Abstract
Ovarian cancer (OC) is one of the most common gynecologic cancer, which has the worst prognosis and highest mortality rate. The lack of curative treatment and the high relapse rate, especially in advanced OC, continues to present a clinical challenge, highlighting the need for new therapeutic strategies. This study was performed to compare the expression of PD-L1 in primary epithelial ovarian cancer (EOC) and their corresponding peritoneal metastases, as well as to evaluate its correlation with clinico-pathological parameters. In total, 194 treatment naïve paired EOC and peritoneal metastasis were analyzed by immunohistochemistry for PD-L1 expression. Clinico-pathological information was available for all patients. Significant differences in PD-L1 expression were found between primary EOC and peritoneal metastasis (p < 0.0001). We found discordant tumor cell PD-L1 expression between primary tumors and corresponding peritoneal metastasis in 34% (66/194) of cases. Furthermore, PD-L1 expression in peritoneal metastasis samples was significantly associated with adverse prognostic factors, such as high proliferative index (Ki67) (p = 0.0039) and high histologic grade (p = 0.0330). In conclusion, the discordance of PD-L1 expression between primary EOC and corresponding peritoneal metastases suggests that its assessment as a potential biomarker for predicting response to anti-PD-L1 therapy may require analysis of metastatic lesions.
Similar content being viewed by others
Introduction
Ovarian cancer (OC) remains the deadliest gynecological malignancy, accounting for ~ 5% of all death from cancer in women1,2. Epithelial ovarian cancers (EOC) are the most common histological subtype, comprising > 95% of OCs3. Majority of the patients with EOC have advanced stage disease at diagnosis, with metastatic lesions, due to absence of specific clinical symptoms and lack of early screening programs4,5,6. Most currently available treatments are not curative for patients with advanced disease, which could explain the low five-year survival rate of less than 30%7. Hence, there is a need for more effective systemic therapies for the management of advanced EOC.
Programmed cell death ligand 1 (PD-L1) has attracted attention as a novel therapeutic target in the context of successful trials in many cancer types8,9,10. PD-1/PD-L1 pathway is considered a critical immune modulatory pathway that inhibits the immune reaction to cancer cells by negatively regulating T-cell functions11,12. Blockade of PD-1/PD-L1 signaling pathway using targeted monoclonal antibodies has become a promising therapeutic modality in cancers, with encouraging anti-tumor activity and an increased survival in several cancers13. Similarly, it has been shown that PD-L1 inhibitors play an important role in the adjuvant therapy of advanced and treatment-resistant OC14,15. Ongoing clinical trials are investigating the efficacy and safety of anti-PD-L1 antibodies in recurrent advanced OC16,17.
The immunohistochemical expression of PD-L1 as a prognostic marker and/or predictor of curative effect of anti-PD-L1 therapy has been investigated in various malignancies including OC18,19,20,21,22,23,24. However, only a few studies have investigated how PD-L1 expression may vary throughout primary tumors or in the primary tumor versus the corresponding metastases25,26,27,28.
This information can expand the potential predictive value for this biomarker and determine whether the expression of PD-L1 is likely to be more informative in primary tumor tissue or from metastatic site. For this reason, we investigated PD-L1 expression in a series of treatment naïve primary EOC and corresponding peritoneal metastasis. Moreover, we also investigated the correlation between PD-L1 expression status and several important clinico-pathological parameters in EOC from Middle-Eastern ethnicity.
Results
Patient characteristics
Median age of the study cohort was 54.5 years (range 19–90 years). High-grade serous carcinoma was the most common histologic subtype, accounting for 64.4% (125/194) of all EOCs. Majority of the patients presented with high FIGO grade (Grade 3–49%; 95/194) and advanced stage (Stage III and IV—91.8%; 178/194) tumors (Table 1).
Distribution of PD-L1 in primary EOC and paired peritoneal metastases
PD-L1 expression was analysed in 194 treatment naïve paired primary EOC and peritoneal metastases tissues using tissue microarray (TMA). Positive expression of PD-L1 in primary tumor and matched peritoneal metastases was 32.5% (63/194) and 45.9% (89/194), respectively. Importantly, the difference in expression of PD-L1 between the primary tumor and paired peritoneal metastases was statistically significant (p < 0.0001) (Table 2, Fig. 1A–D). Among the 63 cases showing positive PD-L1 expression in primary tumor, 43 also had positive expression of PD-L1 in the paired peritoneal metastasis, whereas 20 cases were negative. Of the 131 cases with negative PD-L1 expression in primary tumor, 85 also had negative expression of PD-L1 in the paired peritoneal metastasis and 46 cases were positive for PD-L1 (Table 2). Thus, the concordance rate of PD-L1 expression was 66.0% (128/194). A discrepancy between the primary tumor and metastatic tissue was noted in 34.0% (66/194) cases.
Immunohistochemical analysis of PD-L1 expression in primary EOC and corresponding peritoneal metastasis. EOC array spots showing positive (A) and negative (B) expression of PD-L1 in primary tumor, with the corresponding peritoneal metastatic tissue showing negative (C) and positive (D) expression of PD-L1. 20X/0.70 objective on an Olympus BX 51 microscope. (Olympus America Inc, Center Valley, PA, USA) with the inset showing a 40X 0.85 aperture magnified view of the same TMA spot.
Clinico-pathological associations of PD-L1 expression in primary EOC and paired peritoneal metastases
The associations between PD-L1 expression and clinico-pathological parameters was analysed in the primary tumor and their matched peritoneal metastases. In the primary EOCs, positive PD-L1 expression was associated with lymph node metastasis (p = 0.0112). PD-L1 expression in metastatic tissues was associated with grade 3 tumors (p = 0.0330) and high Ki-67 index (p = 0.0039) (Table 3). Interestingly, PD-L1 expression was not associated with mismatch repair deficiency (dMMR) in both primary tumor as well as peritoneal metastases.
Prognostic impact of PD-L1 expression in primary EOC and paired peritoneal metastases
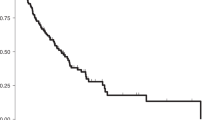
We evaluated the effect of PD-L1 expression on progression-free survival (PFS), overall survival (OS) and disease-specific survival (DSS). PD-L1 expression in both primary tumor and peritoneal metastases was not significantly associated with PFS, OS or DSS (Fig. 2). In the primary EOC tissues, patients with PD-L1 positive tumors (n = 63) had a median follow-up of 22 months (range: 2–153 months) and experienced 43 progression events, whereas patients with PD-L1 negative tumors (n = 131) had a median follow-up of 21 months (range: 2–237 months) and experienced 86 progression events. In the peritoneal metastases tissues, patients with PD-L1 positive tumors (n = 89) had a median follow-up of 22 months (range: 2–237 months) and experienced 54 progression events, whereas patients with PD-L1 negative tumors (n = 105) had a median follow-up of 20 months (range: 2–199 months) and experienced 75 progression events. On multivariate analysis using Cox proportional hazard model for PFS, only stage of tumor was an independent prognostic marker (Table 4).
Survival analysis of PD-L1 protein expression in epithelial ovarian cancer. Kaplan Meier survival plot showing no statistically significant difference between PD-L1 positive and negative tumors in both primary and corresponding peritoneal metastases for progression-free survival (A,B), overall survival (C,D) as well as disease specific survival (E,F).
It is well known that the different histological types of ovarian cancer represent different diseases. Since high-grade serous carcinomas were the predominant histologic subtype in our cohort, we analysed PFS with respect to PD-L1 expression in this subset of patients. Again, PD-L1 expression in both primary tumor and peritoneal metastases was not significantly associated with PFS (Fig. 3).
Discussion
Very promising results have been obtained with immunotherapeutic agents that target PD-1/PD-L1 pathway29,30,31,32. PD-L1 is a crucial immune regulatory factor, and as a receptor for PD-1, it plays an important role in the immune escape mechanism of cancer cells12,33. It is well known that binding of PD-1 with its ligand, PD-L1 impair T-cell activation and differentiation, and there is evidence that tumor-infiltrating immune cells induce cytokines that upregulate PD-L1 expression33,34,35.
We found PD-L1 positivity in 32.5% (63/194) of primary EOCs. PD-L1 expression was significantly associated with lymph node metastasis. Several previous studies have reported on the clinical associations of PD-L1 expression in ovarian cancer patients, but still have not reached consensus. While some studies found PD-L1 expression in OC to be associated with aggressive clinico-pathological features such as higher tumor stage, grade and poor survival21,36,37,38, others have failed to demonstrate this association39,40. Interestingly, a previous meta-analysis has revealed the effect of patients’ ethnicity on prognostic value of PD-L1 expression in OC. Huang et.al. found that PD-L1 expression is a poor prognostic biomarker in Asian population in contrast to the non-Asian patients with OC where PD-L1 is a good prognostic marker41. Unfortunately, PD-L1 expression did not affect the progression-free survival in EOC from the Middle-Eastern ethnicity, which could be due to the inherent biases of the study as discussed later. Also, assessment of PD-L1 expression in immune infiltrates, in addition to tumor cells, might provide a clearer picture with regards to prognosis, as shown by previous studies38,42. However, use of tissue microarray in our study precluded adequate assessment of PD-L1 expression in immune infiltrates.
Recent studies have highlighted the important role of PD-L1 inhibition in the treatment of OC43,44. However, recent evidence has shown that immunohistochemistry (IHC) staining of PD-L1 in OC specimens may not identify all patients who might respond to anti-PD-L1 agents. Indeed, up to 8% of patients with PD-L1 negative tumors were found to have objective response to treatment with anti-PD-L1 agent (Avelumab), whereas many patients who had PD-L1 positive tumors failed to respond16. Another Phase I clinical trial in advanced /recurrent ovarian cancer found that only 25% (2 /8) of patients with PD-L1 positive tumors showed response to Atezolizumab17. A possible explanation could be the effect of tumor heterogeneity on the predictive value of PD-L1 expression. Given the high tumor heterogeneity in OC, testing for PD-L1 in primary tumors alone may not be an accurate reflection of the biology of metastatic tumors that need to be targeted with immunotherapy. Consistent with this hypothesis, previous reports have found discordance between the primary and metastatic tumors in several cancers such as melanoma, renal cell carcinoma and breast cancer25,26,27.
We compared the PD-L1 expression between primary tumor and peritoneal metastasis to evaluate if intra-patient heterogeneity exists in EOC patients. Peritoneum is usually the initial and most common site of metastasis in OC45. The presence of peritoneal metastasis is important for staging, treatment and prognosis of OC patients46,47. In our study, we found discordant tumor cell PD-L1 expression between primary tumors and corresponding peritoneal metastasis in a high proportion of cases (34%). Gottlieb and colleagues28 also compared the concordance rate of PD-L1 expression in the primary ovarian tumors and their matched metastatic deposits from predominantly treatment naïve high grade serous ovarian carcinoma from 21 patients. In contrast to our study, they found a relatively high concordance of PD-L1 expression (76.2%; 16/21) between the two tissues.
In the present study, we found a higher proportion of peritoneal metastatic tumors showing PD-L1 expression compared to primary EOC (p < 0.0001). This suggests the importance of analyzing tissue from metastatic lesions when assessing the predictive value of PD-L1 expression in EOC. Prospective clinical trials might provide further insight and help in selecting patients who could respond to immunotherapy.
Our study also highlights the association between PD-L1 expression and several critical clinico-pathological characteristics in the primary EOC and their matched peritoneal metastasis. We found PD-L1 expression to be associated with aggressive markers such as lymph node metastasis in primary EOC and high Ki-67 index and high grade tumors in metastatic tissues. Although no significant correlation was observed between PD-L1 expression and clinical outcome, the significant association with aggressive clinico-pathological parameters might indirectly suggest a similar association. Furthermore, although studies have previously shown an association between PD-L1 expression and microsatellite instability status48,49, we did not find a similar association in our cohort. This could be partly explained by the very low incidence of dMMR in our cohort (1.6%; 3/194). Also previous studies have shown dMMR to be more common in clear cell OC and associated with PD-L1 in this subset of OC48,50, whereas our cohort had only three cases of clear cell carcinoma. This may have contributed to the lack of association between PD-L1 and dMMR in our study.
While this study provides important information with potential impact in clinical practice for EOC from Middle Eastern ethnicity, it has a few limitations. Firstly, patients were enrolled over a long period of time (28 years), during which the surgical and therapeutic approach may have changed, leading to treatment bias. Secondly, preserved antigenicity and better fixation of peritoneal implants could be a technical confounder leading to higher expression of PD-L1 in peritoneal metastases. However, this is more pronounced in biopsy samples, whereas all our peritoneal metastasis samples were surgically resected specimens, which mitigates this confounding effect to an extent. Thirdly, PD-L1 expression should have ideally been assessed both on tumor cells and immune infiltrates. However, our focus was on the differential expression of PD-L1 between primary tumor and corresponding peritoneal metastases. Hence, PD-L1 expression in immune infiltrates was not assessed.
In conclusion, the discordance in PD-L1 expression between the primary EOC and the matched peritoneal metastasis observed in our study suggests that testing for PD-L1 expression in both metastatic tumors and primary EOC could increase the predictive role of PD-L1 for responders to immunotherapy in these patients.
Methods
Sample selection
One-hundred and ninety-four EOC patients diagnosed between 1989 to 2017 at King Faisal Specialist Hospital and Research Center (Riyadh, Saudi Arabia) with available primary and peritoneal metastases archival tissue samples were included in the study. All the patients were treatment-naïve. Primary tumor samples and the corresponding peritoneal metastases were collected at the same time for all the cases. Clinico-pathological data were collected from case records, the details of which are summarized in Table 1. Progression-free survival was computed from date of surgery for patients who underwent primary cytoreduction to date of disease progression or death from any cause. The median follow-up time was 21 months (range, 2–237 months). Tumors were classified according to WHO Classification of female genital tumors (2020). International Federation of Gynecology and Obstetrics (FIGO) system was used for staging and grading of tumors.
Ethics declarations
Institutional Review Board of King Faisal Specialist Hospital and Research Centre provided ethical approval for the current study. Research Advisory Council (RAC) granted waiver of informed consent for use of retrospective patient case data under project RAC# 2190 015. All the methods were carried out in accordance with relevant guidelines and regulations.
Tissue microarray (TMA) construction and immunohistochemistry (IHC)
Tissue microarray (TMA) format was utilized for immunohistochemical analysis of the EOC samples. TMA was constructed as previously described51. Briefly, modified semiautomatic robotic precision instrument (Beecher Instruments, Woodland, WI) was used to punch tissue cylinders with a diameter of 0.6 mm from representative tumor area of the donor tissue block and brought into the recipient paraffin block. Two 0.6-mm cores of EOC were arrayed from each case.
Tissue microarray slides were processed and stained manually as described previously52. Primary antibody against PD-L1 (E1LN3, 1:100 dilution, pH 9.0, Cell Signaling Technology, Danvers, MA) was used. A membranous and/or cytoplasmic staining was observed. Only the membrane staining was considered for scoring. PD-L1 was scored as described previously39. Briefly, the proportion of positively stained cells was calculated as a percentage for each core and the scores were averaged across two tissue cores from the same tumor to yield a single percent staining score representing each cancer patient. For the purpose of statistical analysis, the scores were dichotomised. Cases showing expression level of ≥ 5% were classified as positive and those with less than 5% as negative.
Mismatch repair (MMR) protein as well as Ki-67 staining and evaluation was done as described previously53,54. MMR protein expression was evaluated using MSH2, MSH6, MLH1 and PMS2 proteins. Tumor was classified as deficient MMR (dMMR) if any of the four proteins showed loss of staining in cancer with concurrent positive staining in the nuclei of normal epithelial cells. Otherwise, they were classified as proficient MMR (pMMR). For Ki-67, nuclear staining was considered as positive. The cutoff for high Ki-67 was taken as more than 30% of tumor nuclei staining in the total tumor area.
IHC scoring was done by two pathologists, blinded to the clinico-pathological characteristics. Discordant scores were reviewed together to achieve agreement.
Statistical analysis
The associations between clinico-pathological variables and protein expression was performed using contingency table analysis and Chi square tests. Mantel-Cox log-rank test was used to evaluated progression-free survival. Two-sided tests were used for statistical analyses with a limit of significance defined as p value < 0.05. Data analyses was performed using the JMP11.0 (SAS Institute, Inc., Cary, NC) software package.
References
Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424 (2018).
Malvezzi, M., Carioli, G., Rodriguez, T., Negri, E. & La Vecchia, C. Global trends and predictions in ovarian cancer mortality. Ann. Oncol. 27, 2017–2025 (2016).
Lheureux, S., Braunstein, M. & Oza, A. M. Epithelial ovarian cancer: evolution of management in the era of precision medicine. CA Cancer J. Clin. 69, 280–304 (2019).
Torre, L. A. et al. Ovarian cancer statistics, 2018. CA Cancer J. Clin. 68, 284–296 (2018).
Abiko, K. et al. PD-L1 on tumor cells is induced in ascites and promotes peritoneal dissemination of ovarian cancer through CTL dysfunction. Clin. Cancer Res. 19, 1363–1374 (2013).
Narod, S. Can advanced-stage ovarian cancer be cured?. Nat. Rev. Clin. Oncol. 13, 255 (2016).
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 69, 7–34 (2019).
Darvin, P., Toor, S. M., Nair, V. S. & Elkord, E. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp. Mol. Med. 50, 1–11 (2018).
Larkin, J. et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N. Engl. J. Med. 373, 23–34 (2015).
Brahmer, J. et al. Nivolumab versus docetaxel in advanced squamous-cell non–small-cell lung cancer. N. Engl. J. Med. 373, 123–135 (2015).
Topalian, S. L., Taube, J. M., Anders, R. A. & Pardoll, D. M. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat. Rev. Cancer 16, 275–287 (2016).
Chen, J., Jiang, C., Jin, L. & Zhang, X. Regulation of PD-L1: a novel role of pro-survival signalling in cancer. Ann. Oncol. 27, 409–416 (2016).
Zou, W., Wolchok, J. D. & Chen, L. PD-L1 (B7–H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci. Transl. Med. 8, 328rv324 (2016).
Brahmer, J. R. et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 366, 2455–2465 (2012).
Hamanishi, J. et al. Safety and antitumor activity of anti–PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. J. Clin. Oncol. 33, 4015–4022 (2015).
Disis, M. L. et al. Efficacy and safety of avelumab for patients with recurrent or refractory ovarian cancer: phase 1b results from the JAVELIN solid tumor trial. JAMA Oncol. 5, 393–401 (2019).
Liu, J. F. et al. Safety, clinical activity and biomarker assessments of atezolizumab from a Phase I study in advanced/recurrent ovarian and uterine cancers. Gynecol. Oncol. 154, 314–322 (2019).
Li, Y. et al. Prognostic impact of programed cell death-1 (PD-1) and PD-ligand 1 (PD-L1) expression in cancer cells and tumor infiltrating lymphocytes in colorectal cancer. Mol. Cancer 15, 55 (2016).
García-Pedrero, J. M. et al. Tumor programmed cell death ligand 1 expression correlates with nodal metastasis in patients with cutaneous squamous cell carcinoma of the head and neck. J. Am. Acad. Dermatol. 77, 527–533 (2017).
Qin, T. et al. High PD-L1 expression was associated with poor prognosis in 870 Chinese patients with breast cancer. Oncotarget 6, 33972 (2015).
Wang, Q., Lou, W., Di, W. & Wu, X. Prognostic value of tumor PD-L1 expression combined with CD8+ tumor infiltrating lymphocytes in high grade serous ovarian cancer. Int. Immunopharmacol. 52, 7–14 (2017).
Muenst, S. et al. Expression of programmed death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancer. Breast Cancer Res. Treat. 146, 15–24 (2014).
Pawelczyk, K. et al. Role of PD-L1 expression in non-small cell lung cancer and their prognostic significance according to clinicopathological factors and diagnostic markers. Int. J. Mol. Sci. 20, 824 (2019).
Reck, M. et al. Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer. N. Engl. J. Med. 375, 1823–1833 (2016).
Yuan, C. et al. Expression of PD-1/PD-L1 in primary breast tumours and metastatic axillary lymph nodes and its correlation with clinicopathological parameters. Sci. Rep. 9, 1–8 (2019).
Madore, J. et al. PD-L1 expression in melanoma shows marked heterogeneity within and between patients: implications for anti-PD-1/PD-L 1 clinical trials. Pigment Cell Melanoma Res. 28, 245–253 (2015).
Callea, M. et al. Differential expression of PD-L1 between primary and metastatic sites in clear-cell renal cell carcinoma. Cancer Immunol. Res. 3, 1158–1164 (2015).
Gottlieb, C. E., Mills, A. M., Cross, J. V. & Ring, K. L. Tumor-associated macrophage expression of PD-L1 in implants of high grade serous ovarian carcinoma: a comparison of matched primary and metastatic tumors. Gynecol. Oncol. 144, 607–612 (2017).
Herbst, R. S. et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515, 563–567 (2014).
Rosenberg, J. E. et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 387, 1909–1920 (2016).
Peters, S. et al. Phase II trial of atezolizumab as first-line or subsequent therapy for patients with programmed death-ligand 1–selected advanced non-small-cell lung cancer (BIRCH). J. Clin. Oncol. 35, 2781 (2017).
Schmid, P. et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N. Engl. J. Med. 379, 2108–2121 (2018).
Chen, N. et al. Upregulation of PD-L1 by EGFR activation mediates the immune escape in EGFR-driven NSCLC: implication for optional immune targeted therapy for NSCLC patients with EGFR mutation. J. Thorac. Oncol. 10, 910–923 (2015).
Abiko, K. et al. IFN-γ from lymphocytes induces PD-L1 expression and promotes progression of ovarian cancer. Br. J. Cancer 112, 1501–1509 (2015).
Quandt, D., Jasinski-Bergner, S., Müller, U., Schulze, B. & Seliger, B. Synergistic effects of IL-4 and TNFα on the induction of B7–H1 in renal cell carcinoma cells inhibiting allogeneic T cell proliferation. J. Transl. Med. 12, 151 (2014).
Hamanishi, J. et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc. Natl. Acad. Sci. 104, 3360–3365 (2007).
Zhu, J., Wen, H., Bi, R., Wu, Y. & Wu, X. Prognostic value of programmed death-ligand 1 (PD-L1) expression in ovarian clear cell carcinoma. J. Gynecol. Oncol. 28 (2017).
Webb, J. R., Milne, K., Kroeger, D. R. & Nelson, B. H. PD-L1 expression is associated with tumor-infiltrating T cells and favorable prognosis in high-grade serous ovarian cancer. Gynecol. Oncol. 141, 293–302 (2016).
Mesnage, S. et al. Neoadjuvant chemotherapy (NACT) increases immune infiltration and programmed death-ligand 1 (PD-L1) expression in epithelial ovarian cancer (EOC). Ann. Oncol. 28, 651–657 (2017).
Kim, H.-S. et al. Expression of programmed cell death ligand 1 and immune checkpoint markers in residual tumors after neoadjuvant chemotherapy for advanced high-grade serous ovarian cancer. Gynecol. Oncol. 151, 414–421 (2018).
Huang, L.-J. et al. Prognostic significance of programmed cell death ligand 1 expression in patients with ovarian carcinoma: a systematic review and meta-analysis. Medicine 97 (2018).
Darb-Esfahani, S. et al. Prognostic impact of programmed cell death-1 (PD-1) and PD-ligand 1 (PD-L1) expression in cancer cells and tumor-infiltrating lymphocytes in ovarian high grade serous carcinoma. Oncotarget 7, 1486 (2016).
Mandai, M. et al. Anti-PD-L1/PD-1 immune therapies in ovarian cancer: basic mechanism and future clinical application. Int. J. Clin. Oncol. 21, 456–461 (2016).
Zhu, H. & Zhang, R. (AME Publ Co Room 604 6-F Hollywood Center, 77–91, QUEENS Road, Sheung Wan …, 2017).
Motohara, T. et al. An evolving story of the metastatic voyage of ovarian cancer cells: cellular and molecular orchestration of the adipose-rich metastatic microenvironment. Oncogene 38, 2885–2898 (2019).
Mutch, D. G. & Prat, J. 2014 FIGO staging for ovarian, fallopian tube and peritoneal cancer. Gynecol. Oncol. 133, 401–404 (2014).
Javadi, S., Ganeshan, D. M., Qayyum, A., Iyer, R. B. & Bhosale, P. Ovarian cancer, the revised FIGO staging system, and the role of imaging. Am. J. Roentgenol. 206, 1351–1360 (2016).
Howitt, B. E. et al. Clear cell ovarian cancers with microsatellite instability: a unique subset of ovarian cancers with increased tumor-infiltrating lymphocytes and PD-1/PD-L1 expression. Oncoimmunology 6, e1277308 (2017).
Marcus, L., Lemery, S. J., Keegan, P. & Pazdur, R. FDA approval summary: pembrolizumab for the treatment of microsatellite instability-high solid tumors. Clin. Cancer Res. 25, 3753–3758 (2019).
Chui, M. H. et al. The histomorphology of Lynch syndrome–associated ovarian carcinomas: toward a subtype-specific screening strategy. Am. J. Surg. Pathol. 38, 1173–1181 (2014).
Siraj, A. et al. Genome-wide expression analysis of Middle Eastern papillary thyroid cancer reveals c-MET as a novel target for cancer therapy. J. Pathol. 213, 190–199 (2007).
Bavi, P. et al. Prevalence of fragile histidine triad expression in tumors from saudi arabia: a tissue microarray analysis. Cancer Epidemiol. Prev. Biomark. 15, 1708–1718 (2006).
Siraj, A. K. et al. Prevalence of Lynch syndrome in a Middle Eastern population with colorectal cancer. Cancer 121, 1762–1771 (2015).
Beg, S. et al. Loss of PTEN expression is associated with aggressive behavior and poor prognosis in Middle Eastern triple-negative breast cancer. Breast Cancer Res. Treat. 151, 541–553 (2015).
Acknowledgements
We thank Padmanaban Annaiyappanaidu and Felisa DeVera for their technical assistance.
Author information
Authors and Affiliations
Contributions
Study concept and design: K.S.A., A.K.S., S.K.P. Executed the study: A.K.S., S.K.P., I.A.A., A.T., F.A.D. Statistical analysis: S.K.P. Drafting the article: K.S.A., A.K.S., S.K.P. Critical revision of the article for important intellectual content, writing of the article, and approval of the final version: K.S.A., A.K.S., S.K.P., I.A.A., A.T., F.A.D.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Parvathareddy, S.K., Siraj, A.K., Al-Badawi, I.A. et al. Differential expression of PD-L1 between primary and metastatic epithelial ovarian cancer and its clinico-pathological correlation. Sci Rep 11, 3750 (2021). https://doi.org/10.1038/s41598-021-83276-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-83276-z
This article is cited by
-
PD-L1 expression in ovarian clear cell carcinoma using the 22C3 pharmDx assay
Diagnostic Pathology (2024)
-
Immunotherapy for ovarian cancer: towards a tailored immunophenotype-based approach
Nature Reviews Clinical Oncology (2024)
-
The roles of PD-L1 in the various stages of tumor metastasis
Cancer and Metastasis Reviews (2024)
-
PD-L1 is highly expressed in ovarian cancer and associated with cancer stem cells populations expressing CD44 and other stem cell markers
BMC Cancer (2023)
-
Comparison of PD-L1 expression and MMR status between primary and matched metastatic lesions in patients with cervical cancer
Journal of Cancer Research and Clinical Oncology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.