Abstract
Cardiac injury among patients with COVID-19 has been reported and is associated with a high risk of mortality, but cardiac injury may not be the leading factor related to death. The factors related to poor prognosis among COVID-19 patients with myocardial injury are still unclear. This study aimed to explore the potential key factors leading to in-hospital death among COVID-19 patients with cardiac injury. This retrospective single-center study was conducted at Renmin Hospital of Wuhan University, from January 20, 2020 to April 10, 2020, in Wuhan, China. All inpatients with confirmed COVID-19 (≥ 18 years old) and cardiac injury who had died or were discharged by April 10, 2020 were included. Demographic data and clinical and laboratory findings were collected and compared between survivors and nonsurvivors. We used univariable and multivariable logistic regression methods to explore the risk factors associated with mortality in COVID-19 patients with cardiac injury. A total of 173 COVID-19 patients with cardiac injury were included in this study, 86 were discharged and 87 died in the hospital. Multivariable regression showed increased odds of in-hospital death were associated with advanced age (odds ratio 1.12, 95% CI 1.05–1.18, per year increase; p < 0.001), coagulopathy (2.54, 1.26–5.12; p = 0·009), acute respiratory distress syndrome (16.56, 6.66–41.2; p < 0.001), and elevated hypersensitive troponin I (4.54, 1.79–11.48; p = 0.001). A high risk of in-hospital death was observed among COVID-19 patients with cardiac injury in this study. The factors related to death include advanced age, coagulopathy, acute respiratory distress syndrome and elevated levels of hypersensitive troponin I.
Similar content being viewed by others
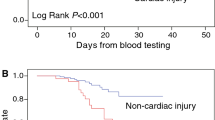
Introduction
Since the coronavirus disease 2019 (COVID-19) outbreak in December 2019, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread all over the world and resulted in considerable mortality worldwide. With the increasing number of confirmed cases and information regarding the clinical features of COVID-19, acute cardiac injury caused by COVID-19 and the high risk of in-hospital death associated with cardiac injury have generated considerable concern.
Previous studies have reported that 7.2–27.8% of COVID-19 patients had myocardial injuries, and the mortality rate was markedly higher in patients with elevated troponin I levels than in patients with normal troponin I levels1,2,3. However, the reason for the high mortality associated with cardiac injury remains unclear. Myocardial injury may only partly explain the cause of death, and there may be multiple factors involved in the death of COVID-19 patients with cardiac injury. The present study aims to analyze data from a single center in Wuhan, China, and explore the potential risk factors for in-hospital death among COVID-19 patients with cardiac injury.
Methods
Study participants
This retrospective cohort study included all in patients with confirmed COVID-19 (≥ 18 years old) and cardiac injury who died or were discharged from Renmin Hospital of Wuhan University between January 20, 2020 and April 10, 2020. The patients in this study were diagnosed with COVID-19 according to the World Health Organization interim guidance4. Patients without hypersensitive troponin I were excluded. This study was approved by the National Health Commission of China and Ethics Commission of Renmin Hospital of Wuhan University (Wuhan, China). All methods were performed in accordance with the relevant guidelines and regulations. The requirement for written informed consent was waived by the Ethics Commission of Renmin Hospital of Wuhan University (Wuhan, China). The patients reported in this manuscript have not been reported in other submissions by me or anyone else.
Data collection
We extracted the electronic medical records of patients included in this study. The demographic characteristics (age and sex), and clinical data (symptoms, comorbidities, laboratory examinations, complications, and treatments) were independently reviewed by 2 investigators.
Laboratory procedures
To confirm COVID-19, the Viral Nucleic Acid Kit (Health) was used to extract nucleic acids from clinical samples according to the kit instructions. Throat swab samples were obtained for SARS-CoV-2 Polymerase Chain Reaction (PCR) examination. A SARS-CoV-2 detection kit (Bioperfectus) was used to detect two target genes, including open reading frame 1ab (ORF1ab) and nucleocapsid protein (N), using real-time reverse transcriptase–polymerase chain reaction. An infection was considered laboratory-confirmed if the ORF1ab and N tests both showed positive results5.
Routine blood examinations included complete blood count, coagulation profile, serum biochemical tests (including renal and liver function, creatine kinase, lactate dehydrogenase, albumin, total bilirubin, hypersensitive troponin I, N-terminal pro-brain natriuretic peptide, C-reactive protein, procalcitonin, CD4 count, CD8 count, interleukin-6 (IL-6), and tumor necrosis factor α). Chest radiographs or CT scans were also acquired for all inpatients. The criteria for discharge were a temperature that had returned to normal for at least 3 days, substantial improvement in both lungs in chest CT, disappearance of clinical symptoms and two negative SARS-CoV-2 RNA tests over an interval of at least 24 h6.
Definitions
Acute kidney injury was identified according to the Kidney Disease: Improving Global Outcomes definition7. Acute cardiac injury was diagnosed if serum levels of cardiac biomarkers (e.g., high- sensitivity cardiac troponin I) were above the 99th percentile of the upper reference limit8. Acute respiratory distress syndrome (ARDS) was diagnosed according to the Berlin definition9. Acute liver injury, defined as either an alanine aminotransferase or aspartate aminotransferase level greater than three times the upper limit of normal10. Coagulopathy was defined as a 3-s extension of prothrombin time or a 5-s extension of activated partial thromboplastin time6.
Statistical analysis
Frequency rates and percentages are used to describe categorical variables, and median and interquartile range (IQR) values are used to describe continuous variables. Continuous variables were compared using the t test and categorical variables were compared using the χ2 test. Univariable and multivariable logistic regression models were used to explore the risk factors for death during hospitalization. Data were analyzed using SPSS (Statistical Package for the Social Sciences) version 13.0 software (SPSS Inc). For all the statistical analyses, p < 0.05 was considered statistically significant.
Result
Patient characteristics
A total of 187 adult patients confirmed with COVID-19 and cardiac injury were hospitalized in Renmin Hospital of Wuhan University between January 20, 2020 and April 10,2020, After excluding 14 patients who were still hospitalised or did not have available key information in their medical records, we included 173 inpatients in the final analysis. Eighty-seven patients died during hospitalization, and 86 were discharged. The median age of the 173 patients was 73.0 years (IQR 64.0–80.5), ranging from 28 to 97 years, and 111 (64.2%) were male (Table 1). The most common symptoms on admission were fever (126 patients [72.8%]), followed by cough (97 patients[56.1%]), dyspnea(60 patients[34.7%], hemoptysis (39 patients [22.5%]), fatigue or myalgia (34 patients [19.6%]), chest pain (27 patients [15.6%]), diarrhea (16 patients [9.2%]), dizziness or headache (10 patients [5.8%]) , and nausea or vomiting (6 patients [3.5%]). Hypertension (72 patients [41.6%]), diabetes (29 patients [16.8%]) and coronary heart disease (29 patients [16.8%]) were the most common comorbidities, followed by cerebrovascular disease (15 patients [8.7%]), chronic obstructive pulmonary disease (9 patients [5.2%]) and chronic kidney disease (8 patients [4.6%]). Chronic liver disease (2 patients [1.2%]), cancer (2 patients [1.2%]) and chronic heart failure (1 patient [0.6%]) were rare. A total of 133 enrolled patients (76.9%) showed bilateral involvement or ground-glass opacities on chest CT scans (Table 1).
Compared with the survivors, the nonsurvivors were older (median [range] age, 76.0 [65–83] years vs 70.5 [61.75–78.25] years; p < 0.05), and more likely to have chest pain (19 of 87 patients [21.8%] vs 8 of 86 patients [9.3%]; p < 0.05), and fatigue or myalgia (21 of 87 patients [24.1%] vs 13 of 86 patients [15.1%]; p < 0.05). The comorbidities were not very different between survivors and nonsurvivors (Table 1).
Laboratory findings
There were numerous differences in laboratory findings between survivors and nonsurvivors. Compared with the survivors, the nonsurvivors showed higher leukocyte, neutrophil, lactate dehydrogenase, total bilirubin, blood urea nitrogen, hypersensitive troponin I, C-reactive protein, procalcitonin, and IL-6 levels, as well as higher levels of D-dimer and lower lymphocyte, platelet count, CD8 count and CD4 counts (all p < 0.05; Table 2). The prothrombin time and activated partial thrombin time were longer in nonsurvivors than in survivors, with significant differences for both (all p < 0.05; Table 2).
Complications and treatments
Common complications among the 173 patients included heart failure (130 patients [75.1%]), ARDS (91 patients [52.6%]), coagulopathy (66 patients [38.2%]), acute kidney injury (65 patients [37.6%]), and acute liver injury (31 patients [17.9%]). Nonsurvivors were more likely to have coagulopathy (45 patients [51.7%] vs 21 patients [24.4%]), ARDS (68 patients [78.1%] vs 23 patients [26.7%]), acute kidney injury (42 patients [48.3%] vs 23 [26.7%]), and acute liver injury (21 patients [24.1%] vs 10 [11.6%]) than survivors. (all p < 0.05; Table 3).
The usage rates of nasal cannula, noninvasive ventilation or high-flow nasal cannula, and invasive mechanical ventilation or ECMO were 79.2% (137 patients), 49.1% (85 patients), and 16.2% (28 patients), respectively. Compared with the survivors, the nonsurvivors required more noninvasive ventilation or high-flow nasal cannula (56 [64.4%] vs 29 [33.7%]; p < 0.001) (Table 3).
Antibiotic and antiviral therapy were the most commonly used treatments (148 [85.5%], and 147 [85%], respectively), followed by intravenous immunoglobulin therapy (130 [75.1%]), corticosteroids (117 [67.6%]), vasoconstrictive agents (75 [43.4%]) and continuous renal replacement therapy (31 [17.9%]). The use of corticosteroids (66 [75.9%] vs 51 [59.3%]), and vasoconstrictive agents (58 [66.7%] vs 17 [19.8%]) was more common in nonsurvivors than in survivors (all p < 0.05; Table 3).
Risk factors for mortality
In the univariable analysis, the odds of in-hospital death were higher in patients with acute liver injury, acute kidney injury, ARDS, or coagulopathy. Lymphopenia, elevated leucocytes, elevated neutrophil count, elevated hypersensitive troponin I, elevated procalcitonin and a prolonged prothrombin time were also associated with death. We included 173 patients with complete data for all variables (87 nonsurvivors and 86 survivors) in the multivariable logistic regression model, and we found that advanced age, ARDS, coagulopathy, and elevated level of hypersensitive troponin I were associated with increased odds of death (Table 4). Age was associated with a 1.12-fold higher risk of death (OR: 1.12; 95% CI: 1.05–1.18), ARDS was associated with a 16.56-fold higher risk of death (OR: 16.56; 95% CI: 6.66–41.2), coagulopathy was associated with a 2.54-fold higher risk of death (OR: 2.54; 95% CI: 1.26–5.12), and hypersensitive troponin I was associated with a 4.54-fold higher risk of death (OR: 4.54; 95% CI: 1.79–11.48). (Table 4).
Discussion
In this study, we included 173 COVID-19 patients with cardiac injury and 87 of whom died. Notably, COVID-19-induced cardiac injury was associated with a high risk of death. By analyzing the risk factors for death, we found that older age, coagulopathy, ARDS and higher hypersensitive troponin I levels were associated with higher odds of in-hospital death.
According to recent studies in China, myocardial injury is independently associated with an increased risk of mortality among COVID-19 patients1,2. However, the reasons for the high mortality associated with cardiac injury are not well described. Advanced age has been reported as an important independent predictor of mortality in patients with COVID-196. Our study confirmed that advanced age was associated with death in COVID-19 patients with cardiac injury. However, there was no difference in comorbidities between survivors and nonsurvivors. These data differ from a recent report that showed that comorbidities may be a risk factor for poor outcome11. Symptoms including fatigue, myalgia and chest pain were more common in patients who died. Nonsurvivors required more noninvasive ventilation or high-flow nasal cannula therapy. Major complications including ARDS, coagulopathy, acute liver injury, and acute kidney injury, occurred more often in nonsurvivors. Additionally, lymphopenia, elevated leukocyte count, elevated neutrophil count, and elevated hypersensitive troponin I were also associated with death.
In our study, nonsurvivors had higher levels of D-dimer, and a longer prothrombin time and activated partial thrombin time, but lower platelet counts than survivors. Coagulation disorders are relatively frequently encountered among COVID-19 patients, especially among those with adverse outcomes. The pathogenetic mechanisms may include endothelial dysfunction, increased consumption of platelets and the decreased production of platelets, von Willebrand factor elevation, Toll-like receptor activation, and tissue-factor pathway activation12. COVID-19 patients with coagulation disorders are at risk of developing disseminated intravascular coagulation (DIC), deep vein thrombosis and pulmonary embolism, and multiorgan infarcts, which increase the risk of death13. These observations were also reported in a previous autopsy study on SARS-CoV-1-infected patients14. Coagulation activation could be related to a sustained systemic pro-inflammatory cytokine release elicited by viral infections. Inflammation not only leads to the activation of coagulation, but coagulation also affects inflammatory activity, suggesting the extensive cross-talk between these two systems15. In a coagulation cascade, IL-6 not only increases the production of fibrinogen in the liver, but also activates the coagulation system, and infusion of a monoclonal anti–IL-6 antibody resulted in the complete abrogation of coagulation activation16.
In our study, the level of IL-6 was much higher in nonsurvivors than in survivors, and IL-6 level appears to be a prognostic indicator of outcome. We also found that markers of inflammatory response, such as leukocytes, neutrophil, C-reactive protein and procalcitonin were significantly increased among nonsurvivors. These abnormalities suggest that the severe inflammatory response may be associated with death among COVID-19 patients with cardiac injury. Previous studies showed that the levels of serum pro-inflammatory cytokines (IL-6 and IFN-α) and chemokines (IL-8, CXCL- 10, and CCL5) were much higher in patients with severe MERS than in patients with mild and moderate disease17,18. Increased levels of proinflammatory cytokines (IFN-γ, IL-1, IL-6, IL-12, and TGFβ) and chemokines (CCL2, CXCL10, CXCL9, and IL-8) were observed in severe SARS patients compared to mild individuals19,20. Inflammatory cytokines can be released into the circulation and induce lung epithelial and endothelial cell apoptosis, which results in vascular leakage, alveolar edema, epithelial cell proliferation and impaired tissue remodeling ultimately resulting in ARDS21. Inflammatory cytokines can also induce hypotension, tissue hypoxia, myocardial dysfunction, and eventually lead to multiple organ dysfunction and DIC22.
We also observed that the CD4 T cell and CD8 T cell counts decreased more in nonsurvivors than in survivors. These abnormalities suggest that mortality may be associated with cellular immune deficiency. Both CD4 and CD8 T cells play a critical role in clearing viruses by eliminating virus-infected cells. Reduction in CD4 and CD8 T cells were associated with severe pneumonia. A previous study showed that a dramatic loss of CD4 T cells and CD8 T cells strongly correlated with the severity of the acute phase of SARS disease in humans23,24. A recent pathological study also found that the peripheral CD4 and CD8 T cell counts were substantially reduced in COVID-19 patients, while their status was overactivated, which accounts for the severe immune injury, in nonsurvivors25. In SARS-CoV-infected mouse models, the depletion of CD4 T and CD8 T cells reduced neutralizing antibody titers in the lungs, delayed virus clearance and further enhanced immune-mediated interstitial pneumonitis26.
Patients with coagulation activation, cellular immune deficiency and high levels of inflammatory cytokines are more likely to experience organ injury and a higher risk of death after COVID-19 infection. In this study, the level of hypersensitive troponin I, and incidence of ARDS, acute liver injury and acute kidney injury were much higher in nonsurvivors. Further multivariable logistic regression analysis showed that advanced age, elevated hypersensitive troponin I, coagulopathy and ARDS were independently associated with an increased risk of death in COVID-19 patients with cardiac injury. These observations suggest that the causes of death among COVID-19 patients with cardiac injury may involve multiorgan dysfunction and coagulation disorders, and myocardial injury may only partly explain the cause of death. Cytokine storms and a series of immune responses, or coagulation disorders may be the pathophysiological mechanism underlying organ injury caused by COVID-19. The respiratory system is the most commonly involved system in COVID-19, and some patients can rapidly develop ARDS. Previous reports showed that the incidence of ARDS was 15.6–31%, higher than that of other organ injuries3,27. The main cause of ARDS may be the injury to the alveolar epithelial cells. A recent pathological study showed evident that ARDS in the lungs was caused by SARS-CoV-2 infection. A few interstitial mononuclear inflammatory infiltrates were found in heart tissue, but no other substantial myocardial damage was observed in a patient with COVID-19, indicating that there were no obvious histological changes seen in heart tissue25. ARDS may be the main cause of death among COVID-19 patients, which is consistent with our research on COVID-19 patients with cardiac injury. The data in this study suggested that coagulopathy and ARDS are valuable warning models for predicting mortality in COVID-19 patients with cardiac injury.
This study provides novel and valuable warning information for physicians to predict the severity of the COVID19 patients with cardiac injury, and makes it possible to identify patients with a high risk of death earlier and provide timely and active management, leading to better clinical outcomes.
Conclusions
A high risk of in-hospital death was shown among COVID-19 patients with cardiac injury in this study. The factors related to death include advanced age, coagulopathy, acute respiratory distress syndrome and elevated levels of hypersensitive troponin I.
Data availability
The datasets generated during or analyzed during the current study are available from the corresponding author on reasonable request.
References
Guo, T. et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 5, 811–818 (2020).
Shi, S. et al. Association of cardiac injury with mortality in hospitalized patients With COVID-19 in Wuhan, China. JAMA Cardiol. 5, 802–810 (2020).
Wang, D., Hu, B. & Hu, C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 323, 1061–1069 (2020).
World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected: interim guidance. Preprint at https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected (2020).
Wang, M, et al. Clinical diagnosis of 8274 samples with 2019-novel coronavirus in Wuhan. Preprint at https://www.medrxiv.org/content/10.1101/2020 (2020).
Zhou, F. et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 395, 1054–1062 (2020).
Khwaja, A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 120, c179–c184 (2012).
Huang, C. et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395, 497–506 (2020).
Ranieri, V. M. et al. Acute respiratory distress syndrome: The Berlin Definition. JAMA 307, 2526–2533 (2012).
Duan, Z. P. et al. Clinical characteristics and mechanism of liver injury in patients with severe acute respiratory syndrome. Chin. J. Hepatol. 11, 493–496 (2003).
Yang, X. et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 8, 475–481 (2020).
Giannis, D., Ziogas, I. A. & Gianni, P. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J. Clin. Virol. 9, 104362 (2020).
Tang, N., Li, D., Wang, X. & Sun, Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 18, 844–847 (2020).
Xiang-hua, Y. et al. Severe acute respiratory syndrome and venous thromboembolism in multiple organs. Am. J. Respir. Crit. Care Med. 182, 436–437 (2010).
Levi, M. & Poll, T. V. Coagulation in patients with severe sepsis. Semin. Thromb. Hemost. 41, 9–15 (2015).
Tanaka, T., Narazaki, M. & Kishimoto, T. Immunotherapeutic implications of IL-6 blockade for cytokine storm. Immunotherapy 8, 959–970 (2016).
Kim, E. S. et al. Clinical progression and cytokine profiles of Middle East respiratory syndrome coronavirus infection. J. Korean Med. Sci. 31, 1717–1725 (2016).
Min, C. K. et al. Comparative and kinetic analysis of viral shedding and immunological responses in MERS patients representing a broad spectrum of disease severity. Sci. Rep. 5, 25359 (2016).
Chien, J. Y., Hsueh, P. R., Cheng, W. C., Yu, C. J. & Yang, P. C. Temporal changes in cytokine/chemokine profiles and pulmonary involvement in severe acute respiratory syndrome. Respirology 11, 715–722 (2006).
Wong, C. K. et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol. 136, 95–103 (2004).
Channappanavar, R. & Periman, S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin. Immunopathol. 39, 529–539 (2017).
Ye, Q., Wang, B. & Mao, J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. J. Infect. 80, 607–613 (2020).
Li, T. et al. Significant changes of peripheral T lymphocyte subsets in patients with severe acute respiratory syndrome. J. Infect. Dis. 189, 648–651 (2004).
Li, T. et al. Rapid loss of both CD4? and CD8? T lymphocyte subsets during the acute phase of severe acute respiratory syndrome. Chin. Med J. 116, 985–987 (2003).
Xu, Z. et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 8, 420–422 (2020).
Channappanavar, R., Zhao, J. & Periman, S. Cell-mediated immune response to respiratory coronaviruses. Immunol Res. 59, 118–128 (2014).
Chen, N. et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 395, 507–513 (2020).
Funding
This work was supported by grants from the National Natural Science Foundation of China [81800444 and 81800431] as well as grants from the Natural Science Foundation of Hubei Province [2018CFB415].
Author information
Authors and Affiliations
Contributions
Y.H. and X.Z. conceived and designed the study, analyzed the data and wrote the manuscript. X.L. revised the manuscript. X.J. provided study oversight and participated in manuscript revision. All authors had access to the study data and approved the decision to submit the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
He, Y., Zheng, X., Li, X. et al. Key factors leading to fatal outcomes in COVID-19 patients with cardiac injury. Sci Rep 11, 4144 (2021). https://doi.org/10.1038/s41598-021-82396-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-82396-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.


