Abstract
The prevalence of metabolic syndrome (MS) is increasing among the elderly, and new lifestyle-based treatment strategies are warranted. We conducted a randomized, double-blind controlled trial of the effects of aquatic exercise (AE) and/or consumption of burdock root extract (BE) on body composition and serum sex hormones, i.e., testosterone, estradiol, sex hormone-binding globulin (SHBG), and dehydroepiandrosterone-sulfate (DHEA-S) in elderly women with MS. The percentage of abdominal fat was decreased in the AE group. Waist circumference was increased in the control (CON) group, but not in the other groups. SHBG and estradiol levels were enhanced by both AE and BE and correlated with changes in fat-related body composition. DHEA-S levels only increased in the BE group, which was consistent with changes in lean body mass. Testosterone levels decreased in the CON group, which correlated with changes in lean body mass, skeletal muscle mass, body fat, and waist circumference. Our findings suggested that the combined AE/BE intervention exerted no synergistic and/or additive effects on any sex-related outcome measures in elderly women with MS.
Similar content being viewed by others
Introduction
Metabolic syndrome (MS) is a group of metabolic disorders including hypertension, insulin resistance, impaired glucose tolerance, and abdominal obesity, and is associated with a high incidence of cardiovascular disease (CVD) and type 2 diabetes1, 2. The prevalence of MS increases with age, and is particularly high among the elderly3, 4, in which the increase in MS-associated risk factors, including central obesity and CVD, is higher in women than that in men5, 6.
In general, postmenopausal women exhibit considerable alterations in sex hormones, which may be related to abdominal adiposity, thus increasing the likelihood of MS. There is increasing evidence that changes in sex hormones contribute to MS pathophysiology7. Dehydroepiandrosterone (DHEA) and its sulfate ester (DHEA-S), the most abundant circulating steroid hormone produced by adrenal glands, are converted to testosterone and estrogen8. Although the precise physiological function of DHEA-S is not fully understood, its serum levels decline with age, and this decrease is associated with increased waist circumference, a diagnostic indicator of MS in elderly women9. Low levels of sex hormone-binding globulin (SHBG), which binds to testosterone and estradiol, are associated with high MS prevalence in postmenopausal women10.
Lifestyle changes commonly recommended as a first-line intervention for MS prevention and treatment include regular physical exercise and a healthy diet11. Several meta-analyses have shown that aerobic exercise has a positive effect on MS profiles, thereby affecting body composition, cardiorespiratory fitness, insulin resistance, as well as sex hormones12,13,14. Although there are controversial issues regarding the effects of physical exercise on sex hormone among elderly women, several studies have revealed that estradiol, testosterone, and SHBG levels are enhanced after adopting an active and healthy lifestyle15,16,17. Additionally, we observed that water-based aquatic exercise (AE), an acceptable compromise between exercise effectiveness and safety intervention for the elderly, improved fitness and vascular-related factors in elderly women without MS18, suggesting that regular AE might also be beneficial in elderly women with MS.
Most studies have demonstrated that appropriate combinations of exercise and diet reduce the clinical prevalence of MS19. However, there is a lack of scientific information regarding the effects on MS of combined interventions involving physical exercise and the consumption of specific dietary components. Among potentially beneficial dietary components, we focused on burdock (Arctium lappa L.), a fusiform brown root containing arctiin, luteolin, and quercetin rhamnoside, which is a remarkable source of proteins, potassium, calcium, and folate, and is rich in phytochemicals20, 21. As a traditional herbal medicine, burdock has been used in Asia as well as western countries for centuries. Each part of the burdock plant has a different composition, especially in terms of bioactive compounds. Indeed, various biologically active compounds, such as terpenoids (beta-eudesmol, C15H24O, present in the fruit), sterols (sitosterol-beta-d-glucopyranoside, C35H60O6, contained in the root), lignans, polyphenols, and fructans, are found in the plant complex22. Burdock possesses antioxidant and anti-inflammatory properties and is reportedly pharmacologically active as an anti-diabetic agent, which improves blood lipid profiles, hypoglycemia, and hyperinsulinemia23, 24. Particularly, one specific burdock component, arctiin, has been found to reduce body weight and adipose tissue through anti-adipogeneic effects by activating the AMP-activated protein kinase (AMPK) pathway in obese mice induced by high-fat diet25. These reports suggest that burdock is potentially beneficial for obesity and diabetes that is closely linked with MS.
Recently, we found that the combination of burdock root extract (BE) and AE exerted positive effects on vascular function and related hormones in elderly women without MS18, 26. Considering the potential health-beneficial effects of the AE and BE, the purpose of this study was to investigate the impact of AE and BE, individually or in combination, on body composition and sex-related hormone levels in elderly women with MS. We hypothesized that a 16-week combined AE/BE intervention in elderly women with MS would either additively or synergistically improve body composition, and induce beneficial changes in the levels of sex-related hormones.
Results
Effects of the interventions on MS criteria parameters
First, there were no significant differences in baseline age, height, and weight, between the control and the intervention groups (Table 1 and Table S1). Regarding MS features, clinical characteristics such as waist circumference, BMI, levels of triglycerides, HDL-C, glucose, and systolic blood pressure and diastolic blood pressure did not differ between groups, and were close to the upper limits established by the National Cholesterol Education Program (NCEP) Adult Treatment Panel (ATP) III criteria27. Thus, all subjects exhibited similar metabolic alterations.
Changes in MS criteria parameters combination interventions are shown in Table 1. A significantly remarkable effect of time was observed for triglycerides (F = 33.003, p = 0.000) and glucose (F = 4.987, p = 0.037). A significant time-group interaction was identified for waist circumference (F = 6.435, p = 0.003), triglycerides (F = 8.162, p = 0.001), HDL-C (F = 3.795, p = 0.026), and glucose (F = 5.446, p = 0.007). Post-hoc analysis revealed that control (CON) women exhibited a significant increase in waist circumference (t = 2.950, p = 0.031), while a significant decrease in triglyceride levels was observed in AE and AE/BE women (t = 3.590, p = 0.007; t = 6.527, p = 0.000). Additionally, AE/BE women showed a significant increase in HDL-C (t = 3.572, p = 0.008), and AE women a significant decrease in glucose levels (t = 3.807, p = 0.004).
Effects of the interventions on body composition
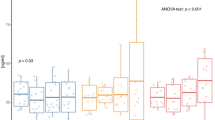
Recently, body composition parameters were found to be closely associated with MS risk28. First, we measured BMI, body fat mass percentage, lean body mass, skeletal muscle mass, abdominal fat (%), and waist circumference before and after the 16-week interventions. The percentage of abdominal fat and waist circumference exhibited a significant time-group interaction (F = 5.950, p = 0.005; F = 6.435, p = 0.003) (Table 1). A significantly remarkable effect of group was observed for the percentage of body fat (F = 3.315, p = 0.041). Post-hoc analysis showed that AE significantly decreased abdominal fat percentage (t = 3.395, p = 0.011) with a large effect size (d = − 1.00). After the intervention period, we observed increase in waist circumference values in the CON group with a large effect size (d = 1.01), but not in the AE, BE, or AE + BE subjects. Furthermore, beneficial changes (Δ) in body composition parameters were observed. The participants undergoing AE demonstrated a significantly greater reduction in the percentage of abdominal fat in comparison to CON subjects (t = 3.894, p = 0.002; Fig. 1a). Moreover, subjects exposed to the AE alone or the AE/BE combination showed a significant decrease in waist circumference, compared to the CON group (t = 3.612, p = 0.005; t = 3.456, p = 0.007, respectively; Fig. 1b). Thus, our result suggest that the intervention of AE and/or BE have positive effects in altering MS risks, particularly in reducing abdominal obesity factors.
Changes in abdominal fat percentage and waist circumference. (a) Significant differences were observed between aquatic exercise (AE) and control (CON) subjects in the delta (Δ) values of % abdominal fat (**p < 0.01). (b) Significant differences were observed between AE + BE (burdock root extract) and CON subjects in the delta (Δ) value of waist circumference (**p < 0.01). Data are expressed as mean ± SD.
Effects of the interventions on sex hormones
Previous reports have suggested that altered levels of endogenous sex hormones are risk factors for MS7. We determined the serum levels of testosterone, estradiol, SHBG, and DHEA-S to investigate the effects of AE and BE interventions on sex hormones. The results are presented in Fig. 2 as a list. A significantly remarkable effect of group for estradiol (F = 4.148, p = 0.019; Fig. 2b) and DHEA-S (F = 3.145, p = 0.048; Fig. 2d), as well as a significantly remarkable effect of time for SHBG (F = 11.607, p = 0.003; Fig. 2c) were observed. Significant time-group interactions were identified for testosterone (F = 4.475, p = 0.15; Fig. 2a), estradiol (F = 5.213, p = 0.008; Fig. 2b), and SHBG (F = 6.517, p = 0.003; Fig. 2c). Post-hoc analysis showed that only CON women exhibited a significant decrease in testosterone levels after the intervention period (t = 3.124, p = 0.021; Fig. 2a), while the AE group showed a large effect size (d = 1.26), but no statistically significant differences. Post-test estradiol levels were higher in the AE (t = 3.407, p = 0.009) compared to the CON group (Fig. 2b). Post-hoc analyses revealed a significant increase in SHBG levels in AE (t = 3.698, p = 0.005) and BE women (t = 3.198, p = 0.018) after the test (Fig. 2c). A significant group effect was observed for post-test serum levels of DHEA-S, as they were higher in BE compared to CON women (t = 2.297, p = 0.015; Fig. 2d). Differences (Δ) between pre and post-exercise levels of sex hormones are presented in Fig. 2. Briefly, Δ-testosterone was found to be higher in both AE (t = 3.262, p = 0.01) and AE + BE (t = 2.867, p = 0.025) compared to CON women (Fig. 2e), and Δ-estradiol was higher in AE (t = 3.385, p = 0.008), BE (t = 2.762, p = 0.031), and AE + BE group (t = 3.117, p = 0.012; Fig. 2f). AE (t = 3.9, p = 0.002) and BE subjects (t = 3.569, p = 0.011) exhibited a significantly greater Δ-SHBG compared to the CON group (Fig. 2g). However, Δ-DHEA-S showed no significant changes between the groups (Fig. 2h).
Effects of 16-week aquatic exercise (AE) and burdock root extract (BE) interventions on serum sex hormones. (a) Testosterone levels in the control (CON) group were decreased after the 16-week intervention period (#p < 0.05). (b) The post-test levels of estradiol were higher in AE (**p < 0.01) compared to the CON group. (c) Sex hormone-binding globulin (SHBG) levels were increased in both AE (##p < 0.01) and BE subjects (#p < 0.05), but not in the AE + BE group. (d) The post-test levels of dehydroepiandrosterone sulfate (DHEA-S) were higher in the BE than in the CON group (*p < 0.05). (e) A significant increase in the delta (Δ) value of testosterone was observed in the AE and AE + BE groups compared to the CON group (*p < 0.05). (f) A significant increase in the delta (Δ) value of estradiol was detected in AE (**p < 0.01), BE (*p < 0.05), and AE + BE women (*p < 0.05) compared to CON women. (g) A significant increase in the delta (Δ) value of SHBG was observed in the AE and BE groups compared to the CON group (*p < 0.05). (h) No significant changes in DHEA-S levels were observed between the groups. Data are presented as mean ± SD.
Correlations between body compositon and sex hormones
To determine whether the levels of specific sex hormones were related to body composition parameters in response to the 16-week intervention, we analyzed the correlation between the Δ values of body composition variables and individual sex hormones, respectively (Table S2 and Fig. 3). Δ-testosterone was positively correlated with Δ-lean body mass (r = 0.534, p = 0.010; Fig. 3a) and Δ-skeletal muscle mass (r = 0.562, p = 0.004; Fig. 3b), and negatively correlated with Δ-% body fat (r = − 0.534, p = 0.007; Fig. 3c), Δ-% abdominal fat (r = − 0.500, p = 0.013; Fig. 3d), and Δ-waist circumference (r = − 0.556, p = 0.005; Fig. 3e). However, Δ-estradiol did not correlate with body composition parameters (Table S2). Moreover, Δ-SHBG was negatively correlated with Δ-% body fat (r = − 0.436, p = 0.033; Fig. 3f) and Δ-% abdominal fat (r = − 0.422, p = 0.040; Fig. 3g). Finally, a significant relationship was observed between Δ-DHEA-S and Δ-lean body mass (r = 0.411, p = 0.046; Fig. 3h).
Correlation between body composition indices and sex hormones. (a–e) Δ-testosterone was correlated with Δ-lean body mass, Δ-skeletal muscle mass, Δ-percentage of body fat, Δ-percentage of abdominal fat and, Δ-waist circumference. (f, g) Δ-SHBG (sex hormone-binding globulin) was correlated with Δ-percentage of body fat and Δ-percentage of abdominal fat. (h) A significant correlation was observed between Δ-DHEA-S (dehydroepiandrosterone sulfate) and Δ-lean body mass. Correlation analysis was performed (Pearson’s correlation coefficients). Each circle represents an individual subject (n = 24).
Discussion
Exercise regimens reportedly exert a significant effect on MS indicators29, and are considered as a valuable non-pharmacological approach to MS management. The aim of this study was to investigate the combined effects of AE and BE on body composition and serum gonadal steroid hormones, as well as their correlation in elderly women with MS. Based on our previous findings18, 26, we hypothesized that the combined effects of AE and BE would lead to additive or synergistic improvement in MS-related body composition and sex hormone levels in elderly women with MS. The results indicated that both AE and BE interventions individually decreased abdominal fat and waist circumference, which induced beneficial changes in the levels of testosterone, estradiol, and SHBG. However, we found no additive or synergistic effect on any of the MS-related outcome measures. Regardless of the contribution of a ceiling effect to these results, we found that AE or BE had potential as non-pharmacological interventions for improving MS symptoms in elderly women.
Abdominal obesity is associated with MS, and is currently the main clinical criterion for the assessment of individual MS risk according to the NCEP-ATP III guidelines30. Waist circumference is widely used as a surrogate marker of abdominal obesity in the elderly, including the elderly constituting the population in Asia31. In this study, the CON group showed a substantial increase in waist circumference after the 16-week intervention period, and both AE and the AE/BE combination resulted in a significant reduction of Δ-waist circumference (Fig. 1b). Notably, a 16-week aqua aerobic exercise regimen has previously been shown to reduce waist circumference in subjects with similar characteristics to our cohort32. However, we found that AE alone, but not BE alone, significantly reduced the percentage of abdominal fat, with a large effect size (Table 1). Therefore, AE may be more effective compared to both BE and the AE/BE combination at reducing MS-related abdominal obesity.
Many studies have reported associations between the levels of serum sex hormones and the risk of MS7, 33. Weinberg et al.10 showed that postmenopausal women with MS exhibited higher blood levels of testosterone (0.2 ng/mL) and estradiol (9.2 ng/mL), consistent with the levels of sex hormones detected in our study. Moreover, in the latter study, acute exercise was found to further increase the levels of these hormones15, while chronic exercise did not affect the levels of these hormones34. We found that in elderly women with MS, the change in testosterone from pre- to post-exercise was significantly greater in the AE and AE + BE groups compared to the CON group (Fig. 2e). All intervention groups showed greater Δ-values for estradiol compared to the CON group. However, the AE/BE combination did not produce any synergistic effect on the level of Δ estradiol (Fig. 2f). In addition to the correlation between body composition parameters and sex hormones, Δ-testosterone significantly correlated with the delta values for waist circumstance, percentage of abdominal fat, percentage of body fat, skeletal muscle mass, and lean body mass (Table S2 and Fig. 3), suggesting that the beneficial effects of AE on abdominal obesity were mainly mediated by changes in testosterone metabolism.
Consistent with its role as a testosterone transporter, SHBG has been associated with the risk of MS. Indeed, low levels of serum SHBG were observed in elderly men and women with MS10, 35. Recently, cross-sectional studies showed that serum levels of SHBG were inversely associated with waist circumference and waist-to-height ratios in Korean and Chinese individuals, respectively36. Thus, since SHBG concentration is a putative biomarker of abdominal obesity-related MS, we investigated the impact of AE and BE interventions on serum SHBG levels. Both interventions separately increased SHBG levels, but their combination did not produce synergistic effects (Fig. 2c). Additionally, SHBG exhibited a higher delta value in the AE and BE groups compared to the CON group (Fig. 2g), which was significantly correlated with Δ-percentage of abdominal fat and Δ-percentage of body fat (Fig. 3g). Recent in vitro experiments in adipocytes and macrophage cells have shown that SHBG protects against inflammation and lipid accumulation37. Further, recent in vivo studies using human SHBG transgenic mice crossed with type-2 diabetic mice (C57BL/ksJ-db/db) have shown that hepatocyte nuclear factor 4 alpha (HNF-4α) and peroxisome proliferator-activated receptor gamma (PPARα) in liver are involved in obesity progression, while human SHBG overexpression partly prevents the increase in body and liver weight, as well as in the proportion of adipose tissue38, 39. Our findings support the notion that AE and BE, by increasing endogenous circulating SHBG, are potential non-pharmacological options against obesity and MS-related fatty liver disease.
A considerable number of studies have documented that low levels of the adrenal steroids, DHEA, and DHEA-S, are associated with risk factors for MS, such as insulin resistance and obesity35, 40, 41. In contrast, DHEA administration led to beneficial effects in obesity-related MS parameters in elderly women42. In line with these studies, we observed alterations in the serum levels of DHEA-S in response to AE and BE interventions. However, these changes were relatively small, and only BE resulted in a clear elevation of DHEA-S levels compared to control subjects. Although we did not detect any significant change in DHEA-S due to the intervention, DHEA-S showed a positive correlation with Δ-lean body mass (Fig. 3h). Considering that DHEA can be converted to testosterone and estradiol43, our findings suggest that changes in the circulating levels of these hormones may contribute to the beneficial effects of AE and BE on abdominal obesity-related MS.
MS is generally caused by a number of pathophysiological mechanisms combined with lifestyle-related factors, such as unhealthy dietary patterns and lack of physical activity44. Among the consequences of the aforementioned lifestyle changes, abdominal obesity is the most predominant causative factor45. Moreover, sex hormone imbalance in the adipose tissue is involved in MS pathophysiology35. We therefore analyzed several parameters to assess the effects of combined BE and AE intervention on the status of abdominal obesity. The percentage of abdominal fat was decreased after AE intervention and waist circumference increased in CON group, but not in the intervention groups (Table 1), suggesting that AE and/or BE had positive effects in inhibiting an increase of MS-related risk factors, particularly abdominal obesity.
A number of studies have investigated the effects of dietary supplements on sex hormones related to conditions of age as well as noncommunicable diseases, because nutrients have important roles for hormone metabolism in the endocrine system46. Furthermore, sex-related endogenous hormones are associated with adiposity in postmenopausal women with diabetes47. A clinical study showed that administration of DHEA had beneficial effects on abdominal fat and insulin sensitivity in elderly subjects with MS48. Supplementation with nutrients containing vitamin E, selenium, vitamin C, and coenzyme Q10 showed no overall significant effects on sex-related hormones49, and vitamin E supplementation alone in elderly subjects did not affect serum levels of DHEA-S50. However, we observed that BE intervention significantly increased DHEA-S levels (Fig. 2d). Although it is uncertain what nutritional component from BE induces the increase in DHEA-S levels, BE supplementation may have similar effects as DHEA replacement therapy, in addition to reducing insulin resistance.
Although our study provides novel lifestyle-based insights into MS management in elderly women, the following limitations must be acknowledged. First, the small sample size does not allow for generalization. However, this study may be an important conceptual basis to explore the combined effects of AE and BE on elderly women with MS in future, larger experiments. Second, we did not investigate dose–response BE effects. Therefore, additional positive effects of BE may be expected from the application of higher BE doses. Further studies are warranted to address these issues.
Conclusions
In summary, this study is the first to investigate the effects of a combination of AE plus the natural dietary component, BE, on body composition and sex hormones in elderly women with MS. Both AE and BE independently improved abdominal fat and waist circumference, and altered the serum levels of sex hormones, such as testosterone and estradiol, which in turn are known to be implicated in central obesity-related MS in elderly women. However, contrary to our hypothesis, the combination of AE and BE did not produce any additive or synergistic effect on the investigated parameters.
Materials and methods
Study participants
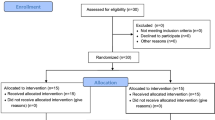
Thirty-two elderly women volunteers with MS from South Korea were initially enrolled (average age: 74.31 ± 5.2). Twenty-four subjects (average age: 75.25 ± 4.96) were finally selected for the 16-week experiment and categorized as follows: (1) control group (CON: n = 7), (2) aquatic exercise group (AE: n = 6), (3) burdock root extract ingestion group (BE: n = 5), and (4) combination of aquatic exercise and burdock root extract ingestion group (AE + BE: n = 6). The baseline characteristics of study participants are shown in Table 1. Before commencement of the study, the participants were informed of the research purpose and intentions, and informed consent forms were obtained from all participants and approved by the National Bioethics Committee of the Pusan National University (PNU IRB/2015_22), in accordance with the Declaration of Helsinki Declaration and the 2010 Consolidated Standards of Reporting Trials statement51. This trial was retrospectively registered in the University Hospital Medical Information Network Clinical Trial Registry (Japan, registration 15/04/2020 UMIN000040170).
Study design
A 16-week intervention study comprising four randomized, double-blind controlled trials investigating the effect of dietary BE supplementation, with or without exercise intervention, was conducted. Pre- and post-tests were performed at the same time each day to minimize temperature-related changes. MS diagnosis was based on the NCEP-ATP III guidelines, recommending the presence of at least three of the following six criteria28. After baseline measurements, all participants were randomly divided into four groups. The following parameters were tested before and after the 16-week intervention: body composition (weight, BMI, % body fat, fat body mass, lean body mass, skeletal muscle mass, % abdominal fat, and waist circumference), and circulating hormones (DHEA-S, SHBG, testosterone, estradiol). For a comprehensive evaluation, the lifestyle habits of the subjects were also monitored by the researchers, and in particular, the control group was encouraged to maintain their usual lifestyle.
Aquatic exercise protocol
We previously reported that aquatic exercise enhanced fitness factors and vascular function in older adults18, 26. Thus, we used the same protocol to the current study. The aquatic exercise program was based on recommendations of the American College of Sports Medicine52, and was scheduled considering the age of subjects. Exercise was performed three times per week for 16-weeks, following a 1–6-week adjustment period at a swimming pool. The program consisted of a 5-min warm-up and a 5-min cool-down exercise session, and a 40-min main exercise session with individualized loads corresponding to 30–40% heart rate reserve (HRR) at a rating of perceived exertion (RPE) of 9–10 for weeks 1–5, 40–50% HRR (RPE 11–12) for weeks 6–10, and 50–60% HRR (RPE 13–14) for weeks 11–16. Heart rates were monitored using a heart rate monitor watch (Polar RS400sd; model APAC, 90026360; Polar, NY, USA) and Borg’s RPE53 was checked twice during the exercise session.
Burdock root extract sampling and ingestion
BE samples were prepared based on methods optimized and described in a previous study18, 26. After the addition of 4 kg of fresh burdock root harvested in the Sancheong region (Gyeongnam, South Korea) and 6000 mL of water to an extractor, extraction was performed for 3 h at 100 °C at a pressure of 0.7 kg/cm2. The main ingredients of BE were water (98.02% ± 0.02%), crude ash (0.10% ± 0.00%), crude fat (1.12% ± 0.00%), crude protein (0.20% ± 0.00%), crude fiber (0.03%), calcium (0.004% ± 0.00%), and phosphorus (0.009% ± 0.00%) (Pukyong National University Feed & Foods Nutrition Research Center, Busan, South Korea). BE administration schedule was based on the advice of an oriental medical doctor. Specifically, the participants consumed one 100-mL dose of BE 3 times a day after each meal (breakfast, lunch, and dinner), for a total of 300 mL of BE per day for 16 weeks.
Body composition and blood biochemical analysis
Participants were advised to refrain from eating after 8:00 p.m. on the day before the test, and the test was performed between 8:00 and 9:00 a.m. according to the procedures recommended by the American College of Sports Medicine54. Bioelectrical impedance, measured with the Inbody 720 device (Biospace, Seoul, Korea), was used to assess body composition. The study participants were instructed to assume a comfortable standing position with their feet slightly apart on the instrument while wearing casual clothing; all metal objects were removed. Blood samples were collected using EDTA tubes and needles at two time points, i.e., before and after the 16-week intervention. After collecting 10 mL of blood from an antebrachial vein, the serum was isolated for analysis of sex hormones.
Statistical analysis
The required sample size was calculated using the G-power version 3.1 Windows program (Kiel University, Kiel, Germany), based on a 0.25-point effect size (default), an alpha level of 0.05, and 40% power55. The results indicated that 20 participants were requisite for the study; assuming a dropout rate of 25%, the sample size was set to 32 participants. All data were expressed as mean ± standard deviation (SD). Two-way repeated ANOVA was performed to evaluate the differences between groups and time for absolute value of body composition and sex hormones, followed by Bonferroni’s multiple comparison tests for post-hoc analysis. One-way ANOVA with Dunnett’s multiple comparison tests was used to analyze the delta (Δ) change. Correlations between body composition and sex hormones were calculated by Pearson’s correlation analysis. A p < 0.05 was considered statistically significant. Effect sizes (Cohen's d) between pre- and post-test data were expressed as mean changes56.
Ethical approval
All procedures and protocols performed in studies involving human participants were by the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and the 2010 Consolidated Standards of Reporting Trials statement46 were approved by the National Bioethics Committee of Pusan National University (PNU IRB/2015_22). This trial was retrospectively registered in the University Hospital Medical Information Network Clinical Trial Registry (Japan, registration 15/04/2020 UMIN000040170).
Data availability
Data and publication materials are available upon request.
Abbreviations
- MS:
-
Metabolic syndrome
- AE:
-
Aquatic exercise
- BE:
-
Burdock root extract
- DHEA-S:
-
Dehydroepiandrosterone sulfate
- SHBG:
-
Sex hormone-binding globulin
References
Butler, J. et al. Metabolic syndrome and the risk of cardiovascular disease in older adults. J. Am. Coll. Cardiol. 47, 1595–1602 (2006).
Ginsberg, H. N. & MacCallum, P. R. The obesity, metabolic syndrome, and type 2 diabetes mellitus pandemic: Part I. Increased cardiovascular disease risk and the importance of atherogenic dyslipidemia in persons with the metabolic syndrome and type 2 diabetes mellitus. J. Cardiometab. Syndr. 4, 113–119 (2009).
Ford, E. S., Giles, W. H. & Dietz, W. H. Prevalence of the metabolic syndrome among US adults: Findings from the Third National Health and Nutrition Examination Survey. JAMA 287, 356–359 (2002).
Saad, M. A. N., Cardoso, G. P., de Martins, W. A., Velarde, L. G. C. & Filho, R. A. D. C. Prevalence of metabolic syndrome in elderly and agreement among four diagnostic criteria. Arq. Bras. Cardiol. 102, 263–269 (2014).
Kim, S. & So, W.-Y. Prevalence and correlates of metabolic syndrome and its components in elderly Korean adults. Exp. Gerontol. 84, 1–6 (2015).
Kangas, P. et al. Changes in hemodynamics associated with metabolic syndrome are more pronounced in women than in men. Sci. Rep. 9, 1–11 (2019).
Kim, C. & Halter, J. B. Endogenous sex hormones, metabolic syndrome, and diabetes in men and women. Curr. Cardiol. Rep. 16, S62-20 (2014).
Labrie, F., Luu-The, V., Labrie, C. & Simard, J. DHEA and its transformation into androgens and estrogens in peripheral target tissues: Intracrinology. Front. Neuroendocrinol. 22, 185–212 (2001).
Barrett-Connor, E. & Ferrara, A. Dehydroepiandrosterone, dehydroepiandrosterone sulfate, obesity, waist-hip ratio, and noninsulin-dependent diabetes in postmenopausal women: The Rancho Bernardo Study. J. Clin. Endocrinol. Metab. 81, 59–64 (1996).
Weinberg, M. E. et al. Low sex hormone-binding globulin is associated with the metabolic syndrome in postmenopausal women. Metabolism 55, 1473–1480 (2006).
Yamaoka, K. & Tango, T. Effects of lifestyle modification on metabolic syndrome: A systematic review and meta-analysis. BMC Med. 10, 1–10 (2012).
Ostman, C. et al. The effect of exercise training on clinical outcomes in patients with the metabolic syndrome: A systematic review and meta-analysis. Cardiovasc. Diabetol. 16, 110 (2017).
Myers, J., Kokkinos, P. & Nyelin, E. Physical activity, cardiorespiratory fitness, and the metabolic syndrome. Nutrients 11, 1652 (2019).
Ennour-Idrissi, K., Maunsell, E. & Diorio, C. Effect of physical activity on sex hormones in women: A systematic review and meta-analysis of randomized controlled trials. Breast Cancer Res. 17, 139–141 (2015).
Copeland, J. L., Consitt, L. A. & Tremblay, M. S. Hormonal responses to endurance and resistance exercise in females aged 19–69 years. J. Gerontol. A Biol. Sci. Med. Sci. 57, B158–B165 (2002).
Kim, J.-W. & Kim, D.-Y. Effects of aerobic exercise training on serum sex hormone binding globulin, body fat index, and metabolic syndrome factors in obese postmenopausal women. Metab. Syndr. Relat. Disord. 10, 452–457 (2012).
Ketabipoor, S. M. & Bulletin, M. J. W. Effect of aerobic exercise in water on serum estrogen and C-reactive protein and body mass index level in obese and normal weight postmenopausal women. Women's Health Bull. 2, e25048.
Ha, M.-S., Kim, J.-H., Ha, S.-M., Kim, Y.-S. & Kim, D.-Y. Positive influence of aqua exercise and burdock extract intake on fitness factors and vascular regulation substances in elderly. J. Clin. Biochem. Nutr. 64, 73–78 (2019).
Anderssen, S. A., Carroll, S., Urdal, P. & Holme, I. Combined diet and exercise intervention reverses the metabolic syndrome in middle-aged males: Results from the Oslo Diet and Exercise Study. Scand. J. Med. Sci. Sports 17, 687–695 (2007).
Barnes, J., Anderson, L. A. & Phillipson, J. D. Herbal Medicines (Pharmaceutical Press, London, 2007).
Ferracane, R., Graziani, G., Gallo, M., Fogliano, V. & Ritieni, A. Metabolic profile of the bioactive compounds of burdock (Arctium lappa) seeds, roots and leaves. J. Pharm. Biomed. Anal. 51, 399–404 (2010).
Chan, Y.-S. et al. A review of the pharmacological effects of Arctium lappa (burdock). Inflammopharmacol 19, 245–254 (2010).
Ahangarpour, A. et al. Antidiabetic, hypolipidemic and hepatoprotective effects of Arctium lappa root’s hydro-alcoholic extract on nicotinamide-streptozotocin induced type 2 model of diabetes in male mice. Avicenna J. Phytomed. 7, 169–179 (2017).
Annunziata, G. et al. Arctium lappacontributes to the management of type 2 diabetes mellitus by regulating glucose homeostasis and improving oxidative stress: A critical review of in vitro and in vivo animal-based studies. Phytother. Res. 33, 2213–2220 (2019).
Min, B., Lee, H., Song, J. H., Han, M. J. & Chung, J. Arctiin inhibits adipogenesis in 3T3-L1 cells and decreases adiposity and body weight in mice fed a high-fat diet. Nutr. Res. Pract. 8, 655–657 (2014).
Ha, M.-S., Kim, J.-H., Kim, Y.-S. & Kim, D.-Y. Effects of aquarobic exercise and burdock intake on serum blood lipids and vascular elasticity in Korean elderly women. Exp. Gerontol. 101, 63–68 (2018).
Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA 285, 2486–2497 (2001).
Xu, T., Liu, J., Liu, J., Zhu, G. & Han, S. Relation between metabolic syndrome and body compositions among Chinese adolescents and adults from a large-scale population survey. BMC Public Health 17, 337 (2017).
Farinha, J. B. et al. Response of oxidative stress and inflammatory biomarkers to a 12-week aerobic exercise training in women with metabolic syndrome. Sports Med. Open 1, 19–10 (2015).
Carr, D. B. et al. Intra-abdominal fat is a major determinant of the National Cholesterol Education Program Adult Treatment Panel III criteria for the metabolic syndrome. Diabetes 53, 2087–2094 (2004).
Yoon, Y. S. & Oh, S. W. Optimal waist circumference cutoff values for the diagnosis of abdominal obesity in Korean Adults. Endocrinol. Metab. 29, 418–426 (2014).
Yoo, Y.-K., Kim, S.-K. & Song, M.-S. Effects of muscular and aqua aerobic combined exercise on metabolic indices in elderly women with metabolic syndrome. J. Exerc. Nutr. Biochem. 17, 133–141 (2013).
Ziaei, S. & Mohseni, H. Correlation between hormonal statuses and metabolic syndrome in postmenopausal women. J. Family Reprod. Health 7, 63–66 (2013).
McTiernan, A. et al. Effect of exercise on serum androgens in postmenopausal women: A 12-month randomized clinical trial. Cancer Epidemiol. Biomark. Prev. 13, 1099–1105 (2004).
Muller, M., Grobbee, D. E., den Tonkelaar, I., Lamberts, S. W. J. & van der Schouw, Y. T. Endogenous sex hormones and metabolic syndrome in aging men. J. Clin. Endocrinol. Metab. 90, 2618–2623 (2005).
Hong, D. et al. Total testosterone and sex hormone-binding globulin are associated with metabolic syndrome independent of age and body mass index in Korean men. Maturitas 74, 148–153 (2013).
Yamazaki, H. et al. Protective effect of sex hormone-binding globulin against metabolic syndrome: In vitro evidence showing anti-inflammatory and lipolytic effects on adipocytes and macrophages. Mediators Inflamm. 2018, 3062319 (2018).
Saéz-López, C. et al. Sex hormone-binding globulin reduction in metabolic disorders May Play a role in NAFLD development. Endocrinology 158, 545–559 (2017).
Saéz-López, C., Rivera-Giménez, M., Hernández, C., Simó, R. & Selva, D. M. SHBG-C57BL/ksJ-db/db: A new mouse model to study SHBG expression and regulation during obesity development. Endocrinology 156, 4571–4581 (2015).
Nestler, J. E., Barlascini, C. O., Clore, J. N. & Blackard, W. G. Dehydroepiandrosterone reduces serum low density lipoprotein levels and body fat but does not alter insulin sensitivity in normal men. J. Clin. Endocrinol. Metab. 66, 57–61 (1988).
Tchernof, A. & Labrie, F. Dehydroepiandrosterone, obesity and cardiovascular disease risk: A review of human studies. Eur. J. Endocrinol. 151, 1–14 (2004).
Gómez-Santos, C., Hernández-Morante, J. J., Tébar, F. J., Granero, E. & Garaulet, M. Differential effect of oral dehydroepiandrosterone-sulphate on metabolic syndrome features in pre- and postmenopausal obese women. Clin. Endocrinol. (Oxf.) 77, 548–554 (2012).
Simard, J. & Gingras, S. Crucial role of cytokines in sex steroid formation in normal and tumoral tissues. Mol. Cell Endocrinol. 171, 25–40 (2001).
van Namen, M., Prendergast, L. & Peiris, C. Supervised lifestyle intervention for people with metabolic syndrome improves outcomes and reduces individual risk factors of metabolic syndrome: A systematic review and meta-analysis. Metabolism 101, 153988–154013 (2019).
Després, J.-P. & Lemieux, I. Abdominal obesity and metabolic syndrome. Nature 444, 881–887 (2006).
Janjuha, R. et al. Effects of dietary or supplementary micronutrients on sex hormones and IGF-1 in middle and older age: A systematic review and meta-analysis. Nutrients 12, 1457–1521 (2020).
Kalyani, R. R. et al. The association of endogenous sex hormones, adiposity, and insulin resistance with incident diabetes in postmenopausal women. J. Clin. Endocrinol. Metab. 94, 4127–4135 (2009).
Villareal, D. T. & Holloszy, J. O. Effect of DHEA on abdominal fat and insulin action in elderly women and men: A randomized controlled trial. JAMA 292, 2243–2248 (2004).
Hoenjet, K. M. J. L. F. et al. Effect of a nutritional supplement containing vitamin E, selenium, vitamin c and coenzyme Q10 on serum PSA in patients with hormonally untreated carcinoma of the prostate: A randomised placebo-controlled study. Eur. Urol. 47, 433–439 (2005).
van Amsterdam, J. et al. The effect of vitamin E supplementation on serum DHEA and neopterin levels in elderly subjects. Int. J. Vitam. Nutr. Res. 75, 327–331 (2005).
Moher, D. et al. CONSORT 2010 explanation and elaboration: Updated guidelines for reporting parallel group randomised trials. Int. J. Surg. 340, c869–c869 (2010).
Chodzko-Zajko, W. J. et al. Exercise and physical activity for older adults. Med. Sci. Sports Exerc. 41, 1510–1530 (2009).
Borg, G. A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 14, 377–381 (1982).
Thompson, P. D., Arena, R., Riebe, D. & Pescatello, L. S. American College of Sports Medicine. ACSM‘s new preparticipation health screening recommendations from ACSM’s guidelines for exercise testing and prescription, ninth edition. Curr. Sports Med. Rep. 12, 215–217 (2013).
Faul, F., Erdfelder, E., Lang, A.-G. & Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 39, 175–191 (2007).
Cohen, J. Statistical Power Analysis for the Behavioral Sciences (Routledge, New York, 1988).
Acknowledgements
The authors are grateful to the research participants for their hard work and cooperation throughout the research process. This research has been partly supported by ARIHHP research Grants of 2019 and 2020 (H.S.), University of Tsukuba and the JST-Mirai Program 2019–2021 (H.S.).
Author information
Authors and Affiliations
Contributions
Conceptualization, M.-S.H. and J.S.Y.; methodology, M.-S.H.; software, M.-S.H. and J.S.Y.; validation, M.-S.H., J.S.Y. and M.C.L.; formal analysis, M.-S.H., J.S.Y. and M.C.L.; investigation, M.-S.H. and W.-M.J.; resources, M.-S.H. and W.-M.J.; data curation, M.-S.H. and J.-J.K.; writing—original draft preparation, M.-S.H. and J.S.Y.; writing—review and editing, K.S. and H.S.; visualization, J.S.Y.; supervision, H.S.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ha, MS., Yook, J.S., Lee, M. et al. Exercise training and burdock root (Arctium lappa L.) extract independently improve abdominal obesity and sex hormones in elderly women with metabolic syndrome. Sci Rep 11, 5175 (2021). https://doi.org/10.1038/s41598-021-84301-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-84301-x
This article is cited by
-
Variations in phytochemical traits, total carbohydrate, and antioxidant activity of Iranian wild populations of greater burdock (Arctium lappa L.)
Genetic Resources and Crop Evolution (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.