Abstract
Fecal immunochemical test (FIT) is widely used as a colorectal cancer screening tool. Antithrombotic drugs may affect the screening performance of FIT for colorectal tumors. The aim of this study was to clarify the effect of antithrombotic agents on FIT accuracy in screening for colorectal neoplasms. This retrospective study enrolled a total of 758 patients who underwent both FIT and total colonoscopy. The effect of antithrombotic drugs on FIT accuracy in detecting colorectal neoplasms (CN), including colorectal cancer (CRC), advanced adenoma (AA), and non-advanced adenoma (NAA), was examined. Of the 758 patients, 144 (19%) received antithrombotic drugs (administration group). In administration group, 61/144 (42%) cases had CN [CRC:14, AA:15, NAA:32] and 217/614 (35%) cases had CN (CRC:43, AA:56, NAA:118) in non-administration group. The prevalence of CN was not significantly different between the two groups (p = 0.1157). There was no significant difference in sensitivity or specificity of the detection of all types of CN with or without taking antithrombotic drugs. Neither the positive predictive value nor negative predictive value of FIT was affected by antithrombotic drug administration. Taking antithrombotic drugs may not have a large impact on sensitivity, specificity, positive predictive value, or negative predictive value of FIT in screening for CN.
Similar content being viewed by others
Introduction
Colorectal cancer (CRC) is one of the most serious health care problems owing to its high incidence, morbidity, and mortality worldwide1. Most CRCs develop from a precancerous lesion, colorectal adenoma2. Therefore, early removal of these precancerous lesions is very significant to decrease CRC incidence and mortality3,4. In addition, highly accurate CRC screening, which can detect the early stages of colorectal neoplasms (CN), is useful to achieve this purpose5.
Fecal immunochemical test (FIT) for occult blood (hemoglobin) is one of the most frequently used CRC screening tools5,6. FIT is a noninvasive examination showing high sensitivity for advanced CN compared to guaiac fecal occult blood testing7. After FIT result is positive, colonoscopy is recommended for follow-up8. The result of quantitative FIT is also helpful when screening the high risk patients who require colonoscopy because increased concentration of fecal hemoglobin is an independent predictor for incident colorectal neoplasia9. Therefore, accurately reflecting the presence of CN in the results of FIT is extremely important in determining the need for colonoscopy and treatment, such as polypectomy, of these lesions.
Regular use of antithrombotic drugs, which are widely prescribed for prophylaxis of cardiovascular and cerebrovascular diseases, is associated with gastrointestinal bleeding10. Intake of antithrombotic drugs thus may facilitate bleeding and affect the efficacy of FIT. A recent cross-sectional study has revealed that regular use of aspirin and direct-acting oral anticoagulant (DOAC) is involved in lower positive predictive value (PPV) of FIT for detection of CRC and advanced adenoma (AA)11. On the other hand, it has also been reported that the usage of DOAC and aspirin did not affect the PPV of FIT for the evaluation of CN12,13,14.
In addition, the effect of antithrombotic drug administration on the sensitivity of FIT for detecting CN is drawing great attention. For instance, it is reported that usage of low-dose aspirin (LDA) may enhance the sensitivity of FIT for detecting AA15, whereas administration of oral aspirin prior to FIT did not significantly increase the test sensitivity for detecting advanced CN in a randomized clinical trial16.
When considering the results of the above clinical trials11,12,13,14, some aspects remain unclear about the effects of antithrombotic agents on colorectal tumor screening using FIT. Several clinical studies have also reported the PPV for colorectal tumors screening using FIT in patients taking antithrombotic drugs, but their range is wide13,14,17,18, while most recent meta-analysis revealed that FIT accuracy is not affected by antithrombotic agents19. Therefore, we considered it is important to reexamine the effects of antithrombotic drugs on the sensitivity and PPV of FIT for detecting CN in medical examination. The aim of the present study was to clarify whether antithrombotic drugs affect detecting performance of FIT for CN.
Methods
Patients
This study protocol was approved by the Institutional Review Board of the Gifu University Hospital and Gihoku Kosei Hospital, Gifu, Japan (27-269). Informed consent was obtained from all patients before total colonoscopy examination for clinical research. The study was carried out in accordance with the Declaration of Helsinki.
Between April 2012 and December 2016, 758 subjects at Gifu University Hospital (98 patients) and Gihoku Kosei Hospital (660 patients) were enrolled. The patients with positive FIT or symptoms such as abdominal discomfort, hematochezia, or constipation were performed colonoscopy. In addition, all patients were undergone FIT before colonoscopy.
Data collection
Patients’ clinical characteristics (age, sex, complication with diabetes mellitus, hypertension, and hyperlipidemia) and information about antithrombotic drugs intake were obtained from electronic medical charts. Antiplatelet agents, LDA, and anticoagulants were defined as antithrombotic drugs. CN were classified into CRC, AA, and non-advanced adenoma (NAA). The definition of AA was as follows: ≥ 10 mm in size, or with a villous component, or a high-grade dysplasia.
As for FIT, patients were instructed to keep the sample collection tube (Alfresa Pharma Co., Ltd, Osaka, Japan) at room temperature according to the manufacturer’s instruction, and after delivering to the lab on the same day of colonoscopy, the samples were analyzed using a discrete automated clinical chemistry analyzer, NS-Plus C15 (Otsuka Electronics Co., Ltd, Osaka, Japan). The test was considered positive at ≥ 20 µg/g, and for 2-sample FIT if one sample tested positive, was considered positive. In addition, we used the same analyzer during the study period (2012–2016). We also completed the FITTER check list recommended by World endoscopy organization.
Outcome measures and statistical analysis
First, the enrolled patients were divided into the administration and non-administration groups of antithrombotic drugs. Categorical variables, which were expressed as the median ± range, were compared between the two groups using a t-test. Next, the sensitivity, specificity, PPV, and negative predictive value (NPV) of FIT of the two groups in the detection of CN were calculated. Differences of these values between the groups were compared using a chi-squared. Differences were considered statistically significant at P-values < 0.05. All statistical analyses were carried out on JMP 10 (SAS Institute Inc., Cary, NC, USA).
Statement of ethics
This study protocol was approved by the Institutional Review Board of the Gifu University Hospital and Gihoku Kosei Hospital, Gifu, Japan (27-269). The study was carried out in accordance with the Declaration of Helsinki.
Results
Comparison of baseline characteristics in patients taking and not taking antithrombotic drugs
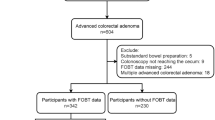
Among the 758 patients who underwent both FIT and total colonoscopy, 144 patients were treated with antithrombotic drugs (administration group). Among them, 129 patients received anti-platelet drugs and 71 patients of them took LDA (Fig. 1). Anticoagulant drugs (warfarin and DOAC) were given to 22 patients (with overlaps) (Table 1). As shown in Table 1, the administration group was significantly older than the non-administration group (75 years vs. 63 years, p < 0.0001) and it had more males (76.4% vs. 57.5%, p < 0.0001). In administration group, 61 cases of CN were there, including 14 of CRC, 15 of AA, and 32 of NAA. 217 cases of CN (43 of CRC, 56 of AA, 118 of NAA) were there in non-administration group. There was no significant difference between the incidences of CN development between the two groups (42.4% vs. 35.3%). Complication rates of diabetes mellitus (31.3% vs. 10.1%, p < 0.0001), hypertension (62.5% vs. 33.7%, p < 0.0001), and hyperlipidemia (31.3% vs. 17.8%, p = 0.0003) were significantly higher in the administration group compared to the non-administration group. The details of the prescription drugs were also listed in Table 1.
Effects of antithrombotic drugs on the diagnostic performance of FIT for CN
We examined whether antithrombotic drugs affect the diagnostic performance of FIT for CN. As shown in Table 2, there was no significant difference in sensitivity or specificity of CN, CRC, CRC plus AA, and NAA, with or without oral medication of antithrombotic drugs. However, the sensitivity tended to be higher regarding CN (62.3% vs. 56.0%, p = 0.3964) and NAA (53.1% vs. 39.8%, p = 0.1775) in the administration group compared to the non-administration group. There was also no significant difference in PPV or NPV; however, the PPV tended to be higher in regard to CN (53.5% vs. 41.2%, p = 0.0604) and NAA (34.0% vs. 21.3%, p = 0.0556) in the administration group compared to the non-administration group.
We next performed a similar analysis for antiplatelet drugs (Table 3) and LDA (Table 4) administered groups. Neither anti-platelet drugs nor LDA administrations affected the sensitivity, specificity, PPV, and NPV of FIT for the detection of CN. However, in patients treated with antiplatelet drugs, the PPV showed a high tendency regarding CN (51.7% vs. 41.2%, p = 0.1360) and NAA (32.6% vs. 21.3%, p = 0.1080) in the administration group compared to the non-administration group. The data of anticoagulant drugs was not examined separately since the number of registered patients was small in the study (n = 22).
Discussion
FIT is accepted as a useful screening tool in reducing the mortality rate of CRC5,6. Currently antithrombotic drugs are widely used for preventing thrombosis in cardiovascular and cerebrovascular diseases. In the present study, the patients receiving antithrombotic drugs were significantly older and had diabetes mellitus, hypertension, and hyperlipidemia. It is well known that lifestyle-related diseases such as diabetes mellitus are significant risk factors for developing CRC20. Therefore, the patients who are taking antithrombotic drugs due to lifestyle-related diseases are more likely to develop colorectal tumors and require more careful screening.
Theoretically, administration of antithrombotic drugs could have the opposite effect in CN screening by FIT. The drugs may facilitate bleeding from non-advanced colorectal neoplastic lesions and thus decreasing the PPV of FIT for CRC and AA. On the other hand, if the drugs stimulate bleeding from advanced colorectal neoplastic lesions, PPV may increase significantly. PPV is one of the useful indexes to evaluate whether antithrombotic agents cause gastrointestinal mucosal damage and influence false positives of FIT.
In the present study, administration of antithrombotic drugs did not affect the sensitivity, specificity, PPV, and NPV of FIT for the detection of CN. These findings were consistent with those of previous reports showing that PPV for CN was not affected by the intake of antithrombotic drugs12,13,14. The PPVs for all colorectal neoplasia (53.5%), CRC (15.5%), CRC plus AA (29.6%), and NAA (34.0%) in patients taking antithrombotic drugs were almost the same levels as those of LDA in the previous reports (17.3–57.0%)13,14,17,18. Therefore, FIT is unlikely to be affected by antithrombotic drugs such as LDA, indicating that there is little need for cessation of these drugs before the test. On the other hand, a recent large cohort study has reported that both aspirin and DOAC users showed lower PPV for CRC and AA compared to non-users of these drugs11. In the study, the PPV for CRC (0.9% vs. 6.8%) and that of AA (20.5% vs. 32.4%) in DOAC users were especially lower compared to the matched non-users11. Therefore, the impact of antithrombotic drugs on FIT, especially in DOAC, needs further examination. Moreover, although we focused on the anti-platelet drugs in the present study, it might be of interest to investigate the effects of other medications on the accuracy of FIT because a number of patients are taking other medications, such as prescriptions for diabetes mellitus, hypertension, and hyperlipidemia.
Detecting adenoma, especially in AA, is involved in the effectiveness of FIT-based CRC screening21. FIT can detect CRC with high sensitivity and specificity; however, these values, especially in sensitivity, are relatively decreased in the detection of AA8,22. Therefore, improvement of the sensitivity of AA might be able to improve the performance of FIT for CRC screening. Usage of antithrombotic drugs, which could facilitate bleeding from adenoma, is expected to enhance the sensitivity of FIT for detecting AA15. In the present study, the sensitivity and PPV tended to be higher in regard to all colorectal neoplasia and NAA in antithrombotic drugs administration group, although there was no statistically significant difference. Removal of as many adenomatous polyps as possible during colonoscopy leads to the prevention of CRC death23. This might indicate that increasing the sensitivity of NAA detection could be used to add value to CRC screening. Further studies are needed to determine whether antithrombotic agents may actually increase the sensitivity of NAA.
The present study has some limitations. First, this was a retrospective study with a small sample size. In particular, due to the limited sample size of the patients that received DOAC and anticoagulant such as warfarin, we could not do a statistical analysis on them. Further multicenter, large-scale studies should be conducted to confirm the results of the present study. Second, it is necessary to verify whether the cutoff value and the kit used in the present study were appropriate. Because the cut-off value may influence the sensitivity and PPV of CRC screening, it is necessary to set a most appropriate value. Patients were enrolled from university and general hospitals in the present study, but the prevalence of colorectal tumors may differ between these hospitals. This is also one of the limitations because the prevalence is a major factor affecting PPV.
In conclusion, the results of our study suggest that antithrombotic drugs may not have a large impact on FIT sensitivity, specificity, PPV, or NPV in screening for CN. Therefore, in patients who take antithrombotic drugs, there may not be as many false positives as previously thought.
Abbreviations
- CRC:
-
Colorectal cancer
- FIT:
-
Fecal immunochemical test
- CN:
-
Colorectal neoplasms
- DOAC:
-
Direct-acting oral anticoagulant
- PPV:
-
Positive predictive value
- AA:
-
Advanced adenoma
- LDA:
-
Low-dose aspirin
- NAA:
-
Non-advanced adenoma
- NPV:
-
Negative predictive value
References
Ferlay, J. et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer. 136(5), E359–E386 (2015).
Brenner, H. et al. Risk of progression of advanced adenomas to colorectal cancer by age and sex: Estimates based on 840,149 screening colonoscopies. Gut 56(11), 1585–1589 (2007).
Winawer, S. J. et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N. Engl. J. Med. 329(27), 1977–1981 (1993).
Kahi, C. J. Reviewing the evidence that polypectomy prevents cancer. Gastrointest. Endosc. Clin. N. Am. 29(4), 577–585 (2019).
Schreuders, E. H. et al. Colorectal cancer screening: A global overview of existing programmes. Gut 64(10), 1637–1649 (2015).
Nakajima, M. et al. Prevention of advanced colorectal cancer by screening using the immunochemical faecal occult blood test: A case–control study. Br. J. Cancer. 89(1), 23–28 (2003).
Wieten, E. et al. Incidence of faecal occult blood test interval cancers in population-based colorectal cancer screening: A systematic review and meta-analysis. Gut 68(5), 873–881 (2019).
Robertson, D. J. et al. Recommendations on fecal immunochemical testing to screen for colorectal neoplasia: A consensus statement by the US Multi-Society Task Force on colorectal cancer. Gastroenterology 152(5), 1217–1237.e3 (2017).
Grobbee, E. J. et al. Association between concentrations of hemoglobin determined by fecal immunochemical tests and long-term development of advanced colorectal neoplasia. Gastroenterology 153(5), 1251–1259.e2 (2017).
Gullov, A. L., Koefoed, B. G. & Petersen, P. Bleeding during warfarin and aspirin therapy in patients with atrial fibrillation: The AFASAK 2 study. Atrial fibrillation aspirin and anticoagulation. Arch. Intern. Med. 159(12), 1322–1328 (1999).
Randel, K. R. et al. Effects of oral anticoagulants and aspirin on performance of fecal immunochemical tests in colorectal cancer screening. Gastroenterology 156(6), 1642–1649.e1 (2019).
Niikura, R. et al. The effects of direct oral anticoagulants, warfarin, aspirin and thienopyridine on the performance of immunochemical, faecal, occult blood tests. Digestion. 100(2), 117–126 (2019).
Mandelli, G. et al. Anticoagulant or aspirin treatment does not affect the positive predictive value of an immunological fecal occult blood test in patients undergoing colorectal cancer screening: Results from a nested in a cohort case–control study. Eur. J. Gastroenterol. Hepatol. 23(4), 323–326 (2011).
Tsuji, Y. et al. Antithrombotic drug does not affect the positive predictive value of an immunochemical fecal occult blood test. Dig. Endosc. 26(3), 424–429 (2014).
Brenner, H., Tao, S. & Haug, U. Low-dose aspirin use and performance of immunochemical fecal occult blood tests. JAMA 304(22), 2513–2520 (2010).
Brenner, H. et al. Effect of a single aspirin dose prior to fecal immunochemical testing on test sensitivity for detecting advanced colorectal neoplasms: A randomized clinical trial. JAMA 321(17), 1686–1692 (2019).
Levi, Z. et al. Sensitivity, but not specificity, of a quantitative immunochemical fecal occult blood test for neoplasia is slightly increased by the use of low-dose aspirin, NSAIDs, and anticoagulants. Am. J. Gastroenterol. 104(4), 933–938 (2009).
Bujanda, L. et al. Effect of oral anticoagulants on the outcome of faecal immunochemical test. Br. J. Cancer. 110(5), 1334–1337 (2014).
Nieuwenburg, S. A. V., Vuik, F. E. R., Kruip, M. J. H. A., Kuipers, E. J. & Spaander, M. C. W. Effect of anticoagulants and NSAIDs on accuracy of faecal immunochemical tests (FITs) in colorectal cancer screening: A systematic review and meta-analysis. Gut 68(5), 866–872 (2019).
Shirakami, Y. et al. Chemoprevention of colorectal cancer by targeting obesity-related metabolic abnormalities. World J. Gastroenterol. 20(27), 8939–8946 (2014).
Haug, U., Knudsen, A. B., Lansdorp-Vogelaar, I. & Kuntz, K. M. Development of new non-invasive tests for colorectal cancer screening: The relevance of information on adenoma detection. Int. J. Cancer. 136(12), 2864–2874 (2015).
Gies, A., Bhardwaj, M., Stock, C., Schrotz-King, P. & Brenner, H. Quantitative fecal immunochemical tests for colorectal cancer screening. Int. J. Cancer. 143(2), 234–244 (2018).
Zauber, A. G. et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N. Engl. J. Med. 366(8), 687–696 (2012).
Acknowledgements
We thank Yukiko Gouda for her secretarial assistance.
Author information
Authors and Affiliations
Contributions
T.I., S.A., M.A. and M.S. was involved in the study concept and design, acquisition of the data, analysis and interpretation of the data, drafting of the manuscript, and overall study supervision. Y.H., T.O., M.M., Y.S., O.Y., E.T., M.I., K.S., K.T. and H.A. were involved in the acquisition of the data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ibuka, T., Adachi, S., Horibe, Y. et al. Effects of antithrombotic drugs on the results of fecal immunochemical test in colorectal neoplasms screening. Sci Rep 11, 4348 (2021). https://doi.org/10.1038/s41598-021-83007-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-83007-4
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.