Abstract
The purpose of this study was to determine whether dynamic elastance EAdyn derived from echocardiographic measurements of stroke volume variations can predict the success of a one-step decrease of norepinephrine dose. In this prospective single-center study, 39 patients with vasoplegic syndrome treated with norepinephrine and for whom the attending physician had decided to decrease norepinephrine dose and monitored by thermodilution were analyzed. EAdyn is the ratio of pulse pressure variation to stroke volume variation and was calculated from echocardiography stroke volume variations and from transpulmonary thermodilution. Pulse pressure variation was obtained from invasive arterial monitoring. Responders were defined by a decrease in mean arterial pressure (MAP) > 10% following norepinephrine decrease. The median decrease in norepinephrine was of 0.04 [0.03–0.05] µg kg−1 min−1. Twelve patients (31%) were classified as pressure responders with a median decrease in MAP of 13% [12–15%]. EAdyn was lower in pressure responders (0.40 [0.24–0.57] vs 0.95 [0.77–1.09], p < 0.01). EAdyn was able to discriminate between pressure responders and non-responders with an area under the curve of 0.86 (CI95% [0.71 to1.0], p < 0.05). The optimal cut-off was 0.8. EAdyn calculated from the echocardiographic estimation of the stroke volume variation and the invasive arterial pulse pressure variation can be used to discriminate pressure response to norepinephrine weaning. Agreement between EAdyn calculated from echocardiography and thermodilution was poor. Echocardiographic EAdyn might be used at bedside to optimize hemodynamic treatment.
Similar content being viewed by others
Introduction
Acute circulatory failure is the result of several pathological mechanisms: hypovolemia, cardiac failure and/or vasoplegia. In the critically ill, arterial hypotension resulting from vasoplegia is a common symptom of a more complex process. Vasopressors are used to treat arterial hypotension, and they are therefore one of the main treatments for acute circulatory failure. Norepinephrine is the first-line vasopressor in intensive care1,2,3. Once the patient is stable and the underlying disease is treated, vascular function is progressively restored, and vasopressor weaning can start. Because prolonged weaning is responsible for unnecessary exposure to vasopressors (and their side effects) and because inappropriate early weaning can lead to arterial hypotension with tissue hypoperfusion, optimal vasopressor weaning is a cornerstone of hemodynamic treatment4,5.
Dynamic elastance is a real time indicator of the interaction between the heart and the vascular system6. Dynamic elastance (EAdyn) might be estimated as the ratio of stroke volume variation (SVV) to pulse pressure variation (PPV). Authors have demonstrated that EAdyn measured by calibrated pulse contour analysis (PCA- EAdyn) and by uncalibrated pulse contour analysis can predict the pressure response to a decrease in norepinephrine7,8,9. Patients with acute circulatory failure and treated with vasopressors are not always monitored by calibrated pulse contour analysis. However, PPV is available for most ICU patients and SVV can be measured by echocardiography. Because the agreement between critical care echocardiography and transpulmonary dilution is moderate10, we aimed to determine whether EAdyn calculated from echocardiographic SVV (TTE- EAdyn) could discriminate pressure response to a decrease in the dose of norepinephrine. Our secondary objective was to determine the agreement between EAdyn measured by calibrated pulse contour analysis and EAdyn measured by echocardiography.
Results
Baseline characteristics
Baseline characteristics are shown in Table 1. The median age was 67 [58–73] years, and 41% of patients had septic shock. Of the 39 patients included in the study, 12 were classified as pressure responders because their MAP decreased more than 10% (Supplementary file 1). The median dose of norepinephrine was 0.2 [0.09–0.38] µg.kg−1 min−1, with no difference between responders and non-responders. The median decrease in norepinephrine was 0.04 [0.03–0.05] µg.kg−1 min−1, again with no significant difference between groups (0.04 [0.03–0.04] vs 0.04 [0.04–0.05] µg.kg−1 min−1, p = 0.20).
Primary outcome
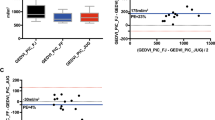
At baseline, TTE-EAdyn was lower in pressure responders (0.40 [0.24–0.57] vs 0.95 [0.77–1.09], p < 0.01) (Table 2), and the reduced dose of norepinephrine significantly decreased the MAP. The decrease in MAP significantly differed between groups (15% (± 5) vs 3% (± 5)). With an AUC of 0.86 (CI95% [0.71 to1.0], p < 0.05), the TTE-EAdyn predicted the decrease in arterial pressure (Fig. 1, Supplementary file 1). The optimal cut-off was of 0.80 with a sensitivity of 92% (CI95% [62–100]), a specificity of 74% (CI95% [54–89]), a positive likelihood ratio of 3.54, and a negative likelihood ratio of 0.11. The bounds for inconclusive TTE-EAdyn were 0.47 and 0.8, and 7 patients (18%) had inconclusive TTE-EAdyn values (Supplementary file 2, Table 3). The PCA-EAdyn predicted the decreased in MAP with an AUC of 0.86 (CI95% [0.67–0.96]). The optimal cut-off was of 0.90.
Agreement between TTE-E Adyn and PCA-E Adyn
The agreement between TTE-EAdyn and PCA-EAdyn is presented in Fig. 2. The median bias was − 0.13 [− 0.39 to − 0.06] with the limit of agreement between − 0.65 and 0.92 (Fig. 2). The concordance correlation coefficient was poor (0.31 [0.13–0.48]).
Bland–Altman plots for the measure of dynamic arterial elastance from echocardiography and from pulse contour analysis (transpulmonary thermodilution). The plain lane represents the median, dashed lines represent the 2.5th and 97.5th percentiles. TEE-EAdyn dynamic elastance calculated from transthoracic echocardiography, PCA-EAdyn dynamic elastance calculated from pulse contour analysis.
Discussion
The results of this study suggest that EAdyn calculated from SVV measured by echocardiography can be used to discriminate pressure response to a decrease in norepinephrine dose. However, we found that there was poor agreement between TTE-EAdyn and PCA-EAdyn despite good ability to predict pressure response.
EAdyn is usually obtained with an invasive hemodynamic monitoring system, but such devices may be unavailable in our patients. On the contrary, point-of-care ultrasound is an extensively used and non-invasive tool11. Thus, extending the validity of EAdyn to echocardiographic measurements means that more patients could benefit from this indicator. Ultrasound is an operator-dependent technique with lower reproducibility than hemodynamic devices. In addition, studies have highlighted inconsistencies between thermodilution and ultrasound for cardiac output monitoring12. In the present cohort, the agreement between echocardiographic EAdyn and EAdyn calculated by thermodilution was low. The bias was negative, meaning that SVV was overestimated by echocardiography. This observation explains why our cut-off was lower than those described by thermodilution7. Our observations are in line with those of De Castro et al., who demonstrated poor agreement between SVV measured with pulse contour analysis and aortic Doppler13. This low agreement may be explained by several factors: the calculation of SVmax and SVmin with echocardiography was manually performed, whereas those calculated by EV100 system may automatically measure, and the time sample to calculate the SVV with the EV1000 system may differ of this of echocardiography.
In practice, EAdyn is an easy-to-read indicator that could be used by the physician to understand the arterial load and its coherence with cardiac status (i.e. ventriculo-arterial coupling) and thus to determine which treatments should be used or withdrawn. High EAdyn might indicate an inappropriately high arterial load compared with stroke volume. According to the concept of ventriculo-arterial coupling, stroke work is optimized when the ventricular and arterial systems are coupled14. Because of its action on both α and β adrenergic receptors15, norepinephrine has an effect on arterial load, cardiac preload and /or cardiac contractility16. Norepinephrine might therefore be associated with both preload and non-preload stroke volume changes17. In patients with high EAdyn, lowering arterial load by decreasing norepinephrine might improve ventriculo-arterial coupling. The optimization of stroke work could compensate for the drop in arterial vasoconstriction, explaining the good tolerance (MAP non-response) to norepinephrine weaning. The method developed by Chen to obtain EAdyn requires complex calculation18 and simplified methods are not reliable in the ICU19; these limits suggest that bedside measurement of EAdyn may be more clinically appropriate than the measurement of ventriculo-arterial coupling. Recently, Monge Garcia et al. have demonstrated that Eadyn was inversely related to ventriculo-arterial coupling6, and that Eadyn could be used to track changes of ventriculo-arterial coupling following hemodynamic treatment.
We acknowledge that this study has some limitations. First, the small sample size limits the power of the analysis, however, the results are significant and the sample size is in accordance with previous studies7,20,21. The sample size also limited the use of parametric approach and prevented the realisation of more specific analysis such as ROC curve comparison between TTE-EAdyn and PCA-EAdyn (or TTE-SVV and PCA-SVV). The monocentric nature of the study limits the external validity, but our results are in accordance with previously reported data7,8,9. Finally, we did not measure ventriculo-arterial coupling.
Conclusion
EAdyn calculated from the echocardiographic estimation of the SVV and the invasive arterial PPV could discriminate pressure response to norepinephrine decrease. Despite the poor agreement between SVV measurements obtained by thermodilution and by echocardiography, EAdyn might be useful at the patient’s bedside to optimize vasopressive treatment.
Methods
Patients
This is an ancillary study using data from a prospective observational cohort. The study objectives and procedures were approved by the local ethics committee (Comité de Protection des Personnes Nord-Ouest II CHU—Place V. Pauchet, 80054 AMIENS Cedex 1). All subjects or next of kin (closest parent, legal guardian) if the patient was unable to be informed, received written information about the study and provided their informed consent to participate. The study was performed in accordance with the ethical standards of the 1964 Declaration of Helsinki. This prospective, observational study was conducted in the intensive care unit (ICU) of the Amiens University Hospital between July 2016 and March 2017. We included patients with a diagnosis of vasoplegic syndrome, treated with norepinephrine, and for whom the attending physician decided to decrease the norepinephrine dosage. Patients treated with epinephrine and/or dobutamine, and patients with arrhythmia, intra-abdominal hypertension or younger than 18 years old were excluded.
Hemodynamic parameters
All patients were monitored with arterial catheterisation, central venous pressure (CVP) and transpulmonary thermodilution (EV1000 system, EDWARDS, Irvine CA, USA). Transthoracic echocardiography (CX50 Ultrasound System and an S5-1 Sector Array Transducer, PHILIPS MEDICAL SYSTEM, Suresnes, France) was performed by a physician with advanced echocardiography certificate. Left ventricular ejection fraction (LVEF) was calculated using Simpson’s method on a four-chamber view. The diameter of the left ventricular outflow tract (LVOT) was measured on a long-axis parasternal view at the time of patient inclusion. The LVOT velocity–time integral (VTILVOT) was measured with pulsed Doppler on a five-chamber apical view. Stroke volume (SV; mL) was calculated as VTIAo × aortic area. Cardiac output (CO) (l/min) was calculated as SV × heart rate (HR). Respiratory variation of SV (SVVTTE) was calculated as: SVVTTE = (SVmax–SVmin)/((SVmax + SVmin)/2))*100. The maximum and minimum SV values were identified during one respiratory cycle (expiratory/inspiratory times), and averaged over 5 cycles to obtain SVmax and SVmin22,23. We calculated the reproducibility of SVVTTE that was 8.1 (± 4.4) %. The physician who performed echocardiography was blinded to the results of the study.
PPV was automatically calculated by the Philips monitoring system, representing the average of five successive values. SVV and PPV were sampled during the same time period on a radial arterial line13. We measured SVV with a calibrated pulse contour analysis (EV1000, Edwards Life science Irvine, software version 4.0), representing the average of three successive values. Echocardiographic dynamic arterial elastance (TTE-EAdyn) was calculated as the ratio of PPV/SVVTTE, and calibrated pulse contour analysis dynamic arterial elastance (PCA-EAdyn) was calculated as PPV/SVV.
Study procedures
The intervention assessed in this study was a one-step decrease in the dose of norepinephrine. The following clinical parameters were recorded for each patient: age, gender, surgical/medical history, and indications for norepinephrine treatment. Hemodynamic variables including HR, systolic arterial pressure (SAP), mean arterial pressure (MAP), diastolic arterial pressure (DAP), central venous pressure (CVP), SVVTTE, SVV, PPV, and cardiac output (CO) were recorded at baseline. The dose of norepinephrine was then decreased. After the hemodynamic variables had stabilised, defined as < 10% variation in MAP over a 30-min period, a second set of measurements (HR, SAP, MAP, DAP, CVP, PPV, SVV, SVVTTE CO) was recorded. Ventilator settings and sedation were kept constant throughout the study period. All patients had mechanical ventilation in volume-controlled mode. Ventilator settings (oxygen inspired fraction, tidal volume, respiratory rate and end positive pressure) were not modified during the study period.
Statistical analyses
Retrospective power analysis shows that a sample of 39 patients enable to demonstrate an area under the receiver-operating-characteristic curve greater than 0.77, a power of 80%, and an α risk of 0.05. The distribution of variables was assessed using a Shapiro–Wilk test. Data are expressed as numbers, proportions (in percent), medians [interquartile range] or as means (standard deviation). The coefficient of variation (CV), precision and least significant change (LSC) for MAP were calculated on the overall population. The LSC was 7% (confidence interval 95% (CI95%): 4–10). Based on LSC, we defined a positive response (pressure responders) as a decrease in MAP of over 10%8. Qualitative data were compared with a chi-squared test or Fisher’s test, and quantitative data were assessed with a Student’s t test or Kruskal–Wallis sum rank test, as appropriate. Paired data were compared with paired Student’s t test or Wilcoxon signed rank test. Changes in hemodynamic variables were expressed as percentages from baseline value. Receiving operator characteristic (ROC) curves were drawn and the areas under the curve (AUC) were calculated. The optimal cut-point was determined using the Youden Method. The concordance correlation coefficient between TTE-EAdyn and PCA-EAdyn was calculated, and the agreement was represented on a Bland–Altman plot. A grey-zone approach was used, and values for which both sensitivity and specificity were lower than 90% were considered inconclusive. The threshold for statistical significance was set at p < 0.05. RSTUDIO (Version 1.1.447-2009-2018 RSTUDIO, Inc.) was used for all statistical analyses.
Ethics approval and consent to participate
This is an ancillary study using data from a prospective observational cohort. The study objectives and procedures were approved by the local ethics committee (Comité de Protection des Personnes Nord-Ouest II CHU—Place V. Pauchet, 80054 AMIENS Cedex 1). All subjects or next of kin (closest parent, legal guardian) if the patient was unable to be informed, received written information about the study and provided their informed consent to participate.
Data availability
All relevant data are within the paper. Raw data are available after notification and authorization of the competent authorities. In France, all computer data (including databases, particular patient data) are protected by the National Commission on Informatics and Liberty (CNIL), the national data protection authority for France. CNIL is an independent French administrative regulatory body whose mission is to ensure that data privacy law is applied to the collection, storage, and use of personal data. As the database of this study was authorized by the CNIL, we cannot make available data without prior agreement of the CNIL. Requests may be sent to: elisabeth.laillet@chu-dijon.fr.
Abbreviations
- AUC:
-
Area under the curve
- CI:
-
Confidence interval
- CO:
-
Cardiac output
- CV:
-
Coefficient of variation
- CVP:
-
Central venous pressure
- DAP:
-
Diastolic arterial pressure
- EAdyn :
-
Dynamic arterial elastance
- HR:
-
Heart rate
- LSC:
-
Least significant change
- LVEF:
-
Left ventricular ejection fraction
- LVOT:
-
Left ventricular outflow tract
- MAP:
-
Mean arterial pressure
- PCA:
-
Pulse contour analysis
- PPV:
-
Pulse pressure variation
- ROC:
-
Receiving operator characteristic
- SAP:
-
Systolic arterial pressure
- SV:
-
Stroke volume
- SVV:
-
Stroke volume variation
- TTE:
-
Transthoracic Echocardiography
- VTILVOT :
-
Left ventricular outflow tract velocity–time integral
References
Rhodes, A. et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 43, 304–377 (2017).
Thiele, H., Ohman, E. M., Desch, S., Eitel, I. & de Waha, S. Management of cardiogenic shock. Eur. Heart J. 36, 1223–1230 (2015).
Duranteau, J. et al. Recommandations sur la réanimation du choc hémorragique. Anesth. Réanimation 1, 62–74 (2015).
Boissier, F. et al. Left ventricular systolic dysfunction during septic shock: the role of loading conditions. Intensive Care Med. 43, 633–642 (2017).
Lamontagne, F. et al. Pooled analysis of higher versus lower blood pressure targets for vasopressor therapy septic and vasodilatory shock. Intensive Care Med. 44, 12–21 (2018).
Monge García, M. I. et al. Dynamic arterial elastance as a ventriculo-arterial coupling index: an experimental animal study. Front. Physiol. 11, 284 (2020).
Guinot, P.-G., Bernard, E., Levrard, M., Dupont, H. & Lorne, E. Dynamic arterial elastance predicts mean arterial pressure decrease associated with decreasing norepinephrine dosage in septic shock. Crit. Care 19, 14 (2015).
Bar, S. et al. Dynamic arterial elastance measured by uncalibrated pulse contour analysis predicts arterial-pressure response to a decrease in norepinephrine. Br. J. Anaesth. 7, 1–7 (2018).
Guinot, P.-G. et al. Monitoring dynamic arterial elastance as a means of decreasing the duration of norepinephrine treatment in vasoplegic syndrome following cardiac surgery: a prospective, randomized trial. Intensive Care Med. 43, 643–651 (2017).
Vignon, P. et al. Hemodynamic assessment of patients with septic shock using transpulmonary thermodilution and critical care echocardiography: a comparative study. Chest 153, 55–64 (2018).
Zieleskiewicz, L. et al. Point-of-care ultrasound in intensive care units: assessment of 1073 procedures in a multicentric, prospective, observational study. Intensive Care Med. 41, 1638–1647 (2015).
Zhang, Y. et al. Ultrasound cardiac output monitor and thermodilution for cardiac function monitoring in critical patients: a Meta-analysis. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 31, 1462–1468 (2019).
De Castro, V. et al. Comparison of stroke volume (SV) and stroke volume respiratory variation (SVV) measured by the axillary artery pulse-contour method and by aortic Doppler echocardiography in patients undergoing aortic surgery. Br. J. Anaesth. 97, 605–610 (2006).
Sunagawa, K., Maughan, W. L. & Sagawa, K. Optimal arterial resistance for the maximal stroke work studied in isolated canine left ventricle. Circ. Res. 56, 586–595 (1985).
Zimmerman, J., Lee, J. P. & Cahalan, M. Vasopressors and Inotropes. in Pharmacology and Physiology for Anesthesia 520–534 (Elsevier, 2019). https://doi.org/10.1016/B978-0-323-48110-6.00025-9.
Annane, D. et al. A global perspective on vasoactive agents in shock. Intensive Care Med. 44, 833–846 (2018).
Guinot, P.-G., Longrois, D., Kamel, S., Lorne, E. & Dupont, H. Ventriculo-arterial coupling analysis predicts the hemodynamic response to norepinephrine in hypotensive postoperative patients: a prospective observational study. Crit. Care Med. 46, e17–e25 (2018).
Chen, C.-H. et al. Noninvasive single-beat determination of left ventricular end-systolic elastance in humans. J. Am. Coll. Cardiol. 38, 2028–2034 (2001).
Nguyen, M. et al. Agreement between different non-invasive methods of ventricular elastance assessment for the monitoring of ventricular–arterial coupling in intensive care. J. Clin. Monit. Comput. https://doi.org/10.1007/s10877-019-00397-7 (2019).
Monge Garcia, M. I., Gil Cano, A. & Gracia Romero, M. Dynamic arterial elastance to predict arterial pressure response to volume loading in preload-dependent patients. Crit. Care 15, R15 (2011).
Huette, P., Abou-Arab, O., Longrois, D. & Guinot, P. G. Fluid expansion improve ventriculo-arterial coupling in preload-dependent patients: a prospective observational study. BMC Anesthesiol. 20, 1–9 (2020).
Guinot, P. G. et al. Respiratory stroke volume variation assessed by oesophageal Doppler monitoring predicts fluid responsiveness during laparoscopy. Br. J. Anaesth. 112, 660–664 (2014).
Guinot, P. G. et al. Ability of stroke volume variation measured by oesophageal Doppler monitoring to predict fluid responsiveness during surgery. Br. J. Anaesth. 110, 28–33 (2013).
Acknowledgments
Thank you to Suzanne Rankin for proofreading and reviewing the English manuscript.
Funding
Support was provided solely from departmental sources. None of the authors have any financial interest in the subject matter, materials or equipment discussed and in competing materials.
Author information
Authors and Affiliations
Contributions
Data acquisition: O.A.A. S.B.; Analysis and interpretation: P.G.G., M.N.; Drafting of the manuscript for important intellectual content: P.G.G., B.B., M.N., H.D. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nguyen, M., Abou-Arab, O., Bar, S. et al. Echocardiographic measure of dynamic arterial elastance predict pressure response during norepinephrine weaning: an observational study. Sci Rep 11, 2853 (2021). https://doi.org/10.1038/s41598-021-82408-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-82408-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.