Abstract
VO2 is a highly correlated electron system which has a metal-to-insulator transition (MIT) with a dramatic change of conductivity accompanied by a first-order structural phase transition (SPT) near room temperature. The origin of the MIT is still controversial and there is ongoing debate over whether an SPT induces the MIT and whether the Tc can be engineered using artificial parameters. We examined the electrical and local structural properties of Cr- and Co-ion implanted VO2 (Cr-VO2 and Co-VO2) films using temperature-dependent resistance and X-ray absorption fine structure (XAFS) measurements at the V K edge. The temperature-dependent electrical resistance measurements of both Cr-VO2 and Co-VO2 films showed sharp MIT features. The Tc values of the Cr-VO2 and Co-VO2 films first decreased and then increased relative to that of pristine VO2 as the ion flux was increased. The pre-edge peak of the V K edge from the Cr-VO2 films with a Cr ion flux ≥ 1013 ions/cm2 showed no temperature-dependent behavior, implying no changes in the local density of states of V 3d t2g and eg orbitals during MIT. Extended XAFS (EXAFS) revealed that implanted Cr and Co ions and their tracks caused a substantial amount of structural disorder and distortion at both vanadium and oxygen sites. The resistance and XAFS measurements revealed that VO2 experiences a sharp MIT when the distance of V–V pairs undergoes an SPT without any transitions in either the VO6 octahedrons or the V 3d t2g and eg states. This indicates that the MIT of VO2 occurs with no changes of the crystal fields.
Similar content being viewed by others
Introduction
Since Morin reported observing the metal-to-insulator transition (MIT) of VO2 in 19591, VO2 has been widely studied to understand the origin of its MIT2,3,4,5,6,7 and to use it in practical applications including smart windows, batteries, transistors, ultrafast switches, and gas sensors8,9,10,11,12,13,14,15. The MIT of VO2 can be induced by different factors such as heat, an electric field, doping, oxygen vacancy, photons, and a magnetic field1,5,6,7,16,17,18,19,20. A typical critical temperature (Tc) of the MIT of VO2 is approximately 68 °C5. However, previous studies showed that the Tc is very sensitive to structural strain21,22,23. Cao and coworkers observed that VO2 beams with multiple domains have different Tc values, resulted in a dull transition22. Since the MIT of VO2 is accompanied by a first-order structural phase transition (SPT) from a monoclinic phase (M1) to a rutile phase (R) via a M2 phase, which is a mixture of the M1 and R phases, the structural changes could be related to the MIT. For the last half decade, arguments have continued as to whether this structural transition directly induces the MIT of VO22,3,4,5,6,7,8,24,25,26. The electrical resistivity change of VO2 between insulator and metallic phases is approximately four orders of magnitude and the MIT is quite abrupt5,7. Many efforts have been made to understand the mechanism of VO2 MIT both theoretically and experimentally2,3,4,5,6,7,8,24,25,26,27,28,29. Many researchers attributed the abrupt MIT of VO2 to the SPT2,3,24,25,30, while others argued that the abrupt MIT can be induced by the change of carriers from holes (insulator) to electrons (metal), supporting the Mott transition of VO28,18. The abruptness of the MIT of VO2 has become a further issue, in addition to its origin.
A single crystal VO2 has a distinct MIT temperature22,31 while the MIT of grained VO2 is dull, occurring over a wide range of temperature7,21. The Tc and the MIT curve of VO2 are very sensitive to structural disorder and strain19,32,33,34. When a VO2 film consists of grains, their structural disorder and distortion can take various forms, resulting in each grain having an individual Tc. Furthermore, Qazilbash demonstrated that the MIT of VO2 could occur at slightly different temperatures for even the same grain by using infrared (IR) mapping measurements5. Structural disorder and defects can prevent the movement of conduction electrons and also demolish a bandgap, creating bands of impurity near the Fermi level35. As a result, the Tc of MIT can shift towards a higher or lower temperature21,22,23. Structural disorder, strain, and defects can be created by different conditions, including impurities, grain boundaries, and lattice mismatch between film and substrate21,22,33. For practical applications of VO2, the Tc, the abruptness, and the resistance difference between metallic and insulating phases of its MIT are the most important parameters.
The Tc and the MIT features of VO2 with various impurities, including Cr, Co, W, Mo, and Ti, have been examined17,37,38,39,40,41,42,43,44. Added impurities in VO2 act as dopants, create structural disorder, and distort the atomic bond lengths17,42,44. Previous studies showed that the Tc of V1−xCrxO2 shifted towards a higher temperature while it was observed at lower temperatures from V1−xCoxO2, V1−xWxO2, and V1−xMoxO2 relative to that of pure VO217,37,38,39,40,41,42,43,44. Cr3+ and Co2+ impurities in VO2 increased and decreased the Tc, respectively, with little effect on the abruptness and the sharpness of the MIT curves38,42, although the chemical valence states of both Cr3+ and Co2+ are smaller than the 4 + of V in VO2. Added impurities in VO2 can influence the density of charge carriers in a conduction band and the structural properties and density of states. The in-situ electrical resistance and X-ray absorption fine structure (XAFS) measurements of Cr- and Co-ion implanted VO2 (Cr-VO2 and Co-VO2) were used to examine the contribution of local structural properties on the MIT of VO2. Since in a single crystal VO2, the correlation of the electrons (Mott insulator), the structural-driven Peierls distortion, and the crystal field effects of metal-oxide octahedrons simultaneously change with MIT at the same temperature, their contributions to MIT are indistinguishable. The contribution of a parameter on MIT can be distinguished from the others only when it does not occur simultaneously with the other parameters.
Results
The temperature-dependent electrical properties of Cr- and Co-VO2
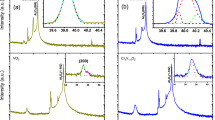
Implanted Cr and Co ions can act as dopants and induce structural defects in VO2. Previous studies showed that the lattice constants of Cr-doped VO2 increased37,39 while the Tc shifted towards a higher temperature38,39. Figure 1 shows the temperature-dependent resistance from the Cr-VO2 films before and after Cr-ions implantation. The typical Tc value of single crystal VO2 is approximately 68 °C5,31. However, the Tc value of a VO2 film is substantially affected by structural strain and disorder and varies according to the substrate21,32,32. The Tc of ~78 °C for the pristine VO2 films in Fig. 1 before the ion implantation is ascribed to structural strain due to a lattice mismatch between the VO2 films and Al2O3 substrates21,33,34. The Tc values of pristine VO2 films in Fig. 2, particularly in Fig. 2(c), are somewhat lower than that in Fig. 1. The Tc values suggest that the growth conditions of VO2 films in Fig. 1 were somewhat different from those in Fig. 2, although the difference was not conscious during growth. A small deviation of the characteristics of the pristine VO2 films does not seriously affect the main conclusions of this study because the changes of MIT features before and after ion implantation are directly compared from the same specimen. The resistance curves of the Cr-VO2 films with a flux of 1012 ions/cm2 show that the Tc values during heating and cooling shift towards lower and higher temperatures relative to those before Cr ion implantation, respectively. As a result, the width of a hysteresis loop from the Cr-VO2 films, particularly with a low energy of Cr ions, is significantly narrower than that of the pristine VO2. For Cr ion fluxes of 1013 ions/cm2 and 5 × 1013 ions/cm2, the resistance curves become similar to that of the pristine VO2. At a flux of 5 × 1013 ions/cm2, the Tc value is ~ 2 degrees higher than that before ion implantation, as shown in Fig. 1(d). This is consistent with the previous studies of V1−xCrxO in which the Tc was shifted towards a higher temperature38,39. The Tc increase of 2 degrees of Cr-VO2 roughly corresponds to the Cr concentration of ~ 3%, compared to a previous study of V1−xCrxO239. The Tc shift and the resistance changes of Cr-VO2 can be understood in terms of structural disorder and doping effects due to implanted Cr ions. The structural damage due to implanted ions is discussed in detail in the supplementary materials.
Temperature-dependent electrical resistance for Cr-VO2 films with a Cr ion energy and a flux of (a) 30 keV and 1012 ions/cm2, (b) 50 keV and 1012 ions/cm2, (c) 50 keV and 1013 ions/cm2, and (d) 50 keV and 5 × 1013 ions/cm2, respectively. Solid lines and circles are the resistances for the same Cr-VO2 film before and after Cr-ion implantation, respectively. XAFS was simultaneously measured at the temperatures of the circles. Red and blue colors indicate the resistance for heating and cooling processes, respectively. The dotted lines are a guide for the eye.
Temperature-dependent electrical resistance for Co-VO2 films with a Co ion energy and a flux of (a) 30 keV and 1013 ions/cm2, (b) 50 keV and 1013 ions/cm2, (c) 70 keV and 1014 ions/cm2, and (d) 100 keV and 1014 ions/cm2, respectively. Solid lines and circles are the resistances for the same Co-VO2 film before and after Co-ion implantation, respectively. XAFS was simultaneously measured at the temperatures of the circles. Red and blue colors indicate resistance for heating and cooling processes, respectively. The dotted lines are a guide for the eye.
Zou and coworkers reported that the width of the hysteresis loop of temperature-dependent resistance for VO2 was reduced by Cr doping and that the MIT features of VO2 disappeared at a Cr concentration ratio of 14%39. The resistance measurements of the Cr- and Co-VO2 films show that the MIT characteristics of VO2 mostly disappear when the flux of Cr and Co ions exceeds 1015 ions/cm2, which corresponds to ~ 50% of the VO2 cells being hit by implanted ions. The probability of a conventional cell of a film being hit by implanted ions can be estimated as \(P\left(x\right)=1-A{\int }_{0}^{x}F\left(x\right)dx\), where F(x) is the distribution function of ions in a film and A is the normalization factor. The details of the probability are described in the supplementary materials. The ion tracks and the local disorder due to implanted ions likely destroy the MIT characteristics. The Tc shift of the Cr-VO2 films with an ion flux of 1012 ions/cm2 suggests that the structural disorder due to ion implantation dominantly contributes to the electrical properties because of the negligible doping effect at a concentration ratio of 0.00023%. The concentration ratio is discussed in the supplementary materials in detail. When the ion flux increases over 1013 ions/cm2, the doping effects due to the implanted ions may influence the MIT of VO2 because of the extremely low charge carrier density of insulating VO2, although structural disorder still dominantly affects the MIT.
Figure 2 shows the temperature-dependent electrical resistance of the Co-VO2 films with different fluxes and energies of Co ions. The distribution of implanted Co ions in VO2 is quite similar to that of Cr ions, as shown in the supplementary materials. When Co ions with a flux of 1013 ions/cm2 and an energy of ≤ 70 keV are implanted on VO2 films, the Tc values for both heating and cooling processes shift towards lower temperatures relative to those before the ion implantation. This is consistent with Co-doped VO242. The width reduction of the resistance hysteresis loop is similar to that of the Cr-VO2 films with low ion fluxes. The resistance curves of the Co-VO2 films with an energy of 30–50 keV and a flux of 1014 ions/cm2 show very weak MIT features near the Tc of 65 °C (data not shown here). When the energy of Co ions is increased over 70 keV with a flux of 1014 ions/cm2, the Co-VO2 films show sharp MIT features, as shown in Fig. 2(c, d). SRIM calculations45 showed that the Co ions with an energy of 30 keV can become concentrated near the surface of a single crystal VO2. Since our VO2 films consist of grains with a mean size of ~ 170 nm36, implanted ions with the energy of 30–50 keV can affect the entire film through the grain boundaries and the lateral surfaces of the grains46. A lack of MIT features in Co-VO2 with a Co ion energy of 30 keV and a flux of 1014 ions/cm2 (data not shown here) can be ascribed to a substantial structural disorder and distortion existing in the entire film due to the ion implantation. Structural disorder and distortion in Cr- and Co-VO2 films which are created due to the implanted ions may not be uniformly distributed and can be more concentrated on near the surface than the bottom because the ion energy of several tens keV is insufficient to create a uniform defect in VO2 films with a mean thickness of ~ 130 nm.
At a Co ion energy of 100 keV, the resistance curves of the Co-VO2 films show sharp MIT features and the Tc values become similar to those before implantation during both heating and cooling. This is substantially different from those for a low energy of Co ions and sharply contrasts to previous works of Co-added VO242. The ion-flux-dependent behavior of Tc values of Co-VO2 is similar to that of Cr-VO2, as shown in Fig. 1. As the flux of both Co and Cr ions exceeds a certain value, Tc shifts towards a higher and lower temperature relative to that for a low flux during heating and cooling, respectively, while the sharpness of MIT is not greatly affected, as shown in Figs. 1 and 2. A similar behavior of Tc was also observed from Ti-added VO244. When the flux of Cr and Co ions with the energy of 30–50 keV is larger than 1014 ions/cm2, the MIT features are significantly diminished. The critical flux of the ions increases when ion energy increases, as shown in Fig. 2. This is an evidence that ions with a lower energy more effectively create structural disorder, particularly near the surface, than the ions with a higher energy.
The temperature-dependent XANES and the pre-edge peaks of Cr-VO2 and Co-VO2
Implanted Cr3+ and Co2+ ions can affect the charge currier density of conduction bands and the local density of states around the V atoms in VO2. X-ray absorption near edge structure (XANES) detects the local density of empty states around a probing atom7. Figure 3 shows XANES from the Cr-VO2 films at the V K edge. The main absorption edge energy near 5478 eV from the Cr-VO2 films is nearly identical to that of a pristine VO2 film, which indicates that the chemical valance state and the 4p states of the V atoms in VO2 are little affected by implanted Cr ions at a flux ≤ 5 × 1013 ions/cm2. The intensity of the pre-edge peak near 5470 eV from the Cr-VO2 films increases dramatically for an ion flux ≥ 1013 ions/cm2, but only increases slightly for a flux of 1012 ions/cm2 relative to that of the pristine VO2. The pre-edge peaks consist of two peaks corresponding to the t2g and eg states of V 3d orbitals of VO2, which are separated by approximately 2.0 eV7. The intensity increase of the pre-edge peak at the V K edge was also observed from Ti-added VO244. Since a lack of doping effects is expected when Ti4+s are replaced at V4+ sites in VO2, the pre-edge peak changes are mainly attributed to structural changes around the V atoms. The position of the pre-edge peak is shifted by approximately 0.5 eV for a Cr ion flux ≥ 1013 ions/cm2 relative to that of the pristine VO2, as shown in Fig. 3 (b). These intensity increases and the position shift of the pre-edge peak might be due to local structural distortion around the V atoms.
(a) Normalized total X-ray absorption (μt) for Cr-VO2 films with different Cr energies and fluxes at the V K edge as a function of the incident X-ray energy at the room temperature. The films are the same as those used for the resistance measurements, as shown in Fig. 1, and (b) is a magnified image of the pre-edge peaks in (a).
Figure 4(a, b) show the temperature-dependent XANES and pre-edge peaks, respectively, from a pristine VO2 film in the temperature range of 30–100 °C. The main absorption edge is almost unaffected by the increasing temperature while the pre-edge shows a temperature-dependent behavior. In a pristine VO2 film, the dull pre-edge peak corresponds to the t2g and eg bands which have the energy difference of ~ 2.0 eV7. The direct band gap of VO2 is ~ 0.65 eV at room temperature47,48 and the Fermi level lies in the lower t2g band2,3,7. Figure 3(d) shows that the intensity of the pre-edge peak decreases and the position shifts towards a higher energy when VO2 is heated from 30 to 100 °C. The separation of the two pre-edge peaks does not change greatly but the peak positions shift by ~ 0.5 eV towards a higher energy when the structural symmetry of VO2 changes from M1 to a rutile (or M2) phase7. The pre-edge peak of the pristine VO2 shows a shift at ~ 68 °C, as shown in Fig. 4(b). This is prior to the Tc of ~ 75 °C. The temperature-dependent behavior of the pre-edge peak is directly related to the local structural changes around the V atoms7.
(a, c) Normalized temperature-dependent XANES (μt) from a pristine VO2 film and the Cr-VO2 film with a Cr ion energy of 30 keV and a flux of 1012 ions/cm2 during heating. (b, d) are the magnified images of the pre-edge peaks in (a, c), respectively. Data in (a, c) are vertically shifted for clarity.
Electrical resistance measurements from Cr-VO2 films with even a low Cr ion flux show substantial changes in the Tc value of the MIT relative to that before ion implantation, as shown in Fig. 1. XANES and the pre-edge peak reveal that a Cr-VO2 film with a small flux of Cr ions shows similar behavior as that of pristine VO2, as shown in Fig. 4(c, d). The pre-edge peak from the Cr-VO2 film shifts at ~ 68 °C and ~ 65 °C while the Tc values of MIT are ~ 71 °C and ~ 65 °C during heating and cooling, respectively, as shown in Fig. 1(a). The pre-edge peak transitions roughly agree with the MITs of the film, although they do not occur simultaneously at the same temperature. This is consistent with pristine VO27. For a Cr ion flux ≥ 1013 ions/cm2, the intensity of the pre-edge dramatically increases relative to that of pristine VO2 while the resistance curves are comparable to that before ion implantation. Figure 5(a, c) show the temperature-dependent XANES of the Cr-VO2 films with a flux ≥ 1013 ions/cm2. The pre-edge peaks of the films show nearly no temperature dependence in the temperature range of 40–100 °C. This sharply contrasts to that of pristine VO2 and Cr-VO2 with a low flux of the Cr ion beam. The resistance curves of the Cr-VO2 films show clear MIT features, as shown in Fig. 1(c, d). This temperature independence of the pre-edge peaks of the Cr-VO2 films is obvious evidence confirming that the pre-edge peak at the V K edge is irrelevant to the MIT of VO2. The pre-edge peak of a single crystal VO2 accidently changes with temperature due to the transition of local structural properties and the local density of states around the V atoms. This study suggests that a structural disorder can remove the correlation between the pre-edge peak and the electrical properties of VO2.
Figure 6(a) shows XANES at the V K edge from Co-VO2 films with different energies and Co ion fluxes. The main absorption edges of the Co-VO2 films are nearly identical to that of pristine VO2. This confirms the permanence of both the chemical valance states and the local density of states around the V atoms in the Co-VO2 films, relative to those of the pristine VO2 film. The intensity of the pre-edge peaks slightly increases at a Co ion flux of 1014 ions/cm2. The XANES of Co-VO2 films is quite different from that of Cr-VO2 films, as shown in Fig. 3. Figure 6(c) shows the temperature-dependent XANES of Co-VO2 with a Co ion energy of 70 keV and a flux of 1014 ions/cm2. No significant changes in the main absorption edge energy of the Co-VO2 film are observed in the temperature range of 30–100 °C. The pre-edge peak behavior of Co-VO2 is similar to that of the pristine VO2 but it is completely different from that of Cr-VO2 with a Cr flux ≥ 1013 ions/cm2. The pre-edge peak shifts at 62 °C towards a higher energy, as shown in Fig. 6(d), which is slightly prior to the Tc of 65 °C, as shown in Fig. 2(c). The different behaviors of the pre-edge peaks from the Cr-VO2 and Co-VO2 films are mainly attributed to the different local structural properties around the V atoms.
(a) Normalized total X-ray absorption (μt) for Co-VO2 films with different Co ion energies and fluxes at the V K edge as a function of the incident X-ray energy at room temperature. The films are the same as those in Fig. 2. (c) Temperature-dependent XANES for Co-VO2 with a Co energy of 70 keV and a flux of 1014 ions/cm2. (b, d) are the magnified images of the pre-edge peaks in (a, c), respectively. Data in (c) are vertically shifted for clarity.
Local structural properties around vanadium atoms of Cr-VO2 and Co-VO2
Extended XAFS (EXAFS), which is small oscillations above the main absorption edge, as partially shown in Figs. 3, 4, 5 and 6, can detect the local structural properties around a probing atom50,51,52. After the atomic background was determined using the IFEFFIT software package53, EXAFS was obtained and analyzed using the standard procedure54. Raw EXAFS in k-space is presented in the supplementary materials. Figure 7 shows Fourier transformed EXAFS from the pristine VO2 and Cr-VO2 films in r-space. The peak positions of EXAFS correspond to the mean atomic distances from a V atom. They are approximately 0.3 Å shorter than the true atomic positions because the phase shift of back-scattered photoelectrons is not accounted for. Figure 7(a, b) show temperature-dependent EXAFS for the pristine VO2 film for heating and cooling, respectively. The two peaks near 1.5 Å correspond to the six V–O pairs of a VO6 octahedron in VO2 at lower temperatures; these become one sharp peak at higher temperatures because VO2 has monoclinic and rutile (or M2) phases at low and high temperatures, respectively. A peak near 3.0 Å, mainly corresponding to eight vertex V atoms, slightly moves towards a longer distance in the rutile (or M2) phase, compared to that in M1. The EXAFS data of a pristine VO2 were quantitatively fitted to the EXAFS theory51 and the fit results are described elsewhere in literatures7,36. The SPTs of the pristine VO2 film are observed at ~ 70 °C and ~ 62 °C for heating and cooling, respectively. The SPT temperatures do not match with either the Tc of MIT or the pre-edge peak transitions. This agrees with previous reports7,33. From the Cr-VO2 film with a Cr ion energy of 50 keV and a flux of 1012 ions/cm2, the SPTs are observed at 68 °C and 63 °C for heating and cooling, respectively, as shown in Fig. 7(c, d). The temperature difference between the SPTs for heating and cooling is approximately 5 °C, which is comparable to the resistance curve after ion-implantation, as shown in Fig. 1(a). This strongly suggests that the electrical property changes and the Tc shifts of Cr-VO2 films are highly related to the structural changes due to ion implantation. In addition, this indicates that the implanted Cr ions with the energy of 50 keV cause structural disorder and distortion around V atoms in the entire Cr-VO2 film.
Magnitude of Fourier transformed EXAFS (|FT(k3χ)|) as a function of the distance from a V atom at different temperatures. (a, b) EXAFS from pristine VO2 for heating and cooling, respectively. (c, d) EXAFS from Cr-VO2 with a Cr ion energy of 50 keV and a flux of 1012 ions/cm2 for heating and cooling, respectively. The data are vertically shifted for clarity.
An obvious MIT is observed from the Cr-VO2 films with a flux of Cr ions ≥ 1013 ions/cm2 with no changes of the pre-edge peaks in the temperature range of 30–100 °C. Figure 8 shows EXAFS from the Cr-VO2 films with a Cr ion flux ≥ 1013 ions/cm2. At this Cr ion flux, there are clear SPTs near 72 °C and 64 °C for heating and cooling, respectively, as shown in Fig. 8(a, b). The SPT temperatures are comparable to the MIT Tc values of 75 °C and 65 °C for heating and cooling, respectively, as shown in Fig. 1(c). This result is substantially different from that of a pristine VO2, which shows very different the Tc values of MIT and SPT during both heating and cooling. The similar Tc values of the MIT and the SPT in Cr-VO2 and Co-VO2 may suggest that Cr- and Co-VO2 films structurally soften relative to a pristine VO2 film due to ion implantation. Figure 8(c, d) show Fourier-transformed EXAFS of Cr-VO2 with a flux of 5 × 1013 ions/cm2. EXAFS shows no SPT of the first two peaks in 1.0–2.0 Å in the temperature range of 40–100 °C, indicating no SPT of the VO6 octahedron in Cr-VO2. There are slight changes in the position and the shape of the EXAFS peaks in the r-range of 2.2–3.3 Å, as shown in Fig. 8(c, d), which correspond to ten V atoms: two V atoms are located above and below along the b-axis and the other eight are located at the vertexes of the rutile VO2. The position and the shape of the V peak near 3.0 Å are evidently changed at the Tc of the SPT of a pristine VO2. A small shift of the V peak of the Cr-VO2 film with a flux of 5 × 1013 ions/cm2 occurs at 73 °C and 61 °C for heating and cooling, respectively. The small shift of the V peak is reproducible and consistent during heating and cooling processes, although it is quite weak. The Tc values of sharp MITs from the Cr-VO2 film are observed at 80 °C and 65 °C for heating and cooling, respectively, as shown in Fig. 1(d). No transitions in either the pre-edge peak or in the V–O distance of the Cr-VO2 film with a flux of 5 × 1013 ions/cm2 are observed, whereas a small shift occurs in the V–V distance. This indicates that both the pre-edge peak and the VO6 octahedron, which are directly related to the crystal field effects, are nearly irrelevant to the MIT of VO2. The structural change of the V sites could drive the MIT of VO2, although the V sites cannot maintain even a correct rutile symmetry above the Tc due to structural disorder. The transitions of the pre-edge peaks and the VO6 octahedrons occur accidentally with the SPT of VO2 crystals because the pre-edge peak is very sensitive to the nearest neighboring atoms around a probing atom and the V–O distance changes with the SPT. The EXAFS of the Cr-VO2 films suggests that a structural change of the V sites is related to the MIT, although the temperatures of the structural changes are not identical to the Tc values of the MIT during both heating and cooling.
Magnitude of Fourier transformed EXAFS (|FT(k3χ)|) as a function of the distance from a V atom at different temperatures. (a, b) EXAFS of Cr-VO2 with a Cr ion energy of 50 keV and a flux of 1013 ions/cm2 for heating and cooling, respectively. (c, d) EXAFS of Cr-VO2 with a Cr ion energy of 50 keV and a flux of 5 × 1013 ions/cm2 for heating and cooling, respectively. The data are vertically shifted for clarity. The vertical dashed lines indicate the position of eight V atoms located at the vertexes of a rutile VO2.
Figure 9(a, b) show temperature-dependent EXAFS from Co-VO2 with a Co ion flux of 1014 ions/cm2. EXAFS shows the SPTs of the films occurring at ~ 62 °C and ~ 56 °C for heating and cooling, respectively, which are comparable to the Tc values of 65 °C and 55 °C, as shown in Fig. 2(c). The SPT of Co-VO2 simultaneously appears at both O and V atomic sites at the same temperature. The SPT of Co-VO2 is similar to that of pristine VO2 but it is quite different from that of Cr-VO2 with a Cr ion flux of 5 × 1013 ions/cm2. The distortion of atomic pairs in Cr-VO2 and Co-VO2 is more obviously seen when the EXAFS data are directly compared to those of pristine VO2, as shown in Fig. 9(c, d). The EXAFS of pristine VO2 shows two obvious peaks in the r-space of 1.0–2.0 Å, which correspond to six V–O pairs. When the ion flux increases, the EXAFS peak intensity is decreased, the shape is deformed, and the positions are shifted relative to those of the pristine VO2. The significant deformation of the first two peaks of the Cr-and Co-VO2 films indicates that the VO6 octahedrons are seriously distorted due to the implanted ions. When the flux of Cr ions is larger than 5 × 1013 ions/cm2, VO2 cannot maintain standard VO6 octahedrons. EXAFS reveals that for an ion energy of 50 keV and a flux of 1013 ions/cm2, the first peaks of Co-VO2 are more distorted than that of Cr-VO2. The second peak of EXAFS at ~ 3.0 Å, which mainly corresponds to eight V atoms at the vertexes of a rutile phase VO2, is also affected by the implanted ions but the distortion of the V–V pairs is less significant than that of the V–O pairs, as shown in Fig. 9(c, d). For a Cr ion flux of 1012 ions/cm2, the intensity and the shape of the second peak from Cr-VO2 with an energy of both 30 keV and 50 keV are similar to those of the pristine VO2, implying that the V sites are slightly affected by the implanted Cr ions. As the ion flux increases, the structural distortion of the V sites also increases in both Cr-VO2 and Co-VO2. When the energy of Co ions becomes 100 keV, structural distortion, particularly at V sites, is somewhat reduced, compared to that at low ion energy, as shown in Fig. 9(d). This is not observed in Cr-VO2. The positions and shapes of the EXAFS peaks from Cr-VO2 with a Cr ion flux of 5 × 1013 ions/cm2 are significantly different from those of other specimens, implying serious distortion existing in all atomic sites. Interestingly, the position and shape of the third two peaks near 4.0 Å in Fig. 9(c, d) are similar to those of the pristine VO2, although the intensity is quite weak. The third peaks mainly correspond to further V atomic shells beyond a conventional cell of a rutile-phased VO2. Those peaks of Cr-VO2 with a flux of 5 × 1013 ions/cm2 show a slight temperature-dependent behavior, as shown Fig. 8(c, d). This is a further evidence that the V sites of Cr-VO3 with a flux of 5 × 1013 ions/cm2 still experience a weak SPT during both heating and cooling processes.
Discussion
Many researchers have observed that the MIT of a single crystal VO2 occurs simultaneously with its SPT at Tc ≈ 68 °C5,29. Since the MIT of VO2 is accompanied by an SPT, the contribution of each structural change, such as VO6 octahedrons, V–V dimers, and vertex V arrays, on the MIT is indistinguishable because the changes occur simultaneously in a single crystal VO2. EXAFS from Cr-VO2 and Co-VO2 films shows independent changes of structural properties of atomic shells during MIT. The direct comparison of electrical resistance and EXAFS measurements suggests that the contribution of the VO6 octahedrons on the MIT is negligible. The energy states of V 3d orbitals are split in the eg and t2g bands due to the crystal field effects of a VO6 octahedron in VO2. In Cr-VO2 with a flux ≥ 1013 ions/cm2 the V–O pairs are significantly disordered, so that the VO6 octahedrons cannot have regular splitting of the eg and t2g bands. This prevents any regular alignment of V 3d orbitals in the specimen and can exclude the possibility of conduction electrons jumping from a lower energy band of the \({d}_{xy}\) and \({d}_{xz}\) orbitals to a higher energy band of the \({d}_{\parallel }\)(\({d}_{{x}^{2}-{y}^{2}})\) to trigger the metallic phase VO2. A lack of temperature-dependent features of the pre-edge peak from Cr-VO2 with a flux ≥ 1013 ions/cm2 is further evidence of no regular splitting of V 3d states because the pre-edge peak corresponds to the eg and t2g bands7. A dramatic increase of the pre-edge peak intensity of Cr-VO2 indicates an increase of local density of states in the V 3d orbitals due to the structural distortion of VO6 octahedrons. The pre-edge peak intensity of the V K edge increased and decreased for V2O5 and V2O3, respectively, relative to that of VO2, because there are more empty states in the V 3d orbitals of V2O5 than of V2O341,55.
The dull pre-edge peak of VO2 at the V K edge consists of two peaks which correspond to the V 3d t2g and eg states, respectively, with an energy gap of ~ 2.0 eV7. XANES cannot detect the direct band gap because of its resolution limit. XANES from pristine VO2 shows that the intensities of the t2g (lower peak) and eg (upper peak) states decreases and increases, respectively, with no change of the band gap between the two states during heating7. As a result, the pre-edge peak seems to be shifted towards a higher energy for heating, as shown in Fig. 4(b, d). The pre-edge peaks of Cr-VO2 with a flux ≥ 1013 ions/cm2 show that the t2g band at ~ 5467.5 eV nearly disappears while the peak intensity of the eg states at ~ 5469.5 eV is very strong with no temperature dependence, as shown in Fig. 5(b, d). For the Cr-VO2 films with a flux ≥ 1013 ions/cm2, the lack temperature dependence of the pre-edge peak strongly implies no changes in the local density of states of the V 3d orbitals in the temperature range of 30–100 °C, although the films experience MIT and SPT. This is evidence indicating that the t2g and eg bands split by the crystal field effects are irrelevant to the MIT of VO2 and that an SPT of short-range orderings around the V atoms in VO2 does not directly contribute to the MIT. EXAFS shows an SPT and no SPT at the O sites of Cr-VO2 with a flux of 1013 and 5 × 1013 ions/cm2, respectively, while an SPT is observed at the V sites for both fluxes. This implies that the pre-edge peak of VO2 is mainly contributed by the nearest neighboring O atoms, rather than by the second neighboring V atoms. This contrasts to the previous studies of the pre-edge peak of transition metals, in which the authors discussed the contribution of the second neighboring atoms on the pre-edge peaks41,44,56.
On the other hand, traditional band theory cannot predict the bandgap of ~ 0.65 eV for M1-phased VO2 at room temperature6. Based on a structural-driven Peierls transition mechanism, two different distances of V–V pairs in M1 VO2 were introduced to understand the insulating phase of VO26,27,57,58,59,60. The dimerization model also cannot explain the measured bandgap of VO2 at room temperature6. Our EXAFS measurements and calculations [Supplementary Materials] on Cr-VO2 with a Cr flux of 5 × 1013 ions/cm2 reveal a substantial amount of structural disorder at both oxygen and vanadium sites. The linear defects created due to the implanted ions are placed perpendicular to the current direction in the DC electrical resistance measurements of the films. Cr-VO2 barely maintains a crystalline structure without regular V–V dimers and does not show an SPT of V–V dimers due to the enormous amount of structural disorder and distortion in the V sites, although there are obvious MITs during both heating and cooling. The distances of the V–V dimers are approximately ~ 2.5 Å and ~ 3.2 Å in the M1 phase and become ~ 2.8 Å in the rutile phase27,36. Our result is further evidence that a V–V dimerization model cannot explain the MIT mechanism of VO2. However, a structural-driven Peierls transition may not be excluded as an explanation for the MIT of VO2 because the EXAFS peaks which mainly correspond to the V sites show a weak SPT at Tc, as shown in Fig. 8(c, d). The sharp MIT features of the resistance curve from Cr-VO2 with a Cr ion flux of 5 × 1013 ions/cm2 strongly suggest a transition of interaction between conduction electrons at Tc. The EXAFS and resistance measurements of the Cr-VO2 film support that the MIT is highly related to the interaction of conduction electrons and is triggered by the alignment of the V atomic arrays near Tc.
Previous studies reported that the Tc values of Cr-added and Co-added VO2 shifted towards higher and lower temperatures, respectively37,38,39,40,42. The Tc values of both Cr- and Co-VO2 films with a flux ≥ 5 × 1013 ions/cm2 shift towards a higher temperature. Since the concentration ratio of the implanted ions is only ~ 0.023% for an ion flux of 1014 ions/cm2, the doping effects of the ions could be negligible. V1−xTixO2 also showed that the Tc decreased and increased for low and high concentrations of Ti4+, respectively44. Ti4+ ions which are mostly replaced at the V4+ sites of VO2 can cause the disorder and distortion of the V sites without doping effects. Previous studies of heavy ion irradiation with high energy on VO2 showed that the resistivity and the Tc value of VO2 were considerably modified due to an extra structural disorder61,62. Hofsäss and coworkers showed that 1 GeV 238U swift heavy ions substantially decreased the Tc value of VO2, although no surface hillocks were observed61. When 200 meV Ag9+-ions with a high flux bombarded VO2, the surface and the crystal symmetry of VO2 were seriously damaged. Both Tc value and resistivity jump size of the MIT of VO2 continuously decreased when the fluence of Ag9+ ions increased62. This result is somewhat different from that of Cr-VO2, Co-VO2, and V1−xTixO2, as discussed above. Since most of implanted Cr and Co ions remain in VO2 films, they play as impurities in addition to the ion tracks. Impurities in VO2 can modify the band structure, contribute the charge carrier density of the conduction band, disturb the SPT, and interrupt the propagation of electrons. When Cr concentration increased in VO2, both lattice constants and structural disorder of V1−xCrxO2 increased, while the Tc value moved towards a higher temperature during both heating and cooling38,39. This is comparable to the Tc behavior of Cr-VO2 with the ion flux of 5 × 1013 ions/cm2.
The resistance and EXAFS measurements of the Cr- and Co-VO2 films with different ion energies and fluxes show that the SPT always occurs before and after the MIT during heating and cooling, respectively. This indicates that a percolation effect is negligible in the systems and that an SPT, particularly the V atomic arrays, is an essential prerequisite for the MIT of VO2. This corresponds to that of the pristine VO2. A few defects in VO2 assist SPTs during heating and cooling whereas many defects interrupt SPTs. As a result, △Tc (Tc heating − Tc cooling) becomes small and large, as shown in Figs. 1 and 2, respectively. When the concentration of defects is larger than a critical value, the MIT of VO2 can be totally destoryed, as reported in previous studies61,62. The total amount of defects in a film due to implanted ions increases with increase in the flux and the penetration depth of the ions because the ions create linear tracks. When the energy of the implanted ions increases, the penetration depth is expanded, leading to the creation of more defects in the film. This scenario is consistent with the results of the Cr- and Co-VO2 films with different energies and the same flux. For an ion flux of 1014 ions/cm2, MIT features from Cr-VO2 are significantly reduced while an obvious MIT is observed from Co-VO2. EXAFS reveals that Cr ions more seriously affect the O sites of VO2 than those of Co ions, as shown in Figs. 8 and 9. The penetration depths of the two ions on VO2 are roughly the same, as shown in the supplementary materials. Researchers observed that the Tc values of V1−xCrxO2 and V1−xCoxVO2 increased and decreased, respectively, relative to that of pristine VO238,39,42, This implies that the contributions of Cr and Co ions on the MIT of VO2 are not the same, as they interrupt and assist the SPT, respectively. EXAFS measurements reveal that Cr ions more effectively destroy the crystalline structure of VO2 than Co ions do. The different effects of Cr and Co ions to VO2 could be attributed to the different radii and the different oxidation states of Cr3+ and Co2+ ions. This study indicates that the Tc of VO2 can be increased or decreased by careful selection of a proper species of ions with different energies and fluxes.
Conclusions
For the Cr and Co ion fluxes ≤ 1014 ions/cm2, both Cr- and Co-VO2 show sharp MIT features near Tc. The Tc of both the Cr- and Co-VO2 films with a low ion flux is lower than that before ion implantation, while it shifts toward a higher temperature for a high ion flux. This indicates that the Tc of VO2 can be engineered by properly selecting the flux, energy, and species of ion beam. Both Cr and Co ions create a substantial amount of structural disorder and distortion in VO2. Based on resistance and EXAFS measurements, model calculations suggest that a sharp and abrupt MIT and SPT can occur in VO2 unless more than 5% of the V sites are disturbed by impurities. Temperature-dependent XANES from Cr-VO2 at the V K edge showed that the pre-edge peak alone cannot fully describe either the MIT or the SPT. Temperature-dependent resistance and EXAFS measurements reveal that crystal field splitting in the VO6 octahedron of VO2 does not play a critical role in the MIT. These study results suggest that an SPT of the V atomic arrays and the interaction of V 3d1 electrons are the necessary conditions for the MIT of VO2, supporting both the structural-driven-Peierls and Mott–Hubbard models. This study also shows that ion-implantation techniques can be widely used to engineer the Tc and the MIT of VO2, and particularly of VO2 nanostructures, without degrading the sharpness of the MIT features23.
Methods
Synthesis of VO2 films
The b-oriented VO2 films were fabricated on α-Al2O3(0001) substrates using direct current (DC)-sputtering deposition from a vanadium target with a purity of 99.95%. The base vacuum of the growth chamber was 10–6 Torr and the pressure was kept at 10–3 Torr during the deposition. Ar gas was used as the plasma and the substrate temperature was maintained at ~ 500 °C. After deposition, the films were annealed at 500 °C for 30 min with a mixture gas flow of Ar: O2 = 300:1. More details of VO2 film fabrication can be found elsewhere in the literatures7,36.
Cr and Co ion implantation on VO2 films
Cr and Co ions with an energy of 30–100 keV and a flux of 1012–1014 ions/cm2 vertically bombarded the VO2 films at room temperature in a vacuum at the Korean Multi-Purpose Accelerator Complex (KOMAC).
In-situ XAFS measurements
Temperature-dependent XAFS measurements were conducted from Cr-VO2 and Co-VO2 films and a pristine VO2 film as a counterpart at the V K edge (5465 eV). XAFS measurements were performed with a fluorescence mode using a Si(111) double-crystal monochromator at beamline 8C of the Pohang Light Source (PLS) and beamline 20-BM of the Advanced Photon Source (APS). The XAFS data were taken with an unpolarized geometry where the angle between the film surface and the incident X-ray beam was fixed at 45 degrees. During in-situ temperature-dependent XAFS measurements, the DC electrical resistance was simultaneously measured from the same specimens7,33. A thermocouple was directly contacted to the surface of a VO2 film to accurately measure the true temperature of the film in real time. The resistance and the temperature were recorded after the temperature at each set temperature was stabilized. The temperature was monitored and controlled within ± 0.1 degree during the XAFS scans and the resistance measurements. Each XAFS scan took approximately 15 min.
DC resistance measurements
Two-probe DC-resistance measurements were performed from pristine, Cr-VO2, and Co-VO2 films before ion-implanted at the applied voltage of 0.5 V using a Keithley 2400 SourceMeter7,36. After the ions were implanted, the resistance measurements were simultaneously performed with in-situ XAFS measurements.
References
Morine, F. J. Oxides which show a metal-to-insulator transition at the Neel temperature. Phys. Rev. Lett. 3, 34 (1959).
Goodenough, J. B. The two components of the crystallographic transition in VO2. J. Solid State Chem. 3, 490 (1971).
Eyert, V. The metal-insulator transitions of VO2: a band theoretical approach, Ann. Phys. (Leipzig) 11, 650 (2002).
Yang, Z. et al. Oxide electronics utilizing ultrafast metal-insulator transitions. Ann. Rev. Mater. Res. 41, 337 (2011).
Qazilbash, M. M. et al. Basov, Mott transition in VO2 revealed by infrared spectroscopy and nano-imaging. Science 318, 1750 (2007).
Shao, Z. et al. Recent progress in the phase-transition mechanism and modulation of vanadium dioxide materials. NPG Asia Mater. 10, 581 (2018).
Hwang, I.-H. et al. The influence of structural disorder and phonon on metal-to-insulator transition of VO2. Sci. Rep. 7, 14802 (2018).
Kim, H.-T. et al. Mechanism and observation of Mott transition in VO2-based two- and three-terminal devices. New J. Phys. 6, 52 (2004).
Shukla, N. et al. A steep-slope transistor based on abrupt electronic phase transition. Nat. Commun. 6, 7812 (2015).
Zhou, J. et al. VO2 thermochromic smart window for energy savings and generation. Sci. Rep. 3, 3029 (2013).
Liang, S. et al. One-step hydrothermal synthesis of W-doped VO2 (M) nanorods with a tunable phase-transition temperature for infrared smart windows. ACS Omega 1, 1139 (2016).
Strelcov, E. et al. Gas sensor based on metal-insulator transition in VO2 nanowire thermistor. Nano Lett. 9, 2322 (2009).
Reddy, C. V. S. et al. Synthesis of VO2 (B) nanorods for Li battery application. Curr. Appl. Phys. 9, 1195 (2009).
Mjejri, I. et al. Vanadium oxides nanostructures: Hydrothermal synthesis and electrochemical properties. Ceram. Int. 40, 1387 (2014).
Cilento, F. et al. Ultrafast insulator-to-metal phase transition as a switch to measure the spectrogram of a supercontinuum light pulse. Appl. Phys. Lett. 96, 021102 (2010).
Liu, M. et al. Terahertz-field-induced insulator-to-metal transition in vanadium dioxide metamaterial. Nature 487, 345 (2012).
Tan, X. et al. Unraveling metal-insulator transition mechanism of VO2 triggered by tungsten doping. Sci. Rep. 2, 466 (2012).
Chen, L. et al. Atomic and electronic structures of charge-doping VO2: first-principles calculations. RSC Adv. 10, 18543 (2020).
Matsuda, Y. H. et al. Magnetic-field-induced insulator–metal transition in W-doped VO2 at 500 T. Nat. Commun. 11, 3591 (2020).
Xing He, X. et al. Photoinduced strain release and phase transition dynamics of solid-supported ultrathin vanadium dioxide. Sci. Rep. 7, 10045 (2017).
Jeong, J. et al. Suppression of metal-insulator transition in VO2 by electric field-induced oxygen vacancy formation. Science 339, 1402 (2013).
J. Cao, J. et al. Strain engineering and one-dimensional organization of metal-insulator domains in single-crystal vanadium dioxide beams, Nat. Nanotech. 4, 732 (2009).
Hongwei, L. et al. Size effects on metal-insulator phase transition in individual vanadium dioxide nanowires. Opt. Express 22, 30748 (2014).
Wentzcovitch, R. M. et al. VO2: peierls or Mott–Hubbard? A view from band theory. Phys. Rev. Lett. 72, 3389 (1994).
Yao, T. et al. Understanding the nature of the kinetic process in a VO2 metal-insulator transition. Phys. Rev. Lett. 105, 226405 (2010).
Kim, H.-T. et al. Monoclinic and correlated metal phase in VO2 as evidence of the Mott transition: coherent phonon analysis. Phys. Rev. Lett. 97, 266401 (2006).
Xu, S. et al. Unified band-theoretic description of structural, electronic, and magnetic properties of vanadium dioxide phases. Phys. Rev. B 95, 125105 (2017).
Brito, W. H. et al. Metal-insulator transition in VO2: a DFT + DMFT perspective. Phys. Rev. Lett. 117, 056402 (2016).
Li, Z. et al. Imaging metal-like monoclinic phase stabilized by surface coordination effect in vanadium dioxide nanobeam. Nat. Commun. 8, 15561 (2017).
Biermann, S. et al. Dynamical singlets and correlation-assisted Peierls transition in VO2. Phys. Rev. Lett. 94, 026404 (2005).
Mun, B. S. et al. Nonpercolative metal-insulator transition in VO2 single crystals. Phys. Rev. B 84, 113109 (2011).
Muraoka, Y. et al. Metal–insulator transition of VO2 thin films grown on TiO2 (001) and (110) substrates. Appl. Phys. Lett. 80, 583 (2002).
Jin, Z. et al. Structural and electrical properties of VO2/ZnO nanostructures. Curr. Appl. Phys. 18, 353 (2018).
Wong, F. J. et al. Epitaxial variants of VO2 thin films on complex oxide single crystal substrates with 3m surface symmetry. J. Crystal Growth 364, 74 (2013).
Hwang, I.-H. et al. Anomalous structural disorder and distortion in metal-to-insulator-transition Ti2O3. J. Appl. Phys. 119, 014905 (2016).
Jin, Z. et al. Synthesis and temperature-dependent local structural and electrical properties of VO2 films. Curr. Appl. Phys. 16, 183 (2016).
Marezio, M. et al. Structural aspects of the metal-insulator transitions in Cr-Doped VO2. Phys. Rev. B 5, 2541 (1972).
Brown, B. L. et al. Electrical and optical characterization of the metal-insulator transition temperature in Cr-doped VO2 thin films. J. Appl. Phys. 113, 173704 (2013).
Zou, Z. et al. Thermochromic, threshold switching, and optical properties of Cr doped VO2 thin films. J. Alloy Compd. 806, 310 (2019).
Marini, C. et al. Optical properties of V1−xCrxO2 compounds under high pressure. Phys. Rev. B 77, 235111 (2008).
Wu, Y. et al. Depressed transition temperature of WxV1−xO2: mechanistic insights from the X-ray absorption fine structure (XAFS) spectroscopy. Phys. Chem. Chem. Phys. 16, 17705 (2014).
Lu, C. et al. Terahertz transmittance of cobalt-doped VO2 thin film: Investigated by terahertz spectroscopy and effective medium theory. IEEE Trans. THz. Sci. Tech. 9, 177 (2019).
Holman, K. L. et al. Insulator to correlated metal transition in V1−xMoxO2. Phys. Rev. B 79, 245114 (2009).
Wu, Y. et al. Decoupling the lattice distortion and charge doping effects on the phase transition behavior of VO2 by titanium (Ti4+) doping. Sci. Rep. 5, 9328 (2015).
Biersack, J. P. et al. A Monte Carlo computer program for the transport of energetic ions in amorphous targets. Nucl. Instrum. Methods 174, 257 (1980).
Park, C.-I. et al. Linear defects and electrical properties of ZnO nanorods. Appl. Phys. Lett. 112, 253101 (2018).
Fu, D. et al. Comprehensive study of the metal-insulator transition in pulsed laser deposited epitaxial VO2 thin films. J. Appl. Phys. 113, 043707 (2013).
Lee, S. et al. Electronic structure and insulating gap in epitaxial VO2 polymorphs. APL Mater. 3, 126109 (2015).
Moatti, A. et al. Electrical transition in isostructural VO2 thin-film heterostructures. Sci. Rep. 9, 3009 (2019).
Sayers, D. E. et al. New technique for investigating noncrystalline structures: Fourier analysis of the extended X-ray-absorption fine structure. Phys. Rev. Lett. 27, 1204 (1971).
Rehr, J. J. et al. Theoretical approaches to X-ray absorption fine structure. Rev. Mod. Phys. 72, 621 (2000).
Han, S.-W. X-ray absorption fine structure and nanostructures. Int. J. Nanotechnol. 3, 396 (2006).
Newville, M. IFEFFIT: interactive XAFS analysis and FEFF fitting. J. Synchrotron Radiat. 8, 322 (2001).
Han, S.-W. et al. Local structure in the stripe phase of La1.6−xSrxNd0.4CuO4, Phys. Rev. B 66, 094101 (2002).
Lu, Y.-R. et al. Atomic and electronic aspects of the coloration mechanism of gasochromic Pt/Mo-modified V2O5 smart films: an in situ X-ray spectroscopic study. Phys. Chem. Chem. Phys. 18, 5203 (2016).
Seo, S.-Y. et al. X-ray absorption fine structure study of cobalt ion distribution in ferromagnetic Zn1−xCoxO films. J. Phys.: Condens. Matter. 25, 256005 (2013).
Laverock, J. et al. Observation of weakened V–V dimers in the monoclinic metallic phase of strained VO2. Phys. Rev. Lett. 121, 256403 (2018).
Holz, A. et al. Theory of discontinuos metal-insulator and spin-dimerization transition in VO2. J. Magn. Magn. Mater. 7, 329 (1978).
Zhang, R. et al. Understanding of metal-insulator transition in VO2 based on experimental and theoretical investigations of magnetic features. Sci Rep. 8, 17093 (2018).
Wall, S. et al. Ultrafast disordering of vanadium dimers in photoexcited VO2. Science 362, 572 (2018).
Hofsäss, H. et al. Tuning the conductivity of vanadium dioxide films on silicon by swift heavy ion irradiation. AIP Adv. 1, 032168 (2011).
Khan, G. R. et al. Augmentation of thermoelectric performance of VO2 thin films irradiated by 200 MeV Ag9+-ions. Radiat. Phys. Chem. 123, 55 (2016).
Acknowledgements
The work was conducted under the auspices of the Basic Science Research Program through the National Research Foundation of Korea government grant funded by the Ministry of Education (Nos. 2017K1A3A7A09016390, 2020K1A3A7A09080403) and the research funds of Jeonbuk National University in 2019. The Cr- and Co-ion implantation was performed at KOMAC in Korea and the XAFS data were collected at the beamline 8C of PLS in Korea and beamline 20-BM of APS in USA. This research used resources of APS, an Office of Science User Facility operated for the U.S. Department of Energy (DOE) Office of Science by Argonne National Laboratory, and was supported by the U.S. DOE under Contract No. DE-AC02-06CH11357, and the Canadian Light Source and its funding partners.
Author information
Authors and Affiliations
Contributions
I.-H.H., C.-I.P., C.-J.S., and S.-W.H. performed in-situ XAFS and electrical resistance measurements. S.Y. and C.-I.P. performed Cr and Co ion-implantation experiments on VO2 and examined the distributions of implanted Cr and Co ions on VO2. I.-H.H. synthesized VO2 films using the DC sputtering deposition, performed DC-electrical resistance measurements, and analyzed the data of the resistance and the XAFS. S.-W.H. designed this study and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hwang, IH., Park, CI., Yeo, S. et al. Decoupling the metal insulator transition and crystal field effects of VO2. Sci Rep 11, 3135 (2021). https://doi.org/10.1038/s41598-021-82588-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-82588-4
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.