Abstract
Treatment-related toxicity is an important component in non-small cell lung cancer (NSCLC) management decision-making. Our aim was to evaluate and compare the toxicity rates of curative and palliative radiotherapy with and without chemotherapy. This meta-analysis provides better quantitative estimates of the toxicities compared to individual trials. A systematic review of randomised trials with > 50 unresectable NSCLC patients, treated with curative or palliative conventional radiotherapy (RT) with or without chemotherapy. Data was extracted for oesophagitis, pneumonitis, cardiac events, pulmonary fibrosis, myelopathy and neutropenia by any grade, grade ≥ 3 and treatment-related deaths. Mantel–Haenszel fixed-effect method was used to obtain pooled risk ratio. Forty-nine trials with 8609 evaluable patients were included. There was significantly less grade ≥ 3 acute oesophagitis (6.4 vs 22.2%, p < 0.0001) and any grade oesophagitis (70.4 vs 79.0%, p = 0.04) for sequential CRT compared to concurrent CRT, with no difference in pneumonitis (grade ≥ 3 or any grade), neutropenia (grade ≥ 3), cardiac events (grade ≥ 3) or treatment-related deaths. Although the rate of toxicity increased with intensification of treatment with RT, the only significant difference between treatment regimens was the rate of oesophagitis between the use of concurrent and sequential CRT. This can aid clinicians in radiotherapy decision making for NSCLC.
Similar content being viewed by others
Introduction
Lung cancer remains the leading cause of cancer mortality worldwide1, the majority of lung cancer is non-small cell lung cancer (NSCLC)2. For patients with unresectable NSCLC, radiation therapy (RT) treatment options include concurrent chemoradiation (CRT), sequential CRT, curative RT and palliative RT.
Although the treatment regimen that provides the highest cure rate for each disease stage is well established, population studies have shown that treatment in NSCLC is consistent with guidelines in only 44–52% of cases3,4,5, and radiotherapy remains underutilised across the world6. While many factors influence the under-utilisation of radiotherapy, an important aspect is clinician concern regarding treatment-related toxicity, where treatments associated with better survival outcomes have increased toxicity. Comorbidity potentially influencing treatment is prevalent in 72%-81% of lung cancer patients7,8,9. This has been associated with reduced likelihood of patients receiving radiotherapy7.
Numerous studies now reported on survival prediction models for NSCLC, two from the MAASTRO group10,11. These both show that even with curative radiotherapy (± chemotherapy), there are different prognostic groups of patients, some who do poorly despite radical RT and some who do well. If clinicians are to use these models then the patient also needs to be informed of toxicity predictions for shared decision making. Some ‘poor risk’ patients may choose to accept higher toxicity rates with curative RT despite small survival gains, and others may not. However, the available literature can be difficult to interpret when quantifying the rate of toxicity between different treatment regimes. Due to the variable toxicity types and rates that is reported in individual trials, better estimates of toxicities would be helpful in guiding clinical management.
The aim of this systematic review and meta-analysis is to evaluate and statistically combine toxicity rates of curative and palliative RT (excluding stereotactic body radiation therapy) with or without chemotherapy for patients with unresectable NSCLC. This information increases the precision of the quantitative estimates of the toxicity rates compared to individual trials.
Methods
A systematic search of electronic databases (MEDLINE, PubMed, EMBASE, and the Cochrane Central Register) was performed using the following terms: non-small cell lung cancer, radiation therapy, radiotherapy, randomised controlled trial, controlled clinical trial, controlled trials, systematic review, and meta-analysis. We included recent studies published between January 2000 and June 2019. Searches were limited to human studies published in English. When multiple studies of the same clinical trial were encountered, the updated results were included. The PRISMA guidelines (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) were used to assist in writing this review12.
References identified by the search strategy were screened independently by two investigators (M.O. and B.L.) to review the trials for eligibility for inclusion and the list of trials eligible for inclusion was agreed.
Inclusion and exclusion criteria
Studies that met the following criteria were included: published randomised trial with greater than 50 patients with unresectable NSCLC undergoing curative and/or palliative RT. Curative RT was defined as a minimum dose of 50 Gy, or its radiobiological equivalent, with or without chemotherapy13. Palliative RT was defined as a dose of less than 50 Gy. Unresectable disease could be medically or surgically inoperable.
We excluded trials with small cell lung cancer or recurrent lung cancer. Patients treated with prior high dose RT in region of lung cancer, stereotactic body radiation therapy (SBRT), protons, carbon-ions, post-operative RT or palliative CRT were also excluded.
Data extraction
The details of included trials were recorded independently by two authors (M.O and B.L) via a data collection template (Appendix 1). Any discrepancy was resolved by consensus with third party (J.L.). Patient and trial characteristics, including disease stage, median age, study type, follow-up and toxicity criteria used were extracted along with summary information on treatment characteristics (treatment regime, chemotherapy type and timing, dose and fractionation). Treatment-related toxicity for each RT regimen was extracted, including the incidence and grade of oesophagitis, pneumonitis, cardiac events, pulmonary fibrosis, radiation myelopathy, neutropenia and/or treatment-related death (TRD).
Statistical analysis
The pooled risk of toxicities by any grade, grade ≥ 3, and treatment related deaths were expressed as the total number of cases for each toxicity outcome divided by the total number of patients treated with the same type of treatment. Treatment regimens were categorised into palliative RT alone, curative RT alone, sequential CRT and concurrent CRT. We performed indirect comparisons to estimate the risk ratio for the comparison between palliative versus curative RT and sequential versus concurrent CRT. The Mantel–Haenszel fixed-effect method was used to obtain the pooled risk ratio and corresponding confidence interval. We used the fixed-effect method for all comparisons for consistency. Statistical heterogeneity was assessed by calculating I2. Cochrane Review Manager version 5.3 (Cochrane Collaboration, Copenhagen, Denmark) was used for the analyses.
Quality assessment
The risk of bias for each trial was assessed using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions14. These include random sequence generation, allocation concealment, incomplete outcome data, selective reporting and other biases (such as method of assessing symptoms).
Results
Eligible studies
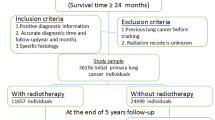
We identified 49 eligible trials15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64 with a total of 10,388 patients, of which 8609 were evaluable for toxicity (Fig. 1). The overall trial characteristics are shown in Table 1. There was variability in the reporting of symptoms, with various versions of 5 different toxicity grading criteria used in 39 of the included trials. 8 trials included stage IV patients accounting for 1835 patients. 5 of these were palliative trials and the remaining 3 trials only had a small proportion (23 patients) of stage IV disease. Treatment characteristics of included trials are summarised in Table 2 demonstrating the heterogeneity with respect to the study design, toxicity scoring criteria, treatment arms, RT dose fractionation and chemotherapy regimen. There was a wide range of RT dose fractionation used, from 10 Gy in 1 fraction for palliative RT, up to 74 Gy in 37 fractions in concurrent CRT. Most chemotherapy regimens were platinum-based. 2 studies assessed elderly patients17,18,60.
PRISMA Flow diagram12 with details of the number of studies identified, screened, assessed and included in the final review.
Treatment-related death
The overall rate of TRD was low on indirect comparisons, highest in concurrent CRT (3.1%), followed by sequential CRT (2.3%), curative radiation alone (2.4%) and palliative radiation (0%). In the 6 trials20,22,27,33,51,53 that directly compared concurrent with sequential CRT, TRD from concurrent was higher than sequential CRT but the difference was not statistically significant (5.1% vs 2.7%, p = 0.05) (Fig. 2). In the one trial30 that compared TRD in sequential CRT with curative RT alone, the rate of TRD was higher in sequential CRT but the absolute difference was small (1.7% vs 0.9%), insufficient for meta-analysis. No trial directly compared palliative with curative RT for TRD.
Oesophagitis
Grade ≥ 3 oesophagitis from concurrent CRT was statistically significantly higher than sequential CRT in 9 trials (22.2% vs 6.4%, p < 0.0001) (Fig. 3A). Any grade oesophagitis from concurrent CRT was also statistically significantly higher than sequential CRT in 3 trials (79.0% vs 70.4%, p = 0.04) (Fig. 3B). 2 trials48,59 compared any grade oesophagitis between curative RT and palliative RT, this was higher in curative but the difference was not statistically significant (35.4% vs 26.6%, p = 0.06) (Fig. 3C). Trials were not sufficient for meta-analysis in other comparison groups in assessing grade ≥ 3 or any grade oesophagitis.
Forest plot showing toxicity risk ratio (RR) for: (A) grade ≥ 3 oesophagitis, comparison between sequential versus concurrent chemoradiation; (B) any grade oesophagitis, comparison between sequential versus concurrent chemoradiation; (C) any grade oesophagitis, comparison between palliative versus curative radiation therapy, generated with Cochrane Review Manager version 5.3
Pneumonitis
In the 7 trials that directly compared concurrent with sequential CRT, Grade ≥ 3 pneumonitis from concurrent was higher than sequential CRT, but not statistically significant (11.1% vs 8.7%, p = 0.26) (Fig. 4A). 2 trials38,53 directly compared any grade pneumonitis, demonstrating the rate from concurrent CRT was not statistically significantly higher than sequential CRT (10.0% vs 5.7%, p = 0.16) (Fig. 4B). Trials were not sufficient for meta-analysis in other comparison groups in assessing grade ≥ 3 or any grade pneumonitis.
Neutropenia
Neutropenia was reported in different time intervals following chemotherapy or not specified. Selective reporting of febrile neutropenia was also identified. 3 trials directly compared Grade ≥ 3 neutropenia between concurrent and sequential CRT. Rates from concurrent was higher than sequential CRT, but not statistically significant (58.2% vs 55.4%, p = 0.56) (Fig. 4C). Trials were not sufficient for meta-analysis in assessing any grade neutropenia.
Cardiac adverse events
4 trials directly compared grade ≥ 3 cardiac events between concurrent and sequential CRT. The rates from concurrent was higher than sequential CRT, but not statistically significant (4.3% vs 1.8%, p = 0.08) (Fig. 4D). Trials were not sufficient for meta-analysis in other comparison groups for grade ≥ 3 or any grade cardiac events.
Pulmonary fibrosis and myelopathy
Pulmonary fibrosis and radiation myelopathy were poorly reported in the studies. Only 7 trials reported pulmonary fibrosis and 9 trials reported myelopathy across all treatment groups; meta-analysis to compare between groups was not feasible. The rate of pulmonary fibrosis (any grade) was higher in the palliative RT and curative RT arms than the sequential CRT and concurrent CRT. This finding is strongly influenced by a single study by Nestle et al. which reported 100% rate of pulmonary fibrosis based on imaging rather than clinical symptoms.
Toxicity stratified by stage
Trials with only stage III NSCLC comparing sequential versus concurrent CRT were analysed (see Fig. 5). The rate of grade ≥ 3 oesophagitis was statistically higher for concurrent CRT (16.6% vs 7.4%, p < 0.0001), whilst the difference in rates of treatment related death, grade ≥ 3 pneumonitis and grade ≥ 3 cardiac events were not statistically significant. Trials were not sufficient for analysis stratified by stage for stages I, II or IV disease.
Forest plot showing toxicity risk ratio (RR) for Stage III only studies, comparison between sequential versus concurrent chemoradiation: (A) treatment-related deaths; (B) grade ≥ 3 oesophagitis; (C) grade ≥ 3 pneumonitis; (D) grade ≥ 3 cardiac event, generated with Cochrane Review Manager version 5.3
Pooled toxicity rates
Overall, the pooled grade ≥ 3 and any grade toxicities rates were lower with sequential compared with concurrent CRT. On pooled comparisons, Grade ≥ 3 (RR 0.75; CI 0.65–0.87) and any grade neutropenia (RR 0.55; CI 0.47–0.64) were significantly less with sequential compared with concurrent CRT. Grade ≥ 3 oesophagitis was also significantly lower with sequential CRT (RR 0.42; CI 0.32–0.54) but not any grade oesophagitis. Any grade cardiac events (RR 0.48; CI 0.23–0.98) and pulmonary fibrosis (RR 0.36; CI 0.20–0.63) were significantly less with sequential compared with concurrent CRT (see Tables 3 and 4).
Any grade toxicities were also lower with palliative compared with curative radiation alone. On pooled comparisons, any grade oesophagitis (RR 0.40; CI 0.32–0.50) and pneumonitis (RR 0.04; CI 0.02–0.09) were significantly less with palliative compared with curative RT alone (see Tables 3 and 4).
The range of reported grade ≥ 3 oesophagitis was 0 to 41.4% for concurrent CRT and 0 to 20.4% for sequential CRT, whilst any grade oesophagitis ranged between 46.4% and 100% for concurrent CRT and 36.4% to 100% in sequential CRT.
Risk of bias
Reporting bias were identified with incomplete data and selective reporting of toxicities in most studies, resulting in an overall high risk of bias. Funnel plots were generated to visually assess for publication bias. Symmetrical funnel plots were obtained for comparison groups (> 5 studies) between sequential and concurrent CRT in grade 3 oesophagitis, pneumonitis and treatment-related deaths.
Discussion
In lung cancer clinical decision making, the consideration of toxicity is essential. As expected, patients receiving palliative RT had lowest toxicity, followed by curative RT alone, sequential and highest with concurrent chemoradiation. The benefit of this review is to provide better estimates of each toxicity effect compared to individual trials.
Acute oesophagitis is one of the main morbidities from lung irradiation. The large differences between individual trials makes it difficult for clinicians to estimate the toxicity in the process of informed consent. The grade ≥ 3 rate with curative RT without chemotherapy is low (0.5%). However, only 4 trials were included, 2 of which included stage I patients only17,19,25,49. Although this toxicity is significantly higher with concurrent CRT, it should not be used alone as a factor to preclude concurrent treatment. Oesophagitis can be managed with nutritional support and admission and rarely leads to late stenosis. In addition, IMRT have reduced the incidence of this65,66. This difference in oesophagitis rates should be considered as oesophagitis may impact on survival67.
Pneumonitis occurs sub-acutely and is the main toxicity of concern as it can result in death. Although the risk of any grade pneumonitis is high for all curative radiotherapy, the risk of Grade 3 + pneumonitis is < 10%. In addition, we found no significant difference between concurrent versus sequential CRT. This suggests that decisions regarding the sequencing of treatment should not be based on the anticipated risk of pneumonitis. However, the increasing use of adjuvant or palliative immunotherapy when combined with prior radiotherapy may potentially increase future pneumonitis risk.
Cardiac toxicity encompasses a range of disorders. Nearly all studies reported cardiac toxicity as a general outcome “cardiac” rather than specifying individual events. The pathophysiology and dose resulting in an event is likely to differ. The risk of grade ≥ 3 toxicity has been correlated with pre-existing cardiac disease and mean heart dose68. In breast cancer, Darby et al. found the rates of major coronary events increased linearly with the mean heart dose by 7.4% per Gray69. Moreover, data from RTOG 0617 showed heart dose is an independent factor for overall survival70. However, a systematic review which includes 3 studies from RTOG 0617 found that heart dose-volume parameters were not consistently associated with survival or cardiac toxicity71. Although reduction of heart dose is ideal, any de-escalation of therapy should be carefully weighed against the resulting inferior cure rates71,72,73,74.
Toxicities for the elderly population are not well established due to the lack of and under-representation in randomised trials. The EORTC and SIOG groups recommended chemotherapy to be considered only in selected fit elderly patients, as the added toxicity may outweigh survival benefit75. In this systematic review, there were only two included studies that specified elderly toxicity rates, reflecting the need for randomised trials in this group to aid determine best suitable treatment.
Only one trial included adjuvant immunotherapy and no palliative immunotherapy was used. The studies included treatment with various radiation technique, dose fractionation, including escalated therapy (radiation dose24,61 or systemic therapy). Due to changes in radiation technique, older studies (prior to 2000) were not included in this review. Advanced radiation technique such as 3-dimentional compared with 2-dimensional palliative RT to improve conformality can reduce toxicities76. Secondary analysis from the RTOG 0617 also confirms that IMRT was associated with lower rates of severe pneumonitis and cardiac doses in locally advanced NSCLC77. We did not review toxicities relating to SBRT or the impact of non-chemotherapy systematic therapy.
There are several limitations which are inherent to systematic reviews of randomised trials78. Whilst the selected good performance status patients may limit the generalisability, the rates reported in this review may be higher due to escalation of treatment in the experimental arms. On the other hand, real-world patients may also have pre-existing comorbidities and other patient factors which could increase toxicity. This review was unable to analyse toxicity rates based on dose-volume parameters due to insufficient data published in the trials included. Moreover, the pooled rates reported are averages of the toxicity from treatment in different stages. This likely results in an overestimation of risk for those with stage I compared to III disease79. The incidence of toxicities reported are crude estimates between the number of patients with toxicities and the total number of patients treated. Actuarial estimates provides a more accurate determination toxicities prevalence80. The findings from this review should be interpreted with some caution.
We included randomised trials as protocols with prospective data generally provides better-quality toxicity data. In concordance with Sivendran et al. on adverse event reporting in cancer clinical trial publications, we identified selectivity and heterogeneity with reporting toxicities in trials81. The quality of studies examined ranged from low to high, contributed by reporting bias. There was variation in timing of reported toxicities, different toxicity grading criteria used and limited studies on quality of life. This highlights the need for trials to report reliable toxicity data, ideally under standardised criteria and in conjunction with the Consolidated Standards of Reporting Trials (CONSORT) recommendations82.
To the best of our knowledge this is the only available review of toxicity data in recent trials that compares and provides estimates of palliative radiotherapy, curative radiotherapy, sequential and concurrent chemoradiotherapy regimens. The only statistically significant difference between treatment regimens was the rate of oesophagitis with concurrent versus sequential CRT. This information is clinically useful and should be considered by clinicians and patients when weighing up the established survival benefits with the toxicity of the different treatment options.
References
Stewart, B. & Wild, C. World Cancer Report (International Agency for Research on Cancer, 2014).
Walters, S. et al. Lung cancer survival and stage at diagnosis in Australia, Canada, Denmark, Norway, Sweden and the UK: A population-based study, 2004–2007. Thorax 68(6), 551–564 (2013).
de Rijke, J. M. et al. Influence of age, comorbidity and performance status on the choice of treatment for patients with non-small cell lung cancer; results of a population-based study. Lung Cancer 46(2), 233–245 (2004).
Duggan, K. J., Descallar, J. & Vinod, S. K. Application of guideline recommended treatment in routine clinical practice: A population-based study of stage I-IIIB non-small cell lung cancer. Clin. Oncol. 28(10), 639–647 (2016).
Potosky, A. L. et al. Population variations in the initial treatment of non-small-cell lung cancer. J. Clin. Oncol. 22(16), 3261–3268 (2004).
Vinod, S. K. International patterns of radiotherapy practice for non-small cell lung cancer. Semin. Radiat. Oncol. 25(2), 143–150 (2015).
Vinod, S. K. et al. Gaps in optimal care for lung cancer. J. Thorac. Oncol. 3(8), 871–879 (2008).
Carrato, A. et al. Clinical management patterns and treatment outcomes in patients with non-small cell lung cancer (NSCLC) across Europe: EPICLIN-Lung study. Curr. Med. Res. Opin. 30(3), 447–461 (2014).
Stevens, W. et al. Lung cancer in New Zealand: patterns of secondary care and implications for survival. J Thorac Oncol 2(6), 481–493 (2007).
Dehing-Oberije, C. et al. Development and external validation of prognostic model for 2-year survival of non-small-cell lung cancer patients treated with chemoradiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 74(2), 355–362 (2009).
Oberije, C. et al. A validated prediction model for overall survival from stage III non-small cell lung cancer: Toward survival prediction for individual patients. Int. J. Radiat. Oncol. Biol. Phys. 92(4), 935–944 (2015).
Moher, D. et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 6(7), e1000097 (2009).
Jones, B. et al. The role of biologically effective dose (BED) in clinical oncology. Clin. Oncol. 13(2), 71–81 (2001).
Higgins, J., et al., Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (Updated July 2019). Cochrane.
Antonadou, D. Radiotherapy or chemotherapy followed by radiotherapy with or without amifostine in locally advanced lung cancer. Semin. Radiat. Oncol. 12(1 Suppl 1), 50–58 (2002).
Antonia, S. J. et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N. Engl. J. Med. 377(20), 1919–1929 (2017).
Atagi, S. et al. Thoracic radiotherapy with or without daily low-dose carboplatin in elderly patients with non-small-cell lung cancer: A randomised, controlled, phase 3 trial by the Japan Clinical Oncology Group (JCOG0301). Lancet Oncol. 13(7), 671–678 (2012).
Atagi, S. et al. Chemoradiotherapy in elderly patients with non-small-cell lung cancer: Long-term follow-up of a randomized trial (JCOG0301). Clin. Lung Cancer 19(5), e619–e627 (2018).
Ball, D. et al. Stereotactic ablative radiotherapy versus standard radiotherapy in stage 1 non-small-cell lung cancer (TROG 0902 CHISEL): A phase 3, open-label, randomised controlled trial. Lancet Oncol. 20(4), 494–503 (2019).
Belani, C. P. et al. Combined chemoradiotherapy regimens of paclitaxel and carboplatin for locally advanced non-small-cell lung cancer: A randomized phase II locally advanced multi-modality protocol. J. Clin. Oncol. 23(25), 5883–5891 (2005).
Belani, C. P. et al. Phase III study of the Eastern Cooperative Oncology Group (ECOG 2597): induction chemotherapy followed by either standard thoracic radiotherapy or hyperfractionated accelerated radiotherapy for patients with unresectable stage IIIA and B non-small-cell lung cancer. J. Clin. Oncol. 23(16), 3760–3767 (2005).
Belderbos, J. et al. Randomised trial of sequential versus concurrent chemo-radiotherapy in patients with inoperable non-small cell lung cancer (EORTC 08972–22973). Eur. J. Cancer 43(1), 114–121 (2007).
Bezjak, A. et al. Randomized phase III trial of single versus fractionated thoracic radiation in the palliation of patients with lung cancer (NCIC CTG SC15). Int. J. Radiat. Oncol. Biol. Phys. 54(3), 719–728 (2002).
Bradley, J. D. et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): A randomised, two-by-two factorial phase 3 study. Lancet Oncol. 16(2), 187–199 (2015).
Cakir, S. & Egehan, I. A randomised clinical trial of radiotherapy plus cisplatin versus radiotherapy alone in stage III non-small cell lung cancer. Lung Cancer 43(3), 309–316 (2004).
Crvenkova, S. Survival and side effects in non-small cell lung cancer patients treated with combination of chemotherapy and conformal radiotherapy. Open Access Maced. J. Med. Sci. 6(12), 2323–2327 (2018).
Curran, W. J. Jr. et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: Randomized phase III trial RTOG 9410. J. Natl. Cancer Inst. 103(19), 1452–1460 (2011).
Edelman, M. J. et al. Randomized phase II study of preoperative chemoradiotherapy +/- panitumumab followed by consolidation chemotherapy in potentially operable locally advanced (stage IIIa, N2+) non-small cell lung cancer: NRG oncology RTOG 0839. J. Thorac. Oncol. 12(9), 1413–1420 (2017).
Erridge, S. C. et al. Symptom control and quality of life in people with lung cancer: A randomised trial of two palliative radiotherapy fractionation schedules. Clin. Oncol. 17(1), 61–67 (2005).
Fairlamb, D. et al. A randomised comparison of radical radiotherapy with or without chemotherapy for patients with non-small lung cancer: Results from the Big Lung Trial. Radiother. Oncol. 75(2), 134–140 (2005).
Falk, S. J. et al. Immediate versus delayed palliative thoracic radiotherapy in patients with unresectable locally advanced non-small cell lung cancer and minimal thoracic symptoms: Randomised controlled trial. BMJ 325(7362), 465 (2002).
Feng, J. et al. S-1 plus cisplatin with concurrent radiotherapy versus cisplatin alone with concurrent radiotherapy in Chinese patients with nonsmall-cell lung cancer: A multicentre randomized controlled trial. Medicine 95(36), e4557 (2016).
Fournel, P. et al. Randomized phase III trial of sequential chemoradiotherapy compared with concurrent chemoradiotherapy in locally advanced non-small-cell lung cancer: Groupe Lyon-Saint-Etienne d’Oncologie Thoracique-Groupe Francais de Pneumo-Cancerologie NPC 95–01 Study. J. Clin. Oncol. 23(25), 5910–5917 (2005).
Fournel, P. et al. Induction or consolidation chemotherapy for unresectable stage III non-small-cell lung cancer patients treated with concurrent chemoradiation: A randomised phase II trial GFPC: IFCT 02–01. Eur. J. Cancer 52, 181–187 (2016).
Gouda, Y. S. et al. Randomized study of concurrent carboplatin, paclitaxel, and radiotherapy with or without prior induction chemotherapy in patients with locally advanced non-small cell lung cancer. J. Egypt Natl. Cancer Inst. 18(1), 73–81 (2006).
Hanna, N. et al. Phase III study of cisplatin, etoposide, and concurrent chest radiation with or without consolidation docetaxel in patients with inoperable stage III non-small-cell lung cancer: The Hoosier Oncology Group and U.S. Oncology. J. Clin. Oncol. 26(35), 5755–5760 (2008).
Hansen, O. et al. A randomized phase II trial of concurrent chemoradiation with two doses of radiotherapy, 60Gy and 66Gy, concomitant with a fixed dose of oral vinorelbine in locally advanced NSCLC. Radiother. Oncol. 123(2), 276–281 (2017).
Huber, R. M. et al. Simultaneous chemoradiotherapy compared with radiotherapy alone after induction chemotherapy in inoperable stage IIIA or IIIB non-small-cell lung cancer: Study CTRT99/97 by the Bronchial Carcinoma Therapy Group. J. Clin. Oncol. 24(27), 4397–4404 (2006).
Jalal, S. I. et al. Updated survival and outcomes for older adults with inoperable stage III non-small-cell lung cancer treated with cisplatin, etoposide, and concurrent chest radiation with or without consolidation docetaxel: Analysis of a phase III trial from the Hoosier Oncology Group (HOG) and US Oncology. Ann. Oncol. 23(7), 1730–1738 (2012).
Johnstone, D. W. et al. Phase III study comparing chemotherapy and radiotherapy with preoperative chemotherapy and surgical resection in patients with non-small-cell lung cancer with spread to mediastinal lymph nodes (N2); final report of RTOG 89-01. Radiation Therapy Oncology Group. Int. J. Radiat. Oncol. Biol. Phys. 54(2), 365–369 (2002).
Kelly, K. et al. Phase III trial of maintenance gefitinib or placebo after concurrent chemoradiotherapy and docetaxel consolidation in inoperable stage III non-small-cell lung cancer: SWOG S0023. J. Clin. Oncol. 26(15), 2450–2456 (2008).
Kramer, G. W. et al. Results of the Dutch National study of the palliative effect of irradiation using two different treatment schemes for non-small-cell lung cancer. J. Clin. Oncol. 23(13), 2962–2970 (2005).
Lee, Y. et al. Incorporating erlotinib or irinotecan plus cisplatin into chemoradiotherapy for stage III non-small cell lung cancer according to EGFR mutation status. Cancer Res. Treat. 49(4), 981–989 (2017).
Liang, J. et al. Etoposide and cisplatin versus paclitaxel and carboplatin with concurrent thoracic radiotherapy in unresectable stage III non-small cell lung cancer: A multicenter randomized phase III trial. Ann. Oncol. 28(4), 777–783 (2017).
Lu, C. et al. Chemoradiotherapy with or without AE-941 in stage III non-small cell lung cancer: A randomized phase III trial. J. Natl. Cancer Inst. 102(12), 859–865 (2010).
Movsas, B. et al. Randomized phase II trial of cisplatin, etoposide, and radiation followed by gemcitabine alone or by combined gemcitabine and docetaxel in stage III A/B unresectable non-small cell lung cancer. J. Thorac. Oncol. 5(5), 673–679 (2010).
Nawrocki, S. et al. Concurrent chemotherapy and short course radiotherapy in patients with stage IIIA to IIIB non-small cell lung cancer not eligible for radical treatment: results of a randomized phase II study. J. Thorac Oncol. 5(8), 1255–1262 (2010).
Nestle, U. et al. A palliative accelerated irradiation regimen for advanced non-small-cell lung cancer vs. conventionally fractionated 60 GY: Results of a randomized equivalence study. Int. J. Radiat. Oncol. Biol. Phys. 48(1), 95–103 (2000).
Nyman, J. et al. SPACE: A randomized study of SBRT vs conventional fractionated radiotherapy in medically inoperable stage I NSCLC. Radiother. Oncol. 121(1), 1–8 (2016).
Pan, Y. et al. Acute esophagitis for patients with local-regional advanced non small cell lung cancer treated with concurrent chemoradiotherapy. Radiother. Oncol. 118(3), 465–470 (2016).
Reinfuss, M. et al. Evaluation of efficacy of combined chemoradiotherapy in locoregional advanced, inoperable non small cell lung cancer (clinical randomized trial). Nowotwory 55(3), 200–206 (2005).
Sasaki, T. et al. A randomised phase II trial of S-1 plus cisplatin versus vinorelbine plus cisplatin with concurrent thoracic radiotherapy for unresectable, locally advanced non-small cell lung cancer: WJOG5008L. Br. J. Cancer 119(6), 675–682 (2018).
Scagliotti, G. V. et al. Docetaxel-based induction therapy prior to radiotherapy with or without docetaxel for non-small-cell lung cancer. Br. J. Cancer 94(10), 1375–1382 (2006).
Sculier, J. P. et al. A phase III randomised study comparing concomitant radiochemotherapy with cisplatin and docetaxel as induction versus consolidation treatment in patients with locally advanced unresectable non-small cell lung cancer. Lung Cancer 117, 32–37 (2018).
Senan, S. et al. PROCLAIM: Randomized phase III trial of pemetrexed-cisplatin or etoposide-cisplatin plus thoracic radiation therapy followed by consolidation chemotherapy in locally advanced nonsquamous non-small-cell lung cancer. J. Clin. Oncol. 34(9), 953–962 (2016).
Senkus-Konefka, E. et al. A prospective, randomised study to compare two palliative radiotherapy schedules for non-small-cell lung cancer (NSCLC). Br. J. Cancer 92(6), 1038–1045 (2005).
Shibamoto, Y. et al. Influence of interfraction interval on the efficacy and toxicity of hyperfractionated radiotherapy in combination with concurrent daily chemotherapy in stage III non-small-cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 50(2), 295–300 (2001).
Su, S. F. et al. Randomized phase II study of pemetrexed-cisplatin or docetaxel-cisplatin plus thoracic intensity-modulated radiation therapy in patients with stage IV lung adenocarcinoma. Am. J. Cancer Res. 9(6), 1235–1245 (2019).
Sundstrom, S. et al. Hypofractionated palliative radiotherapy (17 Gy per two fractions) in advanced non-small-cell lung carcinoma is comparable to standard fractionation for symptom control and survival: a national phase III trial. J. Clin. Oncol. 22(5), 801–810 (2004).
Takigawa, N. et al. Benefits and adverse events among elderly patients receiving concurrent chemoradiotherapy for locally advanced non-small cell lung cancer: analysis of the Okayama Lung Cancer Study Group trial 0007. J. Thorac. Oncol. 6(6), 1087–1091 (2011).
van Diessen, J. et al. The acute and late toxicity results of a randomized phase II dose-escalation trial in non-small cell lung cancer (PET-boost trial). Radiother. Oncol. 131, 166–173 (2019).
Vokes, E. E. et al. Randomized phase II study of cisplatin with gemcitabine or paclitaxel or vinorelbine as induction chemotherapy followed by concomitant chemoradiotherapy for stage IIIB non-small-cell lung cancer: cancer and leukemia group B study 9431. J. Clin. Oncol. 20(20), 4191–4198 (2002).
Yamamoto, N. et al. Phase III study comparing second- and third-generation regimens with concurrent thoracic radiotherapy in patients with unresectable stage III non-small-cell lung cancer: West Japan Thoracic Oncology Group WJTOG0105. J. Clin. Oncol. 28(23), 3739–3745 (2010).
Zatloukal, P. et al. Concurrent versus sequential chemoradiotherapy with cisplatin and vinorelbine in locally advanced non-small cell lung cancer: A randomized study. Lung Cancer 46(1), 87–98 (2004).
Faivre-Finn, C. et al. Concurrent once-daily versus twice-daily chemoradiotherapy in patients with limited-stage small-cell lung cancer (CONVERT): An open-label, phase 3, randomised, superiority trial. Lancet Oncol. 18(8), 1116–1125 (2017).
Turrisi, A. T. 3rd. et al. Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N. Engl. J. Med. 340(4), 265–271 (1999).
Cox, J. D. et al. Interruptions of high-dose radiation therapy decrease long-term survival of favorable patients with unresectable non-small cell carcinoma of the lung: analysis of 1244 cases from 3 Radiation Therapy Oncology Group (RTOG) trials. Int. J. Radiat. Oncol. Biol. Phys. 27(3), 493–498 (1993).
Dess, R. T. et al. Cardiac events after radiation therapy: combined analysis of prospective multicenter trials for locally advanced non-small-cell lung cancer. J. Clin. Oncol. 35(13), 1395–1402 (2017).
Darby, S. C. et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N. Engl. J. Med. 368(11), 987–998 (2013).
Speirs, C. K. et al. Heart dose is an independent dosimetric predictor of overall survival in locally advanced non-small cell lung cancer. J. Thorac. Oncol. 12(2), 293–301 (2017).
Zhang, T. W. et al. Is the importance of heart dose overstated in the treatment of non-small cell lung cancer? A systematic review of the literature. Int. J. Radiat. Oncol. Biol. Phys 104(3), 582–589 (2019).
Wang, K. et al. Cardiac toxicity after radiotherapy for stage III non-small-cell lung cancer: Pooled analysis of dose-escalation trials delivering 70 to 90 Gy. J. Clin. Oncol. 35(13), 1387–1394 (2017).
Guberina, M. et al. Heart dose exposure as prognostic marker after radiotherapy for resectable stage IIIA/B non-small-cell lung cancer: secondary analysis of a randomized trial. Ann. Oncol. 28(5), 1084–1089 (2017).
Tucker, S. L. et al. Impact of heart and lung dose on early survival in patients with non-small cell lung cancer treated with chemoradiation. Radiother. Oncol. 119(3), 495–500 (2016).
Pallis, A. G. et al. Management of elderly patients with NSCLC; updated expert’s opinion paper: EORTC Elderly Task Force, Lung Cancer Group and International Society for Geriatric Oncology. Ann. Oncol. 25(7), 1270–1283 (2014).
McDermott, R. L. et al. Cancer Trials Ireland (ICORG) 06–34: A multi-centre clinical trial using three-dimensional conformal radiation therapy to reduce the toxicity of palliative radiation for lung cancer. Radiother. Oncol. 127(2), 253–258 (2018).
Chun, S. G. et al. Impact of intensity-modulated radiation therapy technique for locally advanced non-small-cell lung cancer: A secondary analysis of the NRG oncology RTOG 0617 randomized clinical trial. J. Clin. Oncol. 35(1), 56–62 (2017).
Diwanji, T. P. et al. Advances in radiotherapy techniques and delivery for non-small cell lung cancer: Benefits of intensity-modulated radiation therapy, proton therapy, and stereotactic body radiation therapy. Transl. Lung Cancer Res. 6(2), 131–147 (2017).
Marks, L. B. et al. Radiation dose-volume effects in the lung. Int. J. Radiat. Oncol. Biol. Phys. 76(3 Suppl), S70–S76 (2010).
Trotti, A. & Bentzen, S. M. The need for adverse effects reporting standards in oncology clinical trials. J. Clin. Oncol. 22(1), 19–22 (2004).
Sivendran, S. et al. Adverse event reporting in cancer clinical trial publications. J. Clin. Oncol. 32(2), 83–89 (2014).
Ioannidis, J. P. et al. Better reporting of harms in randomized trials: An extension of the CONSORT statement. Ann. Intern. Med. 141(10), 781–788 (2004).
Author information
Authors and Affiliations
Contributions
M.O. Wrote the main manuscript text including the preparation of all figures and tables, contributed in initial study concepts and design, literature research, data analysis, statistical analysis. B.L. Participated in literature research, manuscript preparation and editing. J.L. Participated in literature research, manuscript preparation and editing. S.V. Participated in initial study concepts and design, manuscript preparation and editing. W.X. Involved in statistical analysis, manuscript editing. R.Y.A. Participated in initial study concepts and design, manuscript editing. E.H. Participated in initial study concepts and design, data analysis, manuscript preparation and editing.
Corresponding author
Ethics declarations
Competing interests
Dr Hau and Dr Vinod report personal fees from Astra Zeneca, outside the submitted work. Dr Or, Dr Liu, Dr Lam, Dr Xuan and Dr Yeghiaian-Alvandi have nothing to disclose.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Or, M., Liu, B., Lam, J. et al. A systematic review and meta-analysis of treatment-related toxicities of curative and palliative radiation therapy in non-small cell lung cancer. Sci Rep 11, 5939 (2021). https://doi.org/10.1038/s41598-021-85131-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-85131-7
This article is cited by
-
Efficacy and safety of particle therapy for inoperable stage II-III non-small cell lung cancer: a systematic review and meta-analysis
Radiation Oncology (2023)
-
Impact of interventions on the quality of life of cancer patients: a systematic review and meta-analysis of longitudinal research
Health and Quality of Life Outcomes (2023)
-
Stereotactic boost on residual disease after external-beam irradiation in clinical stage III non-small cell lung cancer: mature results of stereotactic body radiation therapy post radiation therapy (SBRTpostRT) study
La radiologia medica (2023)
-
Hypoxia signaling in hepatocellular carcinoma: Challenges and therapeutic opportunities
Cancer and Metastasis Reviews (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.