinicopathological features stratified by age at diagnosis are listed in Table 1.
Survival
The median OS was 14.0 months (range 0–59 months). Univariate regression analyses showed that age, gender, grade, tumor location, stage, T category, N category, and surgery therapies to the primary tumor were significant risk factors of overall survival (P < 0.05). Figure 1 illustrated that the surgery groups showed significantly better OS and CSS than the no surgery groups (P < 0.05).
In the multivariable analysis, compared to surgery groups, the hazard ratios for no surgery groups were 2.856 (95% CI 2.267–3.599; (P < 0.001)) for OS and 3.687 (95% CI 2.561–5.308; (P < 0.001)) for CSS, respectively (Table 2).
Subgroup analysis of the relationship between surgery and survival
Multivariate Cox regression analysis demonstrated that variables, including age, grade, T category, N category, and surgery therapies were all independent prognostic factors of OS and CSS. To rule out the effects of these variables and further validate the effect of surgery on OS and CSS, we conducted the subgroup analysis based on these variables (Table 3). Remarkably, the effect of no surgery was significantly associated with a higher risk of poor CSS in patients aged 75–79 years (HR 5.279; 95% CI 3.111 − 8.956; (P < 0.001)) and 80–84 years (HR 9.641; 95% CI 5.461–17.021; (P < 0.001)), and 85–102 years (HR 6.259; 95% CI 3.700–10.590; (P < 0.001)). Amongst those undergoing surgery, 16 (1.8%) patients died within 30 days after cancer diagnosis and 6 (0.7%), 3 (0.3%), and 7 (0.8%) patients were aged 75–79, 80–84, and 85–102 years old, respectively.
Discussion
Surgery is rarely performed in elderly patients in clinical practice, considering that advanced age is associated with decreased functional status and increased co-morbidity, such as cardiovascular, respiratory, metabolic, hepatic, and renal diseases. Therefore, medical decision about whether elderly patients with OTSCC should undergo surgery or not should be made more carefully.
Surgery is widely believed to be the best way to treat solid tumors, and age shouldn’t be a decisive factor alone in medical decisions16,17. Several studies have reported that elderly patients with lung cancer18, papillary thyroid cancer19, colon cancer20, liver cancer21 and so on should still receive surgery after prognosis assessment and perioperative risk stratification. However, whether surgery should be performed on OTSCC patients aged ≥ 75 years old better was not further analyzed.
In our study, 1064 patients with OTSCC were included, but only 16.8% (179/1064) didn’t receive surgery to primary tumor, which indicated that the management of patients was in favor of surgery because more than 80% of the elderly patients had chosen surgery and the remaining 16.8% of patients might have too many risk factors to be deemed an appropriate surgical candidate. We also found that the rate of surgery declined with advancing age; however, subgroup analysis showed that survival advantage was associated with cancer-directed surgery at all age groups. Soudry et al. found that the 5-year disease-free survival (DFS) was 65% and 58% for patients over 75 and younger patients, and corresponding rates for 5-year disease-specific survival were 69% and 70%, which were not statistically significant. Patients with OTSCC aged 75 years or older should be managed like younger patients and they should be given a chance for treatment in terms of clinical staging and co-morbidities, because their prognosis is not different from that in younger patients22. What’s more, Mukdad et al. found that surgery predicted improved OS and DSS in all groups except young females (≤ 40 years)8. In addition, older age remained an independent risk factor for both OS and CSS even in such an elderly population, which has never been reported individually. Therefore, surgery could be prudently recommended to patients with optimistic life expectancies and carefully selected and closely observed OTSCC patients, including acceptable morbidity, anticipated life span, comorbidities, patient wishes, nutrition, functional status, and social support, could benefit from surgery. Better prognostic and predictive tools for select elderly patients for surgery are needed.
The greatest strength of this study is the population-based nature of the study and the large sample size, which gives insight to the nature of how US doctors treating elderly OTSCC patients. Several limitations of this study should be underlined. First, retrospective analysis may contribute to some bias. Second, due to the data limitations of the SEER database, the lack of data on additional predictors of OS such as comorbidities, performance status, and systemic therapy prevented us to adjust our analyses for these important factors.
Conclusion
Our results showed that a series of factors contributed to poor outcomes in the elderly OTSCC patients, including clinicopathological characteristics and surgical management. Surgical resection is significantly associated with an improved prognosis, but further exploration in larger prospective clinical trials and better prognostic and predictive tools for select old patients for surgery are needed.
Materials and methods
Cohort population
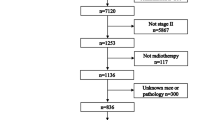
The Surveillance, Epidemiology, and End Results (SEER) program registries collect data on patient demographics; cancer stage, site and type; treatment of the primary tumor; follow-up vital status; and OS and CSS. SEER*Stat Version 8.3.4 (http://www.seer.cancer.gov/seerstat) from the National Cancer Institute was used to identify eligible patients in this study. Tumor staging was assigned with corresponding criteria as described in the classification protocol developed by the American Joint Commission on Cancer (AJCC). Because the SEER database began collecting information on the presence or absence of metastases at the time of diagnosis in 2010, we included patients aged 75 years or older diagnosed with microscopically confirmed OTSCC between 1 January 2010 and 31 December 2014. We selected patients with only one primary malignancy in their lifetime3. A total of 1064 OTSCC patients were included.
Statistical analysis
OS was calculated in months from the date of diagnosis to death, or the date of last follow-up and CSS time from the date of diagnosis to cancer-associated mortality or the date of last follow-up. Survival curves were generated by the Kaplan–Meier method. The Cox proportional hazard model was used to explore the effect of surgery on time to event. All statistical analyses were performed using SPSS (version 24.0) and GraphPad Prism (version 7.0). All reported P-values are two-sided with the level of significance set at 0.05.
Ethical approval and informed consent
All procedures performed in studies involving human participants were in accordance with the ethical standards of Fudan University Shanghai Cancer Center Ethics committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The experimental protocols were also approved by Fudan University Shanghai Cancer Center Ethics committee. Written informed consent was obtained from all individual participants included in the study.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Sasaki, T., Moles, D. R., Imai, Y. & Speight, P. M. Clinico-pathological features of squamous cell carcinoma of the oral cavity in patients <40 years of age. J. Oral Pathol. Med. 34, 129–133 (2005).
Bodner, L., Manor, E., Friger, M. D. & van der Waal, I. Oral squamous cell carcinoma in patients twenty years of age or younger—Review and analysis of 186 reported cases. Oral Oncol. 50, 84–89 (2014).
Li, Y. & Hu, C. Impact of age stratification on the clinicopathological characteristics and survival outcomes on stage IV oral tongue squamous cell carcinomas. Cancer Investig. 38, 565–571 (2020).
Chitapanarux, I. et al. Oral cavity cancers at a young age: Analysis of patient, tumor and treatment characteristics in Chiang Mai University Hospital. Oral Oncol. 42, 82–87 (2006).
Zhang, Y. et al. Clinicopathological characteristics and outcomes of squamous cell carcinoma of the tongue in different age groups. Head Neck 39, 2276–2282 (2017).
Hilly, O. et al. Carcinoma of the oral tongue in patients younger than 30 years: Comparison with patients older than 60 years. Oral Oncol. 49, 987–990 (2013).
Yip, C. et al. Outcomes of oral tongue cancer: Does age matter?. Int. J. Radiat. Oncol. Biol. Phys. 78, S479 (2010).
Mukdad, L. et al. Oral tongue squamous cell carcinoma survival as stratified by age and sex: A surveillance, epidemiology, and end results analysis. Laryngoscope 129, 2076–2081 (2019).
Hoffman, K., Nekhlyudov, L. & Deligdisch, L. Endometrial carcinoma in elderly women. Gynecol. Oncol. 58, 198–201 (1995).
Mundt, A. J., Waggoner, S., Yamada, D., Rotmensch, J. & Connell, P. P. Age as a prognostic factor for recurrence in patients with endometrial carcinoma. Gynecol. Oncol. 79, 79–85 (2000).
Zaino, R. J., Kurman, R. J., Diana, K. L. & Morrow, C. P. Pathologic models to predict outcome for women with endometrial adenocarcinoma: The importance of the distinction between surgical stage and clinical stage—A Gynecologic Oncology Group study. Cancer 77, 1115–1121 (1996).
Alektiar, K. M., Venkatraman, E., Abu-Rustum, N. & Barakat, R. R. Is endometrial carcinoma intrinsically more aggressive in elderly patients?. Cancer 98, 2368–2377 (2003).
Truong, P. T. et al. The effects of age and comorbidity on treatment and outcomes in women with endometrial cancer. Am. J. Clin. Oncol. 28, 157–164 (2005).
Yancik, R. Cancer burden in the aged: An epidemiologic and demographic overview. Cancer 80, 1273–1283 (1997).
Murthy, V. H., Krumholz, H. M. & Gross, C. P. Participation in cancer clinical trials: Race-, sex-, and age-based disparities. JAMA 291, 2720–2726 (2004).
Audisio, R. A. et al. The surgical management of elderly cancer patients; recommendations of the SIOG surgical task force. Eur. J. Cancer 40, 926–938 (2004).
Korc-Grodzicki, B. et al. Surgical considerations in older adults with cancer. J. Clin. Oncol. 32, 2647–2653 (2014).
Wiener, D. C., Argote-Greene, L. M., Ramesh, H., Audisio, R. A. & Jaklitsch, M. T. Choices in the management of asymptomatic lung nodules in the elderly. Surg. Oncol. 13, 239–248 (2004).
Zhou, J. et al. Management of very elderly patients with papillary thyroid cancer: Analysis of outcomes for surgery versus nonsurgery. J. Surg. Res. 256, 512–519 (2020).
Manceau, G., Karoui, M., Werner, A., Mortensen, N. J. & Hannoun, L. Comparative outcomes of rectal cancer surgery between elderly and non-elderly patients: A systematic review. Lancet Oncol. 13, e525–e536 (2012).
Wang, H. Q., Yang, J., Yan, L. N., Zhang, X. W. & Yang, J. Y. Liver resection in hepatitis B related-hepatocellular carcinoma: Clinical outcomes and safety in elderly patients. World J. Gastroenterol. 20, 6620–6625 (2014).
Soudry, E. et al. Squamous cell carcinoma of the oral tongue in patients over 75 years old. Aging Clin. Exp. Res. 23, 231–235 (2011).
Funding
The study was funded by the National Key Technologies Research and Development Program on Prevention and Control of Chronic Non-communicable Diseases (Grant No. 2018YFC1313204).
Author information
Authors and Affiliations
Contributions
All authors have made substantial contributions to all of the following: (1) the conception and design of the study, or acquisition of data, or analysis and interpretation of data, (2) drafting the article or revising it critically for important intellectual content, (3) final approval of the version to be submitted.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, Y., Chu, C. & Hu, C. Effects of surgery on survival of patients aged 75 years or older with oral tongue squamous cell carcinomas. Sci Rep 11, 6003 (2021). https://doi.org/10.1038/s41598-021-85647-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-85647-y
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.