Abstract
A new photoionization scheme accessible by Rhodamine dye lasers is proposed for the isotope separation of 176Lu.
Optimum conditions for the laser isotope separation have been theoretically computed and compared with the previously reported work. The enrichment of ~ 63% can be obtained with > 22 mg/h production rate even when broadband lasers with bandwidth of 500 MHz are employed for the two step excitation. The simplified system requirements for the photoionization scheme combined with a high production rate of 176Lu than previously reported is expected to reduce the global shortage of 176Lu isotope for medical applications.
Similar content being viewed by others
Introduction
Chemistry which is a science of elements, compounds and their separation has immensely helped in the development of mankind. However, separation of isotopes remained a formidable challenge as it is technology intensive; therefore, it is largely limited only to the technologically advanced nations. Some of the isotopes of the low Z-elements could be separated relatively easily by the chemical methods by exploiting the difference in the kinetic isotope effect1. Since the kinetic isotope effect for mid-Z and high-Z elements gradually diminishes with increase in Z, it is necessary to switchover to physical methods for the efficient separation of isotopes of these elements. Isotope separation technology which is largely limited to the developed nations has been applied to the separation of actinides (particularly Uranium) due to its applications in the defence and nuclear industry2. However the real potential of isotope separation for the separation of rest of the isotopes remains unexploited due to limited access to this technology to most of the nations and the complexity of the systems involved.
Among the several methods of the separation of isotopes, gas centrifugation method has evolved as the most commercially viable method, however, apart from the technological challenges, the primary limitation, remains being highly capital intensive. On the other hand, Atomic Vapor Laser Isotope Separation (AVLIS) has evolved as an alternative technique to the gas centrifugation method. AVLIS has significant advantages over gas centrifugation such as being compact, less capital intensive and yielding in high separation factor. If AVLIS technique is appropriately deployed, these systems open up excellent opportunities for the separation of several isotopes other than Uranium.
In daily life, several radioisotopes are being used for medical applications and in particular for cancer diagnosis and therapy3. While many of them are produced as fission products in nuclear reactors; some can be obtained by the irradiation of the precursor (or parent) isotopes. The medical fraternity desires to use nuclear medicine in the highest possible radio-isotopic purity. This demand is normally not met for all the isotopes of nuclear medicine due to challenges in producing the precursor isotopes with a high level of isotopic purity (degree of enrichment) followed by full conversion into the medical isotopes upon irradiation. Among the several isotopes for nuclear medicine, 177Lu isotope has gained lots of attention due to its application in targeted radionuclide therapy4 (TRT). 177Lu is produced in the nuclear reactor by irradiation of its stable parent isotope 176Lu through the nuclear reaction \(^{176}Lu\,{\mathop{\longrightarrow}\limits^{\sigma = 2090 b}} \,{}^{177}Lu\). 177Lu is a radioactive isotope having a half-life of 6.65 days, decays to 177Hf emitting β-particles with energies of 497 keV (76%), 384 keV (9.7%), 176 (12%). 177Hf which is formed in the nuclear excited states decays to the ground state, emitting low energy γ-radiation with energies of 208 keV and 113 keV. With mean β-particle penetration depths of 670 μm, 177Lu is very effective for the treatment of small tumors and metastatic lesions of small size. The low energy γ-radiation emitted by 177Hf is useful for imaging and the studies of bio-distribution and excretion kinetics. Further, large half-life of 177Lu enables shipment to long distances. For these reasons, 177Lu has evolved as the most preferred choice for the targeted radionuclide therapy. However, currently, there is a global shortage of 177Lu isotope primarily due to the shortage of its precursor which is enriched 176Lu isotope. Therefore, there is a need to enhance the production of this isotope to meet the global demand.
Separation of 176Lu (natural abundance = 2.59%) from the other natural major isotope 175Lu (natural abundance = 97.41%) through gas centrifugation is not possible as it has no volatile compounds5. Seemingly, AVLIS remains as the only available option for the enrichment of 176Lu. Kurchatov Institute5,6,7,8,9,10, Russia has extensively worked on the isotope separation of Lu isotopes. The experimental facilities required are technologically most demanding, particularly, with respect to the bandwidth of the excitation lasers, which has to be limited to < 100 MHz level. Such systems cannot be developed by the nations which are not technologically advanced, thus hampering large scale production of enriched 176Lu for medical applications. Therefore, search for suitable photoionization schemes with less stringent requirements and ability to produce at higher production rates is currently on.
In the present work, a new photoionization pathway is proposed for the isotope separation of 176Lu from natural Lu. The proposed photoionization scheme has been theoretically evaluated by computing ionization efficiency and isotope selectivity under various experimental conditions using density matrix formalism.
Results and discussion
The primary limitation in direct irradiation of natural Lu for the production of 177Lu is the low natural abundance of its precursor 176Lu (2.59%). Upon direct irradiation of natural Lu in a medium to high flux reactors (neutron flux 1014–1015 neutrons/cm2-s), the content of 177Lu varies between 0.3 and 1.2% in the irradiated sample (Fig. 1a). When 50% enriched 176Lu is irradiated, it produces 177Lu whose content varies between 6 and 24% (Fig. 1b) in the irradiated sample. Further enrichment of 176Lu does not result in significant improvement in 177Lu content upon irradiation. For example, 100% enriched 176Lu produces 177Lu whose content varies between 12 and 47% (Fig. 1c). Therefore, enrichment of 176Lu to the degree of > 50% can be considered as optimum.
Density matrix formalism provides most accurate description of the laser-atom interaction11. Lineshapes arising in double-resonance12 and triple resonance ionization13 of atoms have been investigated in detail. Recently, investigation of the following photoionization pathway has been carried out for the ionization efficiency and degree of enrichment of 177Lu under various conditions.
540–535 nm scheme
The results obtained were in good agreement with the experimental results14. The optimum conditions for the isotope selective photoionization of 176Lu have been derived. The primary limitation of the scheme for the production of enriched 176Lu is the utilization of narrowband lasers with bandwidth < 100 MHz. Moreover, incorporation of additional apertures along the atomic beam axis is required to limit the Doppler broadening of the atomic beam. This also causes reduction in the throughput of the enriched isotope.
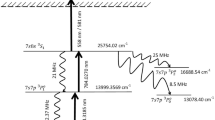
From the energy levels of Lu, it is possible to formulate several photoionization pathways, among them; the following photoionization pathway seems to be particularly promising.
573–560 nm scheme
This photoionization pathway has a number of advantages over the photoionization scheme reported previously5,6,7,8,9,10. They are,
-
1.
The wavelengths of the excitation lasers are easily accessible by high conversion efficiency Rhodamine dye lasers.
-
2.
The large separation between hyperfine components of the Lu isotopes than previously reported enables better enrichment.
-
3.
Possibility of employing relatively broadband lasers for the isotope separation minimises the stringent bandwidth requirements of the excitation lasers.
The hyperfine structure constants of Lu isotopes were measured by Brenner et al.15 and Kuhnert et al.16 for the 0.0 cm−1 and 17,427.28 cm−1 levels. Vergès and Wyart17 have measured hyperfine structure constants of 175Lu isotope for the 35,274.5 cm−1 level using Fourier Transform Infrared Spectrometry, whereas the HFS constants for the 176Lu have not been reported so far. In the absence of hyperfine anomalies15,18, the relationship between the hyperfine structure constants can be expressed as
where A, B are the magnetic dipole and electric quadrupole constants; µ is the magnetic moment, I is the nuclear spin and Q is the quadrupole moment. The hyperfine structure constants for the 176Lu were calculated to be A = 1372.9 MHz and B = − 3538.06 MHz for the 35,274.5 cm−1 level (Table 1).
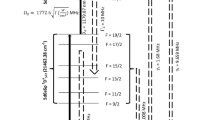
Based on the spectroscopic selection rules, a total of 30 hyperfine excitation pathways are possible in the step wise excitation scheme each denoted by a serial number and have been listed in Table 2 along with their resonance frequencies. Among them, the hyperfine excitation pathway 17/2–17/2–19/2 of 176Lu (listed in serial no 3 in Table 2) lies far away from the hyperfine pathways of the interfering 175Lu isotope. Isotope separation of 176Lu can be accomplished by ionization through the 17/2–17/2–19/2 hyperfine excitation pathway.
In an AVLIS process, when the first and second excitation lasers are tuned to the frequencies ν1 and ν2 respectively, isotope selectivity is defined as
where η is the ionization efficiency of the isotope.
For the case, wherein the abundance of the isotopes is not equal, one need to normalize the selectivity with the abundance of the constituent isotopes; thus, isotope ratio enhancement factor is defined as
Degree of enrichment after laser interaction is calculated using the expression
Two dimensional contour plots of the ionization efficiency of Lu isotopes are plotted in Fig. 2. The resonance frequency positions of all the possible hyperfine excitation pathways are numbered as per the Table 2. Horizontal ridges correspond to the first step excitation and vertical ridges correspond to the second step excitation. Strong horizontal and vertical ridges arise due to the strong transitions of the first and second excitation steps. Enhanced ionization observed at the intersection of horizontal and vertical ridges correspond either to the hyperfine pathway (numbered as per the Table 2) or crossovers. Origin of such crossovers has been discussed in detail in a recent article14. The resonance position of the 17/2–17/2–19/2 of 176Lu has been shown as a “filled circle” in the 175Lu two-dimensional contour plot in Fig. 2a.
Two dimensional contour of the ionization efficiency of (a) 175Lu and (b) and 176Lu for the Doppler free condition and for a laser peak power density of 10 W/cm2 and a bandwidth of 10 MHz for the excitation lasers. The resonance frequency positions of the hyperfine excitation pathways are numbered as per the Table 2. The resonance frequency position of the 17/2–17/2–19/2 of the 176Lu is shown as “filled circle” in (a).
The diagonal ridges correspond to the coherent two photon ionization pathway which is observed when the sum total energy of the two photons is equal to the energy of the upper level. In stepwise excitation schemes, coherent two photon ionization can be observed for the detuning (from the intermediate level) values matching the condition Δ1 = − Δ2. The probability of coherent two photon ionization decreases with the increase in the detuning (|Δ|) of the laser frequency from its resonance transition. For the strong transitions, as in the present case, the diagonal ridge can be observed even for large detuning (Fig. 2).
Diagonal ridges corresponding to the coherent two photon ionization of 175Lu hyperfine excitation pathways numbered as 3 and 6 closely pass through the 17/2–17/2–19/2 hyperfine pathway of 176Lu (Fig. 2a). The two photon sum frequency of 17/2–17/2–19/2 hyperfine pathway of 176Lu is − 8198.1 MHz + 29,358.1 MHz = 21,160.0 MHz (Table 2). The two photon sum frequencies of the hyperfine excitation pathways of 175Lu numbered 3 and 6 ranges between 14,894.1 MHz to 16,945.4 MHz thus have considerable impact on the degree of enrichment of the AVLIS process due to coherent two photon ionization.
Computation of ionization efficiencies and isotope selectivity have been calculated varying the peak power density of excitation and ionization lasers for the Doppler free case and optimum conditions for the enrichment are tabulated in Table 3. From the Table 3, the limit for the bandwidth of the excitation lasers is found to be 500 MHz for obtaining the degree of enrichment of ~ 66%.
Numerical computations of ionization efficiency and degree of enrichment have been carried out for various experimental parameters such as bandwidth of the excitation lasers, peak power density of excitation and ionization lasers and half angular divergence limit to the atomic beam with a limit to the ionization efficiency set to ~ 0.2 and results are tabulated in Table 4. The ionization efficiency of 0.2 corresponds to the ionization of 80% of population available at the ground hyperfine level 17/2. In order to compare the present results with the previously reported data5,14, production rates have been calculated for the atomic number density of 5 × 1014 of Lu corresponding to the number density of 1.3 × 1013 for 176Lu. Further, the laser-atom interaction length is considered as 200 mm with a diameter of 10 mm. It can immediately be observed that for the case of lasers with a bandwidth of 100 MHz, the degree of enrichment reaches to > 99% for all angular divergence values. While for the case of laser bandwidth of 250 MHz, the degree of enrichment degrades to ~ 94%; for higher angular divergence values of the atomic beam, the degree of enrichment marginally deteriorates to ~ 93%. The increase in the angular divergence of the atomic beam has little impact on the degree of enrichment, which implies that under such conditions, larger production rates can be obtained without significant deterioration in the degree of enrichment. Even in the case of lasers with bandwidth of 500 MHz, a degree of enrichment of ~ 63% can be achieved with no limits on the angular divergence.
The present results are compared with the previously reported5,14 values in Table 5. For the case of lasers with bandwidth of 100 MHz, even for the maximum possible half angular divergence value (90°), the degree of enrichment is found to be > 99%, which is nearly 3 times higher than the previously reported 540–535 nm scheme by D’yachkov5 et al. It is not possible to enrich the 176Lu isotope > 5% using broadband lasers having a width of 500 MHz using the 540–535 nm photoionization scheme. However, lasers with bandwidth of 500 MHz can be used in the present 573–560 nm scheme and a degree of enrichment of ~ 63% can be achieved with a production rate > 22 mg / hour which is 6 times higher than the previously reported rate of 3.7 mg/h. Thus the proposed scheme enables production of 176Lu with higher production rate with relatively broad band lasers.
Effect of unknown parameters
In atomic vapor laser isotope separation process, all the atomic parameters such as isotope shifts, hyperfine structures, lifetimes, branching ratios, decay to trapped, autoionization states and their cross-sections; laser parameters such as peak power density, pulse width, bandwidth, repetition frequency; source parameters such as temperature, angular divergence all influence the overall degree of enrichment and the production rate. For the case of the 573–560 nm scheme, some of the spectroscopic parameters have not been measured so far and hence are not known. The effect of these parameters on the degree of enrichment and the resultant production rates is discussed below.
-
(a)
Isotope shift for the 560 nm transition
Isotope shift for the \(5d6s6p\,{^{4}{F}_{3/2}^{o}}\left(17427.28 \,\text{cm}^{-1}\right)\,{\mathop{\longrightarrow}\limits^{{560.3114 \,\text{nm}}}}\, 6s{6p}^{2}\,{^{4}{P}_{5/2}}\left(35274.5 \,\text{cm}^{-1}\right)\) transition has not been reported so far. Isotope shift of a transition comprises of field shift and the mass shift components which can be expressed as19
where F, M are the field shift and mass shift parameters, \({\delta \langle {r}^{2}\rangle }^{\mathrm{175,176}}\) is the difference in the mean square nuclear charge radii.
For the case of Lutetium isotopes, the difference in the mean square nuclear charge radii \({\delta \langle {r}^{2}\rangle }^{\mathrm{175,176}}\) = 0.041 fm2 is small19, resulting in small field shift. The mass shift is also small owing to the small value of the factor \(\left(\frac{{M}^{175}- {M}^{176}}{{M}^{175}.{M}^{176}}\right)\). As a result, the isotope shift between 176 and 177Lu is small i.e., about ~ 100 MHz. Large isotopic selectivity of the present scheme is due to the large spread of the hyperfine spectrum of 176Lu, therefore, the influence of the variation in isotope shift is not of much significance.
-
(b)
Einstein’s coefficient for the \(5{{d}}6{{s}}6{{p}}\,{^{4}{{{F}}}_{3/2}^{{{o}}}}\left(17427.28\,{{{c}}{{m}}}^{-1}\right)\,{\mathop{\longrightarrow}\limits^{{560.3114 {{n}}{{m}}}}}\,{\to } 6{{s}}{6{{p}}}\,^{2}\,{^{4}{{{P}}}_{5/2}}\,\left(35274.5\,{{\text{cm}}}^{-1}\right)\) transition
Einstein’s A coefficient for the transition has not reported so far. However, the typical values reported for the 4P fine structure level were reported to be in the range of 3 × 106 to 2 × 107. A mean value of 1.3 × 107 has been taken for the computations. Due to this uncertainty in the value of Einstein’s A coefficient, the optimum peak power density of the second excitation transition may vary between 20 and 97 W/cm2 which can easily be achieved for the pulsed lasers. Due to the large spread of the hyperfine spectrum of 176Lu isotope and the separation of its 17/2–17/2–19/2 hyperfine excitation pathway from the 175Lu spectrum, the power broadening (due to increase in the laser peak power density) varies the ionization efficiency rather than the selectivity.
-
(c)
Autoionization states and cross-sections
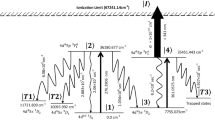
The non-availability of data on the autoionization states and their ionization cross-sections is the primary impediment to the proposed 573–560 nm scheme. Most of the previous work20,21 carried out have reported the data pertaining to the Rydberg series and autoionization states originating from the 5d6s6p 2Do3/2,5/2, 5d6s6p 4Do3/2,5/2 and 6s2nd 2D3/2,5/2, 6s2ns 2S1/2 states. D’yachkov et al.5,10 have found a strong autoionization level connecting to the 5d6s7s 4D3/2 upper level of the 540–535 nm scheme. Further experimental studies on the search for suitable autoionization states connecting to the 6s6p2 4P5/2 upper level of the 573–560 nm scheme is necessary.
Conclusion
A new photoionization pathway has been proposed for the isotope separation of 176Lu precursor isotope. The optimum conditions for the isotope separation of 176Lu have been obtained through density matrix formalism. It has been shown that it is possible to obtain an enrichment of > 99% with high production rates of 19 mg/h when the excitation lasers with bandwidth of 100 MHz are employed. Even when broadband dye lasers of bandwidth of 500 MHz are used, the degree of enrichment of ~ 63% can be obtained with a production rate of > 22 mg/h. This significantly eases the restrictions on the bandwidth of the excitation lasers and Doppler broadening enabling high production rates than previously reported, thus expected to minimise the global shortage of the176Lu precursor isotope for the production of the 177Lu medical isotope.
References
Semiokhin, I. A. Chemical methods of stable isotope separation. J. Radioanal. Nucl. Chem. 205, 201–213 (1996).
Cohen, K. & Murphy, G. M. The theory of isotope separation as applied to the large scale production of U235 (Mcgraw-Hill Book Company Inc, 1951).
Ignac, F. Atlas of clinical nuclear medicine 3rd edn. (CRC Press, 2014).
Dash, A., Raghavan, M., Pillai, A. & Knapp, F. F. Jr. Production of 177Lu for targeted radionuclide therapy: available options. Nucl. Med. Mol. Imaging 49, 85–107 (2015).
Dyachkov, A. B. et al. Selective photoionisation of lutetium isotopes. Quantum Electron. 42, 953–956 (2012).
D’yachkov, A. B. et al. Study of a selective photoionization scheme of 177Lu. Opt. Spectrosc. 125, 839–844 (2018).
D’yachkov, A. B. et al. Photoionization spectroscopy for laser extraction of the radioactive isotope 177Lu. Appl. Phys. 121B, 425 (2015).
Dyachkov, A. B. et al. Effect of amplified spontaneous emission on selectivity of laser photoionisation of the 177Lu radioisotope. Quantum Electron. 46, 574–577 (2016).
Ageeva, I. V. et al. Laser photoionisation selectivity of 177Lu radionuclide for medical applications. Quantum Electron. 49, 832–838 (2019).
D’yachkov, A. B. et al. A study of laser photoionization of 177mLu nuclear isomer. Opt. Spectrosc. 128, 6–11 (2020).
Shore, B. W. The theory of coherent atomic excitation vol 1-simple atoms and fields (Wiley, 1990).
Bushaw, B. A., Nörtershäuser, W. & Wendt, K. Lineshapes and optical selectivity in high-resolution double-resonance ionization mass spectrometry. Spectrochim. Acta 54B, 321–332 (1999).
Nörtershäuser, W., Bushaw, B. A., Müller, P. & Wendt, K. Line shapes in triple resonance ionization spectroscopy. Appl. Opt. 39, 5590–5600 (2000).
Suryanarayana, M. V. Isotope selective three-step photoionization of 176Lu. J. Opt. Soc. Am. 38B, 353–370 (2021).
Brenner, T., Buttgenbach, S., Rupprecht, W. & Traber, F. Nuclear moments of the low abundant natural isotope 176Lu and hyperfine anomalies in the lutetium isotopes. Nuclear Phys. 440A, 407–423 (1985).
Kuhnert, A., Nunnemann, A. & Zimmermann, D. Investigation of the hyperfine structure and isotope shift of the 542.2 nm line of Lu. J. Phys. 16B, 4299–4303 (1983).
Vergès, J. & Wyart, J.-F. Infrared emission spectrum of lutecium and extended analysis of Lu I. Phys. Scr. 17, 495 (1978).
Georg, U. et al. Laser spectroscopy investigation of the nuclear moments and radii of lutetium isotopes. Eur. Phys. J. A 3, 225–235 (1998).
King, W. H. Isotope shiftsin atomic spectra (Springer, 1984).
Xu, C. B. et al. The study of autoionizing states of lutetium atoms by resonance ionization spectroscopy. J. Phys. B At. Mol. Opt. Phys. 26, 2821–2835 (1993).
Li, R. et al. Even-parity Rydberg and autoionizing states of lutetium by laser resonance-ionization spectroscopy. Phys. Rev. A 95, 052501 (2017).
Acknowledgements
Author acknowledges the support of Computational Analysis Division, Bhabha Atomic Research Centre, Visakhapatnam for providing the Super Computer Facility for this work.
Author information
Authors and Affiliations
Contributions
This is the complete indigenous work of the only author M.V.S. No other contributor to this work is ignored.
Corresponding author
Ethics declarations
Competing interests
The author declares no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Suryanarayana, M.V. Isotope separation of 176Lu a precursor to 177Lu medical isotope using broadband lasers. Sci Rep 11, 6118 (2021). https://doi.org/10.1038/s41598-021-85414-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-85414-z
This article is cited by
-
Laser isotope separation of 176Lu through off-the-shelf lasers
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.