Abstract
Tardigrades constitute a micrometazoan phylum usually considered as taxonomically challenging and therefore difficult for biogeographic analyses. The genus Pseudechiniscus, the second most speciose member of the family Echiniscidae, is commonly regarded as a particularly difficult taxon for studying due to its rarity and homogenous sculpturing of the dorsal plates. Recently, wide geographic ranges for some representatives of this genus and a new hypothesis on the subgeneric classification have been suggested. In order to test these hypotheses, we sequenced 65 Pseudechiniscus populations extracted from samples collected in 19 countries distributed on 5 continents, representing the Neotropical, Afrotropical, Holarctic, and Oriental realms. The deep subdivision of the genus into the cosmopolitan suillus-facettalis clade and the mostly tropical-Gondwanan novaezeelandiae clade is demonstrated. Meridioniscus subgen. nov. is erected to accommodate the species belonging to the novaezeelandiae lineage characterised by dactyloid cephalic papillae that are typical for the great majority of echiniscids (in contrast to pseudohemispherical papillae in the suillus-facettalis clade, corresponding to the subgenus Pseudechiniscus). Moreover, the evolution of morphological traits (striae between dorsal pillars, projections on the pseudosegmental plate IV’, ventral sculpturing pattern) crucial in the Pseudechiniscus taxonomy is reconstructed. Furthermore, broad distributions are emphasised as characteristic of some taxa. Finally, the Malay Archipelago and Indochina are argued to be the place of origin and extensive radiation of Pseudechiniscus.
Similar content being viewed by others
Introduction
Tardigrades represent a group of miniaturised panarthropods1, which is recognised particularly for their abilities to enter cryptobiosis when facing difficult or even extreme environmental conditions2. Their relationships with Arthropoda and Onychophora are a subject of long-standing debate3, although the sister position with respect to these two lineages is the most popular hypothesis4. Internal tardigrade relationships are enigmatic likewise, despite the fact that the effort to decipher their phylogeny has been on an increase in the last decade5,6,7,8. The insufficient understanding of evolutionary relationships between tardigrade species (Darwinian shortfall) is not the only gap in our knowledge9, as it results from the scarcity of described tardigrade species10 (Linnean shortfall). The other parallel problem is that tardigrade distribution on the globe is vastly unknown11 (Wallacean shortfall), and many old records are actual misidentifications caused by poor understanding of how to dissect intraspecific and interspecific variability12,13,14. Currently, tardigradologists aim at unravelling biodiversity patterns within the phylum with the application of modern molecular tools, i.a.15,16,17.
One of the most taxonomically absorbing conundrums of recent years concerns the genus Pseudechiniscus18, the echiniscid genus extremely scarce in morphologically informative traits19 after the erection of Acanthechiniscus to accommodate the distinct evolutionary history and morphology of some species earlier attributed to Pseudechiniscus20. The recent disclosure of the molecular diversity within Pseudechiniscus21, with an overview of morphological criteria21,22,23,24 and an integrative re-description of the nominal species Pseudechiniscus suillus23 constituted the turning point in the classification of this taxon. Perhaps the two most significant findings of these studies were the discovery of the crucial taxonomic role of ventral sculpturing patterns22,23,24, independently shown also for Hypechiniscus25, and the distinguishing of two phylogenetic lineages concordant with the shape of the cephalic papillae (secondary clavae)21, which is a unique state in the entire family, as other echiniscid genera are homogeneous in this criterion19. The suillus-facettalis lineage exhibits pseudohemispherical (illusively dome-shaped) papillae (e.g. P. insolitus or P. jubatus, Fig. 1A–B), whereas members of the novaezeelandiae line exhibit dactyloid papillae (e.g. P. novaezeelandiae or P. juanitae, Fig. 1C–D). Cesari et al.21 implied that these lines correspond with potential subgenera, simultaneously noting that the genetic evidence was insufficient at the time to erect them. From the biogeographic point of view, Pseudechiniscus can be superficially considered as impractical due to extreme morphological stasis26 that hampers correct identification of species and impedes the delimitation of geographic ranges.
Exemplary representatives of the former suillus-facettalis lineage: (A) Pseudechiniscus (P.) insolitus (paratype, Spain), (B) Pseudechiniscus (P.) jubatus (paratype, Russia), and the novaezeelandiae lineage: (C) Pseudechiniscus (M.) novaezeelandiae (Te Ika-a-Māui), (D) Pseudechiniscus (M.) juanitae (Colombia). Arrowheads indicate cephalic papillae. Scale bars = 10 μm.
In this study, we sequenced 65 populations of Pseudechiniscus obtained from around the world, although with over 70% of the dataset originated from the Palaearctic and Indomalayan regions. Three nuclear markers (18S rRNA, 28S rRNA, ITS-1) were used for the phylogeny reconstruction and species delimitation. Phylogenies were later exploited in a number of analyses, including biogeographic methods: S-DIVA27 and BioGeoBEARS28, ancestral state reconstruction29 of pivotal taxonomic traits; ecological niche modelling30 for some widely distributed species, and global species richness estimation in Pseudechiniscus. Thanks to these analyses, we present new hypotheses on the origin of the genus, stress the importance of the tropical zone (especially Southeast Asia) in the diversification not only for Pseudechiniscus, but tardigrades in general. We also formulate new research topics that appear as important directions in studying their diversity.
Results
Phylogeny and taxonomy
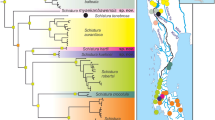
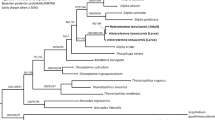
Overall, both Bayesian and Maximum Likelihood approaches gave well-resolved and largely congruent phylogenies (Fig. 2). The monophyly of Pseudechiniscus received a maximal support, and the genus was clearly divided in two large, deeply divergent clades, coherent with the suillus-facettalis (Fig. 1A–B) and novaezeelandiae morphotypes (Fig. 1C–D); see identical division in the COI phylogeny (Supplementary Material 2). Notably, branches in the latter are visibly longer than in the suillus-facettalis line. Moreover, the only inconsistent topology occurred in the novaezeelandiae line, in which ML recovered the following arrangement of species: ((((P. cf. angelusalas (((P. sp. 6 ((P. cf. saltensis (P. sp. 7 + P. sp. 8)))), but with weak support. On the basis of ITS-1 tree, the number of species estimated by bPTP varied between 23 and 43, with the average of 30. Both ML and the simple heuristic search indicated the existence of 26 species (Fig. 2), which was congruent with classical taxonomic identification. The only exception was P. cf. saltensis, divided into two putative species in the Bayesian implementation of the Poisson Tree Processes (bPTP), although with weak support (0.50). However, we classified populations AR.251 and 266 as morphologically homogeneous and largely corresponding with the original description31. Consequently, the number of Pseudechiniscus species utilised in our phylogenetic reconstruction was determined as 25.
Phylogeny of the genus Pseudechiniscus based on the concatenated matrix (18S rRNA+28S rRNA+ITS-1). Values at nodes separated by forward slashes signify BEAST Bayesian posterior probability (BI), MrBayes Bayesian posterior probability, and bootstrap values (ML), respectively. Maximum supports, i.e. 1.00 for BI and 100 for ML, are indicated by asterisks. Support values for tip nodes (within intra-specific phylogenetic structure) are not shown for simplicity. Topologies of all consensus trees were identical, with the exception of a single clade, in which ML gave incongruent results (marked with hashtags). The scale refers to the BEAST consensus tree. Values in round brackets and in the superscript at each species name refer to the support they received in the bPTP delimitation method (maximum values, i.e. 1.00, are indicated by asterisks). Exemplary SEM microphotographs show the dactyloid papillae in Meridioniscus subgen. nov. (Pseudechiniscus (M.) cf. angelusalas) and pseudohemispherical papillae in the subgenus Pseudechiniscus (Pseudechiniscus (P.) cf. ehrenbergi). Scale bars in the photos in μm.
Given that the phylogeny was compatible with morphological variation, we herein divide Pseudechiniscus in two subgenera: the nominal Pseudechiniscus (corresponding to the suillus-facettalis group) and Meridioniscus subgen. nov. (the novaezeelandiae group).
Subgenus: Pseudechiniscus Thulin, 191118.
Type species: Pseudechiniscus suillus32.
Composition: P. alberti, P. asper, P. beasleyi, P. bidenticulatus, P. brevimontanus, P. chengi, P. clavatus sp. dub., P. ehrenbergi, P. facettalis, P. insolitus, P. jubatus, P. lacyformis, P. lalitae, P. megacephalus sp. dub., P. nataliae, P. occultus, P. papillosus, P. pseudoconifer, P. ramazzottii, P. scorteccii, P. shintai, P. suillus, P. xiai.
Diagnosis: Small echiniscids with black crystalline eyes. Only cephalic cirri present. Cephalic papillae pseudohemispherical (laterally attached to the head surface). Pseudosegmental plate IV’ present. Dorsal sculpturing of the Pseudechiniscus type, i.e. consisting of endocuticular pillars; usually lacking pores. Striae usually absent. Ventral plates absent.
Remarks: Tumanov22 indicated dubious character of P. clavatus. Not only the shape of both primary and secondary clavae is atypical, but also the pseudosegmental plate IV’ is unidentifiable in the original description based on juvenile specimens33. Therefore, we ascertain that P. clavatus is a nomen dubium and this name should not be used in modern literature. We are in agreement with Tumanov regarding the status of P. jiroveci sp. dub. and P. marinae. Contrarily to Grobys et al.23, we are of the opinion that P. megacephalus does not belong to a different genus. The alleged mushroom-shaped cephalic papillae with a peduncle-like bases are unknown in any other echiniscid species19, making this trait most likely artefactual rather than autapomorphic34. Consequently, P. megacephalus is designated as a nomen dubium. Pseudechiniscus pulcher should be included within Antechiniscus22. P. transsylvanicus has long cirri C and the body ca. 350 μm long35, that is much larger than the typical length for Pseudechiniscus, which can reach up to 250 μm, but usually is below 200 μm. Moreover, cirriform trunk appendages are absent in Pseudechiniscus s.s.22, thus P. transsylvanicus almost certainly belongs in a different echiniscid genus, but the currently available data preclude its transfer. The same is for P. bispinosus characterised by two rigid, long spines C36. Pseudechiniscus shilinensis has an inadequate description preventing its taxonomic identification37, and is designated here as a nomen dubium. Finally, the following species are unassignable to subgenera as their descriptions do not specify the cephalic papillae shape: P. dicrani and P. marinae38,39, thus they are tentatively retained in the subgenus Pseudechiniscus until type or fresh material is available.
Subgenus: Meridioniscus subgen. nov. Gąsiorek, Vončina & Michalczyk.
Type species: Pseudechiniscus novaezeelandiae40.
Composition: P. angelusalas, P. bartkei, P. conifer, P. dastychi, P. indistinctus, P. juanitae, P. novaezeelandiae, P. quadrilobatus, P. saltensis, P. santomensis, P. spinerectus, P. titianae, P. yunnanensis.
Diagnosis: As for the nominal subgenus, except the cephalic papillae which are dactyloid, and attached to the body cuticle only at their bases. Striae present, usually large and well-developed.
Etymology: From Latin meridionalis = southern + ending -iscus derived from Echiniscus, the first established echiniscid genus, literally meaning “an echiniscid from the South”. The name refers to the geographic origin of the known species belonging to the new subgenus, that most likely has its roots in the Southern Hemisphere and tropical/subtropical regions of the world.
Remarks: Pseudechiniscus angelusalas, P. dastychi and P. indistinctus were incorrectly assigned to the suillus-facettalis line by Roszkowska et al.24,41. The original description of P. yunnanensis does not specify the shape of cephalic papillae42, but the development of evident striae clearly indicates its affinity within Meridioniscus subgen. nov.
The estimation of the number of Pseudechiniscus species to be described by the year 2050 was performed using the exponential model, by applying the best-fit curve to data on cumulative species richness. Curve was described by the formula y = 0.5791e0.2491x, where x signified the subsequent decades since the description of P. (P.) suillus in 1853 until now. The diagram showing the predicted increment in the number of Pseudechiniscus species is shown in Fig. 3.
Cumulative species richness of Pseudechiniscus since the description of the nominal Pseudechiniscus suillus with predicted species numbers until 2059. Red bars indicate the predicted number of species in the future decades. The decade 2020–2029 is the only one with two bars: the first bar signifies the number of described species (blue portion) + the predicted number of species (red portion), whereas the second (grey) bar signifies the total number of known and undescribed species that were uncovered in this study. See sections Remarks in the Results for the taxonomy and our additional remarks on the validity of some species, echoing Tumanov22 and Grobys et al.23 findings.
Morphological evolution and the evolution of reproductive mode
Firstly, we assessed the phylogenetic signal using 1000 most credible BEAST trees, taking four key taxonomic traits and reproductive mode into consideration: (1) the shape of cephalic papillae, (2) the type of ventral ornamentation pattern, (3) the development of projections on the posterior margin of the pseudosegmental plate IV’, (4) the development of striae and (5) the presence of males in a population. Of these, p-value for Pagel’s λ was > > 0.05 for traits 3. and 5., thus they were discarded from further analyses. Subsequently, BayesTraits implemented in RASP was used to reconstruct ancestral states along phylogenetic lineages of Pseudechiniscus. The analysis showed that the ancestor of Pseudechiniscus had elongated (dactyloid) cephalic papillae, therefore the pseudohemispherical papillae of the nominal subgenus should be considered autapomorphic (Fig. 4A; Pagel’s λ1 = 1.00, p < 0.001). Furthermore, ancestors of Pseudechiniscus and its subgenera were most likely characterised by complex ventral ornamentation patterns (Figs. 4B, 5A; Pagel’s λ2 = 1.00, p < 0.001), but parallel simplification of the ventral sculpturing (Fig. 5B) occurred at least twice in the course of evolution: (I) in the tropical lineage comprising P. (M.) quadrilobatus and P. (M.) sp. 1, and (II) in the Palaearctic lineage represented by P. (P.) sp. 9 and P. (P.) suillus. Finally, striae were shown as absent in ancestors of Pseudechiniscus and its nominal subgenus, being a derived and a conservative trait in Meridioniscus subgen. nov. (Figs. 4C; Pagel’s λ4 = 0.94, p < 0.001). Striae did, however, evolve independently also in two lineages of the subgenus Pseudechiniscus: P. (P.) sp. 12 and P. (P.) sp. 17 (fully developed and incipient, respectively), and they are partially reduced in two species of Meridioniscus subgen. nov. (P. (M.) sp. 1 and P. (M.) sp. 6).
Biogeography
S-DIVA and ancestral range reconstruction under the DIVALIKE model gave similar results (Figs. 6–7) which suggested that the ancestor of Pseudechiniscus was of the West Palaearctic-Oriental origin, which is not a conclusive inference (see Discussion for the issue of geographic sampling bias, especially the lack of data for Australian lineages). However, the ancestors of the two subgenera were clearly identified as originating from the West Palaearctic (Pseudechiniscus) and Oriental/Indomalayan region (Meridioniscus subgen. nov.). Although the majority of lineages within Meridioniscus subgen. nov. share the Oriental origin, the picture is more complex in the subgenus Pseudechiniscus, as the basal lineages originated in the West Palaearctic, but the remaining ones have a composite character (not discussed in detail since a broader sampling within Pseudechiniscus is likely to give disparate results in the deep nodes within the subgenera compared to our study). Moreover, two species have wide tropical distributions: P. (M.) cf. angelusalas, with pronounced diphyletic structure (Afrotropical and Oriental lineages), and P. (P.) cf. ehrenbergi, with two clades of mixed origin (Figs. 6–7). The analysis under the DIVALIKE model revealed two points of vicariance in Meridioniscus subgen. nov., and six analogous points in Pseudechiniscus. Additionally, there were four points of dispersal in Meridioniscus subgen. nov. (from the Oriental region or within this area), and six analogous points in the subgenus Pseudechiniscus (between many regions). The modelling of potential distributions based on available incidence data was performed for two species with molecularly verified records from various realms: P. (M.) cf. angelusalas and P. (P.) cf. ehrenbergi (Fig. 8). Whereas the distribution of P. (M.) cf. angelusalas was inferred as almost strictly subtropical-pantropical, the modelled distribution of P. (P.) cf. ehrenbergi extended towards temperate regions in the Americas (Cascade Range and Patagonia) and in Western Europe (Fig. 8).
Historical biogeography of Pseudechiniscus reconstructed using BioGeoBEARS under the DIVALIKE model. Nodal pie charts show the relative probability of historical geographic ranges according to the in-figure colour legend. Pie charts with bolded margins denote points of dispersal, whereas these with discontinuous margins indicate a vicariant origin. Black colour signifies unexplained origin.
Ecological biogeography of two Pseudechiniscus species with pantropical distributions – geographic ranges predicted by ecological niche modelling for: (A) P. (M.) cf. angelusalas, (B) P. (P.) cf. ehrenbergi. Suitability determines whether a given area is characterised by favourable conditions for one of the species (maximal suitability = 1) or by allegedly inhospitable conditions (minimal suitability = 0). Generated using Maxent, ver. 3.4.1103 [https://biodiversityinformatics.amnh.org/open_source/maxent/] with kuenm package104.
Discussion
Systematic and morphological studies with the application of phylogenetic and statistical methods on meiofaunal phyla, represented e.g. by tardigrades43, are notoriously laborious and challenging. The finding of abundant populations of Pseudechiniscus allowed for the confirmation of hypotheses21,22 that its two evolutionary lineages correspond with some morphological traits and can be concurrently elevated to the rank of subgenera. Among the examined traits, the shape of cephalic papillae was recovered as the most conservative character, followed by the development of striae, and the most labile traits, namely the type of ventral ornamentation (Fig. 4) and the development of projections on the posterior margin of the pseudosegmental plate IV’. Pseudohemispherical cephalic papillae occur only in the subgenus Pseudechiniscus, but their hemispherical appearance under LCM makes them superficially similar to truly hemispherical papillae in Parechiniscus19, Cornechiniscus19,44, Mopsechiniscus45 or Proechiniscus19. Moreover, the cephalic papillae in Novechiniscus, being very wide and with additional cuticular ring at the base, but at the same time pointed at their tips46, are intermediate in shape between the spherical and dactyloid types. All other echiniscid genera have elongated papillae, present also in Oreellidae47. However, Carphaniidae do not have papillae47,48, and Echiniscoididae have strongly modified papillae that are comparatively large with respect to the size of the head and are hemispherical in shape49,50,51. Not excluding the homologous character of the shape of the cephalic papillae in some Echiniscidae and Echiniscoididae, we are inclined to hypothesise that hemispherical and pseudohemispherical papillae are derived states, present only in some lineages of the clade Echiniscoidea.
As noted by Tumanov22, the presence of striae in the dorsal sculpturing correlates with phylogeny. Striae are a derived state present in Meridioniscus subgen. nov. and only rarely occur in the subgenus Pseudechiniscus (Fig. 4C). However, some of the examined species, for which multipopulation data are available, exhibit intraspecific variability in the development of striae (e.g. P. (P.) sp. 9 and P. (M.) cf. angelusalas, see Supplementary Material 2). Their homoplasious convergent nature is supported by the independent development in several not directly related lineages within Echiniscidae, e.g. in Cornechiniscus44, Stellariscus52, or some Echiniscus species, such as E. rackae53. Importantly, the ancestor of the genus Pseudechiniscus did not exhibit striae, thus its dorsal sculpturing probably resembled that in Hypechiniscus25. Such a type of sculpturing, composed solely of endocuticular pillars, is plesiomorphic for Echiniscidae in general. Consequently, we recognise the development of pores or a sponge layer as advanced characters that evolved convergently in the Bryodelphax and Echiniscus lineages44.
Ventral ornamentation pattern only recently was found to be pivotal in the taxonomy of Pseudechiniscus22,23,24,41, albeit a reticulated design was reported in many species long before these analyses54. Although taxonomically informative, this trait is not easily identifiable and requires an optical equipment of high quality and properly fixed specimens to comprehensively describe the belts of pillars and connective points. Moreover, inter-population variability was observed in some widely distributed species, such as Pseudechiniscus (M.) cf. angelusalas, P. (M.) sp. 4 or P. (P.) cf. ehrenbergi, evidenced particularly in the intensity of sculpturing in the intersegmental areas between the subsequent pairs of limbs. Moreover, our populations of P. (P.) suillus exhibited reduced ventral sculpturing, whereas the redescription provides an intricate pattern in this species23. The absence of ventral ornamentation is a derived character in the genus Pseudechiniscus, so far observed only in P. (M.) quadrilobatus (replaced by a system of epicuticular thickenings known in Cornechiniscus44). This species has strongly sclerotised armour, and the examination of the type material of P. alberti, also having unusually for the genus sclerotised plates55, would be desirable to adjudicate whether the reduction of ventral sculpturing is correlated with the strengthening of the dorsal armour.
Projections, in the form of teeth or lobes, occur on the posterior margin of pseudosegmental plate IV’ in both Pseudechiniscus subgenera. The variability in the development of the projections varies between species, as some are known to have relatively invariant projections, especially taxa having large teeth, like P. (M.) novaezeelandiae or P. (M.) spinerectus56,57, but some other, particularly species with weakly elevated teeth or lobes, such as P. (P.) asper or P. (M.) santomensis41,58, are characterised by a considerable number of intermediate stages: from the fully developed projections to an almost completely smooth edge of the plate. Such variability was also observed in the newly sequenced species, such as e.g. P. (M.) sp. 6 and P. (P.) sp. 17. Another interesting finding is the presence of the clade P. (M.) quadrilobatus + P. (M.) sp. 1 in the ancestral state reconstruction analysis (Fig. 4), indicating spurless claws as autapomorphic. Noteworthy, the Javanese P. (P.) bidenticulatus also has spurless claws59, suggesting its affinity to this clade and a potential transfer to Meridioniscus. Internal claws with primary spurs are plesiomorphic to Echiniscidae, and the loss of spurs evolved several times in different genera, e.g. in Cornechiniscus44. Irrespectively of this, Pseudechiniscus exhibits a tendency towards miniaturisation of spurs and their further merging with the claw branch, especially in the Asian lineages41.
Our analyses show that Pseudechiniscus biogeography is complex and requires a detailed interpretation. Our initial assumption was in accordance with the ‘everything is everywhere, but environment selects’ hypothesis60,61, i.e. that the majority of species may have wide geographic distributions, but are limited by climate, and if exceeding a single biogeographic region62, then they should occur in realms with similar habitats (e.g. Oriental – Afrotropical – Neotropical). Premises fur such zero hypothesis are numerous: (1) we have already demonstrated a pantropical distribution for an echiniscid species14, which we expect to be the only pattern of a widespread geographic range in the tropical region (in other words, e.g. a combination Nearctic-Afrotropical should be considered as rather non-parsimonious and unlikely); (2) a wide distribution was shown for Pseudechiniscus (P.) ehrenbergi, which inhabits both the Western (Italy) and the Eastern (Mongolia) Palaearctic21,24; (3) Meridioniscus subgen. nov. was preliminarily identified in our samples from Chile, Colombia, French Guyana, Kenya, Tanzania and India (not sequenced due to the scarcity of individuals; see also an unidentified species from Argentina in Tumanov22), but never in a much richer material from the Holarctic (see below for the case of P. (M.) indistinctus); contrarily, individuals of the subgenus Pseudechiniscus were found worldwide, and particularly frequently in Europe, including Iceland, Germany, Sweden, Poland, Croatia, or Italy (not sequenced for identical reasons as above). These observations implied a certain degree of regionalisation at least for one of the evolutionary lineages of the genus Pseudechiniscus; (4) much better studied micrometazoans, such as mites, are known to be geographically regionalised, with a low proportion of pantropical and cosmopolitan species inhabiting a given region (up to only 10–15% of the entire fauna63,64); similar values were reported for collembolans (20%)65, but see the contrasting evidence for gastrotrich66 or proturan67 tropical faunae. In our dataset, out of 14 tropical Pseudechiniscus species, only three (P. (P.) cf. ehrenbergi, P. (M.) cf. angelusalas and likely P. (M.) quadrilobatus, see Supplementary Material 2) have wide geographic ranges (21%). Thus, it seems that regional endemism prevails over pantropical or cosmopolitan ranges of Pseudechiniscus spp. In other words, only two of the analysed species, P. (P.) cf. ehrenbergi, P. (M.) cf. angelusalas, exhibit genetically verified geographic ranges that are in agreement with the ‘everything is everywhere, but environment selects’ hypothesis (in the case of species with preferences for tropical habitats, pantropical distributions are predicted by the hypothesis).
The analyses indicated the West Palaearctic-Oriental (Indomalayan) origin of Pseudechiniscus, which is not surprising given the geographically biased dataset. It is probable that sequencing Antarctic (post-Gondwanan)16 and Australian representatives of Pseudechiniscus may affect the inference about the ancestral geographic range. Although Pseudechiniscus (M.) dastychi and P. (M.) titianae are autochthonous for Antarctica and there are some barcodes available for these species20,24, they are non-homologous with our DNA sequences, thus we were not able to include them in the reconstructions. However, it is Australia, where the crucial phyletic lineages of the genus are most likely to be found. This is so, because ancient and highly diversified lineages are known within many animal groups in Australia68,69,70,71. Unluckily, the Australian tardigrade fauna is almost unknown, although there are at least few Pseudechiniscus species representing both subgenera present on this continent72.
The West Palaearctic and Oriental regions were recovered as ancestral for the subgenus Pseudechiniscus and for Meridioniscus subgen. nov., respectively (Figs. 6–7). Moreover, the first subgenus exhibits a cosmopolitan distribution, whereas the second is primarily tropical/subtropical-Gondwanan (although we do not preclude its presence in e.g. the Mediterranean Palaearctic). A puzzling exception to this pattern is the genetically verified presence of P. (M.) indistinctus in the southern part of Norway (western Scandinavian Peninsula)24, but the tropical origin of Meridioniscus subgen. nov. does not exclude dispersal to the Holarctic. Placing this species in the generic phylogeny will allow for the clarification of its affinities. Comparatively longer branches in Meridioniscus subgen. nov. may be caused by two factors: (1) undersampling of lineages, or (2) a relatively slower rate of speciation and extinction, accompanied by morphological stasis, suggesting bradytely (arrested evolution) in this evolutionary line73. This hypothesis finds support in the main retained plesiomorphy of the subgenus – dactyloid cephalic papillae (Fig. 4A).
We stress that ancestral area reconstructions using BioGeoBEARS (Fig. 7) indicated the Oriental region to be the centre of dispersal of Meridioniscus subgen. nov. lineages (four points of dispersal), which stays in agreement with the wide geographic distributions of P. (M.) quadrilobatus (potential dispersal to Neotropics from tropical Asia) and P. (M.) cf. angelusalas (potential dispersal to the Afrotropic from tropical Asia). Among the sequenced 25 species, only one representative of the subgenus Pseudechiniscus exhibited a similarly wide tropical distribution (P. (P.) cf. ehrenbergi). This means that only ca. 12% of species in our dataset show signs of pantropical ranges, and we anticipate this fraction to be similar in many other cosmopolitan tardigrade genera (based also on the echiniscid-rich material from South Africa, data in preparation). The analyses predicted that P. (M.) cf. angelusalas and P. (P.) cf. ehrenbergi are likely to be pantropical (which resembles the analyses conducted on Echiniscus lineatus14), with the majority of suitable areas present in the subtropical and tropical zones, and highly suitable areas in Western Europe for P. (P.) cf. ehrenbergi. This species most likely inhabits also the Palaearctic, as indicated by COI data, placing our sequences with those published previously21,24 in one clade (Supplementary Material 2). We also stress the evident importance of mountain ranges throughout the modelled geographic ranges (Fig. 8) for the presence of both taxa, which is in line with the known pattern of tardigrade diversity and endemism increasing to some extent in relation to the elevation74 (also observed in many other animal groups75).
Pseudechiniscus is the second most speciose echiniscid genus after Echiniscus23, and its taxonomy was for long considered disorganised and distrustful. For example, a considerable fraction of literature reports of Pseudechiniscus constitute the records of P. (P.) suillus and P. (P.) facettalis11, two allegedly cosmopolitan species. Paradoxically, it is likely that they both have restricted geographic ranges, as their loci typici are at high elevations in the mountains of West Palaearctic23,32 and in Greenland76, i.e. places that favour typical stenotherms rather than eurytopic species. Our model (Fig. 3) predicts that by the mid of twentieth century, the number of described Pseudechiniscus species will be likely at least doubled, approaching the currently known number of Echiniscus species. In fact, it would not be surprising if it is actually Pseudechiniscus that will have the status of the most speciose echiniscid, but testing this supposition requires strict taxonomic regime, that is redescriptions of “old” taxa, e.g. P. (P.) facettalis or P. (M.) conifer, joined with descriptions of new species always associated with DNA barcodes. This will help to avoid synonymies and will also allow for supplementation of generic phylogenetic tree, as well as for more detailed biogeographic analyses and testing of the hypotheses put forward in this study. Beside of the abovementioned Australia, we foresee that sampling in the Oriental region may bring further improvement to our understanding of the natural history of Pseudechiniscus. Recently, Borneo and Indochina, i.e. areas that arose at the border between Laurasia and Gondwana, were argued as crucial for Oriental biodiversity77. This goes in tandem with what we discovered for Pseudechiniscus, thus tardigradologists interested in taxonomy and phylogeny of the genus are likely to benefit from focusing on the Indopacific area.
Material and methods
Sampling, microscopy and imaging
Pseudechiniscus specimens were extracted from 65 samples collected throughout the world (Supplementary Table 1). Each population was divided into at least two groups destined for DNA sequencing and light microscopy analyses. If a population was sufficiently abundant, specimens were preserved also for scanning electron microscopy. Permanent slides were made from some individuals using Hoyer’s medium and later examined under an Olympus BX53 light microscope with phase contrast (PCM), associated with an Olympus DP74 digital camera. Supplementary figures illustrating dorsal/ventral sculpturing of females and males (provided always in this order) were assembled in Corel Photo-Paint X6, ver. 16.4.1.1281. For structures that could not be satisfactorily focused in a single light microscope photograph, a stack of 2–6 images was taken with an equidistance of ca. 0.2 mm and assembled manually into a single deep-focus image.
Cephalic papillae (secondary clavae) morphotypes
Although all species in the genus Pseudechiniscus have elongated cephalic papillae, the subgenera Pseudechiniscus and Meridioniscus subgen. nov. differ in the orientation and attachment of the papillae to the cuticle of the body, which results in their very much different appearance under LCM (Fig. 9)22. Specifically, papillae in Meridioniscus, as in the great majority of Echiniscidae, are attached to the body only by their base and they appear finger-like under both SEM and LCM (Fig. 9A–D). However, in the subgenus Pseudechiniscus, the papillae are attached to the head also along their side (Figs. 9E–H). This peculiar longitudinal attachment is clearly visible in SEM (Fig. 9F,H), but most often makes these papillae appear in LCM as hemispherical (dome-shaped)21 (Fig. 9E,G). Thus, under LCM, cephalic papillae in the subgenus Pseudechiniscus resemble those in Cornechiniscus (Fig. 9I–J) and Mopsechiniscus. However, the papillae in the two latter genera are flattened and widened from the apex, thus they are indeed hemispherical (dome-shaped) and are visible as such in both SEM and LCM. Thus, in order to avoid confusion when describing the shape of cephalic papillae in the subgenus Pseudechiniscus, and to differentiate them from the truly hemispherical (dome-shaped) papillae in Cornechiniscus (Fig. 9I–J) and Mopsechiniscus45, we propose to term them as pseudohemispherical.
Three morphotypes of cephalic papillae in Echiniscidae: Dactyloid papillae, present in the subgenus Meridioniscus and the great majority of echiniscids, i.e. finger-like papillae attached to the body cuticle only at their bases, visible as such both under PCM and SEM: (A) P. (M.) cf. saltensis (AR.251, PCM, dorso-ventral projection), (B) P. (M.) sp. 5 (MU.001, SEM, ventral projection), (C) P. (M.) cf. angelusalas (ZA.177, PCM, lateral projection), (D) P. (M.) cf. angelusalas (ZA.177, SEM, lateral projection); Pseudohemispherical papillae, present only in the subgenus Pseudechiniscus, i.e. finger-like papillae attached to the body cuticle at their bases but also longitudinally, along their length, which makes them appear under PCM as hemispheres/domes, but the actual finger-like shape of the papillae is clearly visible under SEM: (E) P. (P.) cf. ehrenbergi (ID.546, PCM, dorso-ventral projection), (F) P. (P.) sp. 9 (TN.018, SEM, frontal projection), (G) P. (P.) sp. 9 (ES.202, PCM, dorso-lateral projection), (H) P. (P.) sp. 9 (TN.018, SEM, lateral projection); Hemispherical papillae, i.e. truly hemispherical/dome-shaped papillae, flattened and widened but attached to the body cuticle only at their bases, visible both under PCM and SEM (present in Cornechiniscus, Mopsechiniscus, Parechiniscus, Mopsechiniscus and Proechiniscus): (I) Cornechiniscus madagascariensis (paratype, PCM, ventral projection), (J) Cornechiniscus madagascariensis (ET.007, SEM, lateral projection). Figs I–J from44. Scale bars in μm.
Comparative material
We borrowed and examined the following type specimens of Pseudechiniscus species: P. bartkei, P. gullii, P. insolitus, P. jubatus, P. nataliae, P. quadrilobatus, P. ramazzottii, P. santomensis, P. spinerectus. They were used to determine their subgeneric affinity and to formulate taxonomic notes (Supplementary Material 2).
Estimating species richness
In order to predict species richness in the genus, described Pseudechiniscus species were categorised into cumulative species richness increasing since the description of P. suillus in 185332. Then, a logarithmic best-fit curve was assigned to data to infer a probable progress in the taxonomic descriptions of new taxa. We decided to use this simple method in order to obtain conservative estimates of potential species richness78.
Genotyping
Prior to DNA extraction, all specimens were examined in vivo under PCM. Individual DNA extractions were made from animals and/or exuviae following recent protocols79,80. Three nuclear DNA markers were sequenced: 18S rRNA, 28S rRNA and ITS-1. All fragments were amplified using the primers listed in Supplementary Table 3. We attempted to sequence the mitochondrial COI, but obtained good quality chromatograms only for ca. one third of the analysed populations (see Supplementary Table 3 for all used primer combinations). Nevertheless, GenBank accession numbers for obtained COI sequences are presented alongside the nuclear markers in Supplementary Table 4, and in cases of successful COI amplification, the barcode was used to verify species identities in our dataset. Sequencing products were read with the ABI 3130xl sequencer at the Molecular Ecology Lab, Institute of Environmental Sciences of the Jagiellonian University. Sequences were processed in BioEdit ver. 7.2.581.
Species delimitation
For the purpose of molecular species discrimination, ITS-1-based (710 bp) phylogenetic tree was reconstructed using IQ-TREE82,83 under the best-fit GTR + F + G4 model indicated by ModelFinder84. A bPTP model was used85 with the following priors: tree rooted on several outgroup genera (see below), 100 thousand MCMC generations and 0.1 burn-in. Results of bPTP are presented on the concatenated phylogenetic trees as the clades recovered in both analyses were identical (topology of deeper nodes is irrelevant from the perspective of species delimitation).
Phylogenetics
The 18S rRNA and 28S rRNA sequences were aligned using the default settings and the G-INS-I method of MAFFT version 786,87 [https://mafft.cbrc.jp/alignment/server/] and manually checked in BioEdit. We were not able to use sequences from earlier studies20,21 as they represented non-homologous fragments of both markers (however, all available COI sequences were separately aligned with two outgroup taxa: Acanthechiniscus islandicus and Echiniscus testudo). ITS-1 sequences were aligned using ClustalW Multiple Alignment tool88 implemented in BioEdit. Seven outgroup taxa representing various echiniscid genera were chosen: Bryodelphax australasiaticus, Diploechiniscus oihonnae, Echiniscus succineus, Echiniscus testudo, Hypechiniscus gladiator, Mopsechiniscus granulosus, Testechiniscus spitsbergensis. Then, the aligned sequences were trimmed to: 884 bp (18S rRNA), 713 bp (28S rRNA), 710 bp (ITS-1), and subsequently concatenated using SequenceMatrix89 (we concatenated sequences exclusively within specimens, i.e. all three concatenated markers always originated from a single animal). Phylogeny of the genus Pseudechiniscus was reconstructed using Maximum Likelihood (ML) and Bayesian Inference (BI) methods. IQ-TREE was applied in ML, and Model-Finder indicated the retention of three separate partitions90: SYM+I+G4 (18S rRNA), SYM+I+G4 (28S rRNA), GTR+F+G4 (ITS-1); K3Pu+F+I+G4 model was applied in the COI phylogeny presented in Supplementary Material 2. BI was performed both in MrBayes91 and BEAST92. In MrBayes, random starting trees were used and the analysis was run for ten million generations, sampling the Markov chain every 1000 generations. An average standard deviation of split frequencies of < 0.01 was used as a guide to ensure the two independent analyses had converged. The program Tracer v.1.693 was then used to ensure Markov chains had reached stationarity and to determine the correct “burn-in” for the analysis, which was the first 10% of generations. The effective sample size values were greater than 200, and a consensus tree was obtained after summarising the resulting topologies and discarding the “burn-in”. The original matrix was also analysed using BEAST in order to obtain a set of Bayesian phylogenetic trees needed for biogeographic analyses. Four clock and tree prior combinations were chosen and ran in parallel: (a) random local clock94 with the coalescent tree prior, (b) random local clock with the speciation: Yule process as the tree prior, (c) strict clock95 with the coalescent tree prior and (d) strict clock with the speciation: Yule process as the tree prior. Tree searches ran for 10 million generations, sampling a tree each 1000 steps. These trees were summarised with the TREEANNOTATOR software (distributed with BEAST) [https://beast.community/treeannotator], removing the first 1000 trees. Eventually, combinations (a) and (b) were chosen for further analyses as Markov chains did not reach stationarity in the latter options. All final consensus trees were viewed and visualised in FigTree v.1.4.3 (http://tree.bio.ed.ac.uk/software/figtree). Character states were coded for populations (Supplementary Material 2) and tested for phylogenetic signal96 with a consensus tree in RASP97,98 with implemented R packages 'adephylo'99 and 'geiger'100. Only traits indicated as phylogenetically significant were reconstructed on the simplified trees (single lineage representing each species to ensure that the polytomies at the tip nodes were absent) with BayesTraits29 implemented in RASP.
Historical biogeography
1000 most credible BEAST trees were used in RASP analyses. Areas were coded for species and defined broadly as classical biogeographic realms62, with the reservation that Palaearctic was divided into West and East Palaearctic as traditionally separated by the Ural Mountains, Caspian Sea and Zagros Mountains. Both S-DIVA and ancestral area reconstruction in BioGeoBEARS were ran, the latter under the DIVALIKE model101 as we rejected the DEC+J model with the highest AICc_wt score since it has been criticised due to theoretical problems102.
Ecological biogeography
We used the ecological niche modelling (ENM) approach to predict the current potential distribution of P. (M.) cf. angelusalas and P. (P.) cf. ehrenbergi. The ENM was performed with the use of Maxent algorithm, ver. 3.4.1103, with the kuenm package104, in R105. To eliminate a potential bias of clustered occurrences, the datasets were filtered so that there was only one record per a 5 arc-min cell for each species. Thus, only 15 occurrence records for P. (M.) cf. angelusalas and 12 records for P. (P.) cf. ehrenbergi were used in modelling (see Supplementary Material 5 for the full list of records). We used the bioclimatic variables available in WorldClim version 2.1106 [http://www.worldclim.com/version2], with a 5-arc-minute resolution, as environmental variables for Maxent modelling. Out of 19 available variables, we excluded those that combined temperature and precipitation (bio8, bio9, bio18 and bio19), because they displayed artificial discontinuities between adjacent grid cells in some areas, which could introduce artefacts to modelling107. From the remaining 15 bioclimatic variables, we removed the highly correlated ones (Spearman rank correlation value <|0.85|) and selected those with the highest importance based on jack-knife procedure (regularised training gain) for each species (Supplementary Tables 6–7). Finally, we used six variables in the ENM: bio2 (mean diurnal range), bio4 (temperature seasonality), bio10 (mean temperature of the warmest quarter), bio12 (annual precipitation), bio14 (precipitation of the driest month) and bio15 (precipitation seasonality). Based on these variables and randomly selected 60% of occurrence records, we created 255 candidate models for each species combining 17 values of regularisation multiplier (0.1–1.0 at intervals 0.1, 2–6, 8 and 10) and 15 combinations of four feature classes (linear = l, quadratic = q, product = p, and hinge = h). Then, all candidate models were evaluated based on the partial ROC approach108 and predictive power of model based on omission rates109, using the test data subset (40% of species records not included in training data). Statistically significant models with omission rates ≤ 10% were selected as the best models. The 10 best models for P. (M.) cf. angelusalas and 4 for the P. (P.) cf. ehrenbergi were selected according to these criteria (Supplementary Table 8). For each parameter setting selected as the best, we created 10 bootstrap replicates of models with complemental log–log (cloglog) output format103. Models were calibrated and projected using the whole world as a training area and all runs were set with 500 iterations and 10,000 background points. Final maps of potential distribution of both species were created in QGIS110 by averaging all best models. The map showing similarities in the distribution of both species is provided in Supplementary Material 9.
Data availability
Data generated or analysed during this study are included in the published article and its supplementary information files. All unique sequences are deposited in GenBank (i.e. if several specimens from one population shared an identical haplotype, only one sequence was uploaded).
References
Gross, V. et al. Miniaturization of tardigrades (water bears): Morphological and genomic perspectives. Arthr. Struct. Dev. 48, 12–19 (2019).
Møbjerg, N. et al. Survival in extreme environments – on the current knowledge of adaptations in tardigrades. Acta Physiol. 202, 409–420 (2011).
Giribet, G. & Edgecombe, G. D. Current understanding of Ecdysozoa and its internal phylogenetic relationships. Integr. Comp. Biol. 57, 455–466 (2017).
Campbell, L. I. et al. MicroRNAs and phylogenomics resolve the relationships of Tardigrada and suggest that velvet worms are the sister group of Arthropoda. Proc. Natl Acad. Sci. USA 108, 15920–15924 (2011).
Jørgensen, A., Møbjerg, N. & Kristensen, R. M. Phylogeny and evolution of the Echiniscidae (Echiniscoidea, Tardigrada) – an investigation of the congruence between molecules and morphology. J. Zool. Syst. Evol. Res. 49(Suppl. 1), 6–16 (2011).
Bertolani, R. et al. Phylogeny of Eutardigrada: new molecular data and their morphological support lead to the identification of new evolutionary lineages. Mol. Phyl. Evol. 76, 110–126 (2014).
Fujimoto, S., Jørgensen, A. & Hansen, J. G. A. molecular approach to arthrotardigrade phylogeny (Heterotardigrada, Tardigrada). Zool. Scr. 46, 496–505 (2017).
Gąsiorek, P., Stec, D., Morek, W. & Michalczyk, Ł. Deceptive conservatism of claws: distinct phyletic lineages concealed within Isohypsibioidea (Eutardigrada) revealed by molecular and morphological evidence. Contrib. Zool. 88, 78–132 (2019).
Hortal, J. et al. Seven shortfalls that beset large-scale knowledge of biodiversity. Ann. Rev. Ecol. Evol. Syst. 46, 523–549 (2015).
Bartels, P. J., Apodaca, J. J., Mora, C. & Nelson, D. R. A global biodiversity estimate of a poorly known taxon: phylum Tardigrada. Zool. J. Linn. Soc. 178, 730–736 (2016).
McInnes, S. J. Zoogeographic distribution of terrestrial/freshwater tardigrades from current literature. J. Nat. Hist. 28, 257–352 (1994).
Morek, W., Stec, D., Gąsiorek, P., Surmacz, B. & Michalczyk, Ł. Milnesium tardigradum Doyère, 1840: The first integrative study of interpopulation variability in a tardigrade species. J. Zool. Syst. Evol. Res. 57, 1–23 (2019).
Gąsiorek, P., Blagden, B. & Michalczyk, Ł. Towards a better understanding of echiniscid intraspecific variability: A redescription of Nebularmis reticulatus (Murray, 1905) (Heterotardigrada: Echiniscoidea). Zool. Anz. 283, 242–255 (2019).
Gąsiorek, P. et al. Echiniscus virginicus complex: the first case of pseudocryptic allopatry and pantropical distribution in tardigrades. Biol. J. Linn. Soc. 128, 789–805 (2019).
Cesari, M., McInnes, S. J., Bertolani, R., Rebecchi, L. & Guidetti, R. Genetic diversity and biogeography of the south polar water bear Acutuncus antarcticus (Eutardigrada : Hypsibiidae) – evidence that it is a truly pan-Antarctic species. Invertebr. Syst. 30, 635–649 (2016).
Guidetti, R., McInnes, S. J., Cesari, M., Rebecchi, L. & Rota-Stabelli, O. Evolutionary scenarios for the origin of an Antarctic tardigrade species based on molecular clock analyses and biogeographic data. Contrib. Zool. 86, 97–110 (2017).
Stec, D., Krzywański, Ł, Zawierucha, K. & Michalczyk, Ł. Untangling systematics of the Paramacrobiotus areolatus species complex by an integrative redescription of the nominal species for the group, with multilocus phylogeny and species delineation in the genus Paramacrobiotus. Zool. J. Linn. Soc. 188, 694–716 (2020).
Thulin, G. Beiträge zur Kenntnis der Tardigradenfauna Schwedens. Ark. Zool. 7, 1–60 (1911).
Kristensen, R. M. Generic revision of the Echiniscidae (Heterotardigrada), with a discussion of the origin of the family. In Biology of Tardigrada (ed. Bertolani, R.) 261–335 (U.Z.I. Modena, 1987).
Vecchi, M. et al. Integrative systematic studies on tardigrades from Antarctica identify new genera and new species within Macrobiotoidea and Echiniscoidea. Invertebr. Syst. 30, 303–322 (2016).
Cesari, M. et al. An integrated study of the biodiversity within the Pseudechiniscus suillus–facettalis group (Heterotardigrada: Echiniscidae). Zool. J. Linn. Soc. 188, 717–732 (2020).
Tumanov, D. V. Analysis of non-morphometric morphological characters used in the taxonomy of the genus Pseudechiniscus (Tardigrada: Echiniscidae). Zool. J. Linn. Soc. 188, 753–775 (2020).
Grobys, D. et al. High diversity in the Pseudechiniscus suillus–facettalis complex (Heterotardigrada: Echiniscidae) with remarks on the morphology of the genus Pseudechiniscus. Zool. J. Linn. Soc. 188, 733–752 (2020).
Roszkowska, M. et al. Integrative description of five Pseudechiniscus species (Heterotardigrada: Echiniscidae: the suillus-facettalis complex). Zootaxa 4763, 451–484 (2020).
Gąsiorek, P. et al. New Asian and Nearctic Hypechiniscus species (Heterotardigrada: Echiniscidae) signalise a pseudocryptic horn of plenty. Zool. J. Linn. Soc. (in press).
Fontoura, P. & Morais, P. Assessment of traditional and geometric morphometrics for discriminating cryptic species of the Pseudechiniscus suillus complex (Tardigrada, Echiniscidae). J. Zool. Syst. Evol. Res. 49(Suppl. 1), 26–33 (2011).
Yu, Y., Harris, A. J. & He, X. S-DIVA (Statistical Dispersal-Vicariance Analysis): a tool for inferring biogeographic histories. Mol. Phyl. Evol. 56, 848–850 (2010).
Matzke, N. J. Probabilistic historical biogeography: new models for founder- event speciation, imperfect detection, and fossils allow improved accuracy and model-testing. Front. Biogeogr. 5, 242–248 (2013).
Pagel, M., Meade, A. & Barker, D. Bayesian estimation of ancestral character states on phylogenies. Syst. Biol. 53, 673–684 (2004).
Phillips, S. J., Anderson, R. P. & Schapire, R. E. Maximum entropy modelling of species geographic distributions. Ecol. Model. 190, 231–259 (2006).
Rocha, A., Doma, I., Gonzalez-Reyes, A. & Lisi, O. Two new tardigrade species (Echiniscidae, Doryphoribiidae) from Salta province (Argentina). Zootaxa 4878, 267–286 (2020).
Ehrenberg, C. G. Diagnoses novarum formarum. Verhandl. König. Preuss. Akad. Wiss Berlin 8, 526–533 (1853).
Mihelčič, F. Zwei neue Tardigradenarten aus Spanien. Zool. Anz. 155, 309–311 (1955).
Mihelčič, F. Beitrag zur Systematik de Tardigraden . Arch. Zool. Ital. 36, 57–103 (1951).
Iharos, A. Zwei neue Tardigraden-Arten. Zool. Anz. 115, 218–220 (1936).
Murray, J. Some South African Tardigrada. J. R. Microsc. Soc. 12, 515–524 (1907).
Yang, T. Three new species and one new record of the Tardigrada from China. Acta Hydrobiol. Sin. 26, 504–507 (2002).
Mihelčič, F. Beiträge zur Kenntnis der Tardigrada Jugoslawiens. Zool. Anz. 121, 95–96 (1938).
Bartoš, E. Eine neue Tardigradenart aus der Tschechoslowakei. Zool. Anz. 106, 175–176 (1934).
Richters, F. Beitrag zur Kenntnis der Moosfauna Australiens und der Inseln des Pazifischen Ozeans. Zool. Jahrb. Abt. Syst. Ökol. Geogr. Tiere 26, 196–213 (1908).
Vončina, K., Kristensen, R. M. & Gąsiorek, P. Pseudechiniscus in Japan: re-description of Pseudechiniscus asper Abe et al., 1998 and description of Pseudechiniscus shintai sp. nov. Zoosyst. Evol. 96, 527–536 (2020).
Wang, L. Tardigrades from the Yunnan-Guizhou Plateau (China) with description of two new species in the genera Mixibius (Eutardigrada: Hypsibiidae) and Pseudechiniscus (Heterotardigrada: Echiniscidae). J. Nat. Hist. 43, 2553–2570 (2009).
Hulings, N. C. & Gray, J. S. A manual for the study of meiofauna. Smithson. Contrib. Zool. 78, 1–84 (1971).
Gąsiorek, P. & Michalczyk, Ł. Revised Cornechiniscus (Heterotardigrada) and new phylogenetic analyses negate echiniscid subfamilies and tribes. R. Soc. Open Sci. 7, 200581 (2020).
Dastych, H. Notes on the revision of the genus Mopsechiniscus (Tardigrada). Zool. Anz. 240, 299–308 (2001).
Rebecchi, L., Altiero, T., Eibye-Jacobsen, J., Bertolani, R. & Kristensen, R. M. A new discovery of Novechiniscus armadilloides (Schuster, 1975) (Tardigrada, Echiniscidae) from Utah, USA with considerations on non-marine Heterotardigrada phylogeny and biogeography. Org. Divers. Evol. 8, 58–65 (2008).
Binda, M. G. & Kristensen, R. M. Notes on the genus Oreella (Oreellidae) and the systematic position of Carphania fluviatilis Binda, 1978 (Carphaniidae fam. nov., Heterotardigrada). Animalia 13, 9–20 (1986).
Binda, M. G. Risistemazione di alcuni Tardigradi con l’instituzione di un nuovo genere di Oreellidae e della nuova famiglia Archechiniscidae. Animalia 5, 307–314 (1978).
Kristensen, R. M. & Hallas, T. E. The tidal genus Echiniscoides and its variability, with erection of Echiniscoididae fam. n. (Tardigrada). Zool. Scr. 9, 113–127 (1980).
Møbjerg, N., Kristensen, R. M. & Jørgensen, A. Data from new taxa infer Isoechiniscoides gen. nov. and increase the phylogenetic and evolutionary understanding of echiniscoidid tardigrades (Echiniscoidea: Tardigrada). Zool. J. Linn. Soc. 178, 804–818 (2016).
Møbjerg, N., Jørgensen, A. & Kristensen, R. M. Ongoing revision of Echiniscoididae (Heterotardigrada: Echiniscoidea), with the description of a new interstitial species and genus with unique anal structures. Zool. J. Linn. Soc. 188, 663–680 (2020).
Gąsiorek, P., Suzuki, A. C., Kristensen, R. M., Lachowska-Cierlik, D. & Michalczyk, Ł. Untangling the Echiniscus Gordian knot: Stellariscus gen. nov. (Heterotardigrada: Echiniscidae) from Far East Asia. Invertebr. Syst. 32, 1234–1247 (2018).
Dastych, H. Echiniscus rackae sp. n., a new species of Tardigrada from the Himalayas. Entomol. Mitt. Zool. Mus. Hamburg 8, 246–250 (1986).
McInnes, S. J. Tardigrades from Signy Island, South Orkney Islands, with particular reference to freshwater species. J. Nat. Hist. 29, 1419–1445 (1995).
Dastych, H. Two new species of Tardigrada from the Canadian Subarctic with some notes on sexual dimorphism in the family Echiniscidae. Entomol. Mitt. Zool. Mus. Hamburg 8, 319–334 (1987).
Pilato, G., Binda, M. G. & Lisi, O. Remarks on some Echiniscidae (Heterotardigrada) from New Zealand with the description of two new species. Zootaxa 1027, 27–45 (2005).
Pilato, G., Binda, M. G., Napolitano, A. & Moncada, E. Notes on South American tardigrades with the description of two new species: Pseudechiniscus spinerectus and Macrobiotus danielae. Trop. Zool. 14, 223–231 (2001).
Fontoura, P., Pilato, G. & Lisi, O. First record of Tardigrada from São Tomé (Gulf of Guinea, Western Equatorial Africa) and description of Pseudechiniscus santomensis sp. nov. (Heterotardigrada: Echiniscidae). Zootaxa 2564, 31–42 (2010).
Bartoš, E. Die Tardigraden der Chinesischen und Javanischen Moosproben. Acta Soc. Zool. Bohem. 27, 108–114 (1963).
Beijerinck, M.W. De infusies en de ontdekking der backteriën. Jaarboek van de Koninklijke Akademie v. Wetenschappen. Amsterdam: Müller (1913).
Baas-Becking, L. G. M. Geobiologie of inleiding tot de milieukunde (W.P. Van Stockum & Zoon, 1934).
Wallace, A. R. The geographical distribution of animals: with a study of the relations of living and extinct faunas as elucidating the past changes of the Earth’s surface (Macmillan and Company, 1876).
Niedbała, W. The ptyctimous mites fauna of the Oriental and Australian regions and their centres of origin (Acari: Oribatida). Genus Suppl. 10, 1–493 (2000).
Niedbała, W. Ptyctimous mites (Acari: Oribatida) of South Africa. Ann. Zool. 56(Suppl. 1), 1–97 (2006).
Janion-Scheepers, C., Deharveng, L., Bedos, A. & Chown, S. Updated list of Collembola species currently recorded from South Africa. ZooKeys 503, 55–88 (2015).
Kisielewski, J. Inland-water Gastrotricha from Brazil. Ann. Zool. 43(Suppl. 2), 1–168 (1991).
Tuxen, S. L. Ecology and zoogeography of the Brazilian Protura (Insecta). Stud. Neotrop. Fauna Environ. 12, 225–247 (1977).
Greenslade, P. Why are there so many exotic springtails in Australia? A review. Soil Org. 90, 141–156 (2018).
Smit, H. Australian water mites of the subfamily Notoaturinae Besch (Acari: Hydrachnidia: Aturidae), with the description of 24 new species. Int. J. Acarol. 36, 101–146 (2010).
Moir, M. L., Brennan, K. E. C. & Harvey, M. S. Diversity, endemism and species turnover of millipedes within the south-western Australian global biodiversity hotspot. J. Biogeogr. 36, 1958–1971 (2009).
Harvey, M. S., Abrams, K. M., Beavis, A. S., Hillyer, M. J. & Huey, J. A. Pseudoscorpions of the family Feaellidae (Pseudoscorpiones: Feaelloidea) from the Pilbara region of Western Australia show extreme short-range endemism. Invertebr. Syst. 30, 491–508 (2016).
Claxton, S.K. The taxonomy and distribution of Australian terrestrial tardigrades. PhD thesis, Macquarie University: Sydney (2004).
Simpson, G. G. Tempo and mode in evolution (Columbia University Press, 1944).
Dastych, H. The Tardigrada of Poland. Monogr. Faun. Pol. 16, 1–255 (1988).
Peters, M. K. et al. Predictors of elevational biodiversity gradients change from single taxa to the multi-taxa community level. Nat. Commun. 7, 13736 (2016).
Petersen, B. The tardigrade fauna of Greenland. Medd. Grønl. 150, 1–94 (1951).
de Bruyn, M. et al. Borneo and Indochina are major evolutionary hotspots for Southeast Asian biodiversity. Syst. Biol. 63, 879–901 (2014).
Ugland, K. I., Gray, J. S. & Ellingsen, K. E. The species–accumulation curve and estimation of species richness. J. Anim. Ecol. 72, 888–897 (2003).
Casquet, J. T., Thebaud, C. & Gillespie, R. G. Chelex without boiling, a rapid and easy technique to obtain stable amplifiable DNA from small amounts of ethanol-stored spiders. Mol. Ecol. Resour. 12, 136–141 (2012).
Stec, D., Kristensen, R.M. & Michalczyk, Ł. An integrative description of Minibiotus ioculator sp. nov. from the Republic of South Africa with notes on Minibiotus pentannulatus Londoño et al., 2017 (Tardigrada: Macrobiotidae). Zool. Anz. 286, 117–134 (2020).
Hall, T. A. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41, 95–98 (1999).
Nguyen, L.-T., Schmidt, H. A., von Haeseler, A. & Minh, B. Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum likelihood phylogenies. Mol. Biol. Evol. 32, 268–274 (2015).
Hoang, D. T., Chernomor, O., von Haeseler, A., Minh, B. Q. & Vinh, L. S. UFBoot2: Improving the ultrafast bootstrap approximation. Mol. Biol. Evol. 35, 518–522 (2018).
Kalyaanamoorthy, S., Minh, B. Q., Wong, T. K. F., von Haeseler, A. & Jermiin, L. S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Meth. 14, 587–589 (2017).
Zhang, J., Kapli, P., Pavlidis, P. & Stamatakis, A. A general species delimitation method with applications to phylogenetic placements. Bioinformatics 29, 2869–2876 (2013).
Katoh, K., Misawa, K., Kuma, K. & Miyata, T. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 30, 3059–3066 (2002).
Katoh, K. & Toh, H. Recent developments in the MAFFT multiple sequence alignment program. Brief. Bioinform. 9, 286–298 (2008).
Thompson, J. D., Higgins, D. G. & Gibson, T. J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22, 4673–4680 (1994).
Vaidya, G., Lohman, D. J. & Meier, R. SequenceMatrix: concatenation software for the fast assembly of multi-gene datasets with character set and codon information. Cladistics 27, 171–180 (2011).
Chernomor, O., von Haeseler, A. & Minh, B. Q. Terrace aware data structure for phylogenomic inference from supermatrices. Syst. Biol. 65, 997–1008 (2016).
Ronquist, F. & Huelsenbeck, J. P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19, 1572–1574 (2003).
Suchard, M.A. et al. Bayesian phylogenetic and phylodynamic data integration using BEAST 1.10 Virus Evol. 4, vey016 (2018).
Rambaut, A., Suchard, M.A., Xie, D. & Drummond, A.J. Tracer v1.6 (2014). Available from http://beast.bio.ed.ac.uk/Tracer.
Drummond, A. J. & Suchard, M. A. Bayesian random local clocks, or one rate to rule them all. BMC Biol. 8, 114 (2010).
Ferreira, M. A. R. & Suchard, M. A. (2008) Bayesian analysis of elapsed times in continuous-time Markov chains. Can. J. Stat. 36, 355–368 (2008).
Münkemüller, T. et al. How to measure and test phylogenetic signal. Meth. Ecol. Evol. 3, 743–756 (2012).
Yu, Y., Harris, A. J., Blair, C. & He, X. J. RASP (Reconstruct Ancestral State in Phylogenies): a tool for historical biogeography. Mol. Phyl. Evol. 87, 46–49 (2015).
Yu, Y., Blair, C. & He, X. J. RASP 4: ancestral state reconstruction tool for multiple genes and characters. Mol. Biol. Evol. 37, 604–606 (2020).
Jombart, T., Balloux, F. & Dray, S. adephylo: new tools for investigating the phylogenetic signal in biological traits. Bioinformatics 26, 1907–1909 (2010).
Pennell, M. W. et al. geiger v2.0: an expanded suite of methods for fitting macroevolutionary models to phylogenetic trees. Bioinformatics 30, 2216–2218 (2014).
Matzke, N. J. Model selection in historical biogeography reveals that founder-event speciation is a crucial process in island clades. Syst. Biol. 63, 951–970 (2014).
Ree, R. H. & Sanmartín, I. Conceptual and statistical problems with the DEC+J model of founder-event speciation and its comparison with DEC via model selection. J. Biogeogr. 45, 741–749 (2018).
Phillips, S. J., Anderson, R. P., Dudík, M., Schapire, R. E. & Blair, M. E. Opening the black box: an open-source release of Maxent. Ecography 40, 887–893 (2017).
Cobos, M. E., Peterson, A. T., Barve, N. & Osorio-Olvera, L. kuenm: an R package for detailed development of ecological niche models using Maxent. PeerJ 7, e6281 (2019).
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/ (2020).
Fick, S. E. & Hijmans, R. J. WorldClim 2: new 1km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 37, 4302–4315 (2017).
Escobar, L. E., Lira-Noriega, A., Medina-Vogel, G. & Peterson, A. T. Potential for spread of the white-nose fungus (Pseudogymnoascus destructans) in the Americas: use of Maxent and NicheA to assure strict model transference. Geospat. Health 9, 221–229 (2014).
Peterson, A. T., Papeş, M. & Soberón, J. Rethinking receiver operating characteristic analysis applications in ecological niche modeling. Ecol. Model. 213, 63–72 (2008).
Anderson, R. P., Lew, D. & Peterson, A. T. Evaluating predictive models of species’ distributions: criteria for selecting optimal models. Ecol. Model. 162, 211–232 (2003).
QGIS Development Team. QGIS Geographic Information System. Open Source Geospatial Foundation Project (2020).
Acknowledgements
Sample collectors are gratefully acknowledged alphabetically: Maciej Barczyk, Brian Blagden, Olena Garmish, Andrzej Kaźmierski, Reinhardt M. Kristensen, Łukasz Krzywański, Grzegorz Kwiatkowski, Jamila Marnissi, Witold Morek, Diane Nelson, Artur Oczkowski, Aleksandra Rysiewska, Daniel Stec, Bartłomiej Surmacz and Wojciech Witaliński. Roberto Guidetti and Giovanni Pilato kindly made tardigrade collections deposited in Modena and Catania available for examination. Alejandro López-López is acknowledged for advice on phylogenetics and two anonymous reviewers are thanked for improving this manuscript. The study was supported by the Polish Ministry of Science and Higher Education via the Diamond Grant (grant no. DI2015 014945 to PG, supervised by ŁM), and Polish National Science Centre via the ‘Preludium’ (grant no. 2019/33/N/NZ8/02777 awarded to PG, supervised by ŁM) and ‘Sonata Bis’ programmes (grant no. 2016/22/E/NZ8/00417 awarded to ŁM). PG is a recipient of the ‘Etiuda’ (2020/36/T/NZ8/00360, funded by the National Science Centre) and ‘Start’ stipends (START 28.2020, funded by the Foundation for Polish Science). Open-access publication of this article was funded by the BioS Priority Research Area under the program “Excellence Initiative – Research University” at the Jagiellonian University in Kraków, Poland.
Author information
Authors and Affiliations
Contributions
P.G. conceived and designed the study, gained funding, acquired, analysed and interpreted data, drafted the manuscript, and prepared figures; K.V. acquired, analysed and interpreted data, and prepared figures; K.Z. analysed data and prepared figures; Ł.M. conceived and designed the study, gained funding, interpreted data and drafted the manuscript, and prepared figures. All authors amended the original version of the work, read it and approved the submission for review.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gąsiorek, P., Vončina, K., Zając, K. et al. Phylogeography and morphological evolution of Pseudechiniscus (Heterotardigrada: Echiniscidae). Sci Rep 11, 7606 (2021). https://doi.org/10.1038/s41598-021-84910-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-84910-6
This article is cited by
-
Novel integrative data for Indomalayan echiniscids (Heterotardigrada): new species and old problems
Organisms Diversity & Evolution (2024)
-
Diversification rates in Tardigrada indicate a decreasing tempo of lineage splitting regardless of reproductive mode
Organisms Diversity & Evolution (2022)
-
The importance of being integrative: a remarkable case of synonymy in the genus Viridiscus (Heterotardigrada: Echiniscidae)
Zoological Letters (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.